Abstract
Background
Staphylococcus epidermidis and S. aureus have been identified as the most common bacteria responsible for sub-clinical and overt breast implant infections and their ability to form biofilm on the implant as been reported as the essential factor in the development of this type of infections. Biofilm formation is a complex process with the participation of several distinct molecules, whose relative importance in different clinical settings has not yet been fully elucidated. To our knowledge this is the first study aimed at characterizing isolates causing breast peri-implant infections.
Results
Thirteen S. aureus and seven S. epidermidis causing breast peri-implant infections were studied.
Using the broth microdilution method and the E-test, the majority of the strains were susceptible to all antibiotics tested. Methicillin resistance was detected in two S. epidermidis. All strains had different RAPD profiles and were able to produce biofilms in microtitre plate assays but, while all S. aureus carried and were able to express icaA and icaD genes, this was only true for one S. epidermidis. Biofilm development was glucose- and NaCl-induced (5 S. aureus and 1 S. epidermidis) or glucose-induced (the remaining strains). Proteinase K and sodium metaperiodate treatment had different effects on biofilms dispersion revealing that the strains studied were able to produce chemically different types of extracellular matrix mediating biofilm formation.
All S. aureus strains harboured and expressed the atlA, clfA, FnA, eno and cna genes and the majority also carried and expressed the sasG (10/13), ebpS (10/13) genes.
All S. epidermidis strains harboured and expressed the atlE, aae, embp genes, and the majority (six strains) also carried and expressed the fbe, aap genes.
Genes for S. aureus capsular types 5 and 8 were almost equally distributed. The only leukotoxin genes detected were lukE/lukD (6/13).
Conclusions
S. aureus and S. epidermidis breast peri-implant infections are caused by heterogeneous strains with different biofilm development mechanisms.
Since the collagen adhesin (cna) gene is not ubiquitously distributed among S. aureus, this protein could have an important role in the cause of breast peri-implant infections.
Electronic supplementary material
The online version of this article (doi:10.1186/s12866-015-0368-x) contains supplementary material, which is available to authorized users.
Keywords: Implant infections, Biofilm, Staphylococcal infections, ica gene, biofilm-related genes, MSCRAMMs
Background
Immediate breast reconstruction, using tissue expanders and implants, has become a standard of care for almost all women requiring mastectomy for breast cancer [1-3]. However, after implantation, patients may experience local complications during the ensuing years, with peri-implant infection being one of the most common problems, with an infection rate ranging from 0 to 29 percent [4].
The most traditional approach to severe or refractory breast periprosthetic infection remains removal of the device. However, removal makes subsequent reinsertion and re-expansion more difficult, since the soft tissue retracts rapidly to close the expanded pocket [5].
The majority of cases reported identify Staphylococcus epidermidis and S. aureus as the most common bacteria responsible for sub-clinical and overt breast implant infections.
Bacteria may reach the implants during or after placement and their ability to form biofilms on the implant has been reported as the essential factor in the development and persistence of infection. Biofilm formation is a complex process with the participation of several distinct molecules, whose relative importance in different clinical settings has not yet been fully elucidated.
Specific staphylococcal surface proteins impacting adhesion to abiotic surfaces (AtlA, AtlE, Bap/Bhp) as well as a vast array of proteins called MSCRAMMs (Microbial Surface Components Recognizing Adhesive Matrix Molecules) promoting colonization of medical implants have been identified [6]. In S. aureus and S. epidermidis, production of an extracellular polysaccharide promoting intercellular adhesion (PIA), also called polymeric N-acetyl-glucosamine (PNAG) is currently the best-characterised biofilm mechanism [7]. PIA/PNAG is synthesised by enzymes encoded by the ica operon.
Once this operon is activated, four proteins are transcribed icaA, ica D, icaB and icaC. The expression of icaA alone induces low enzymatic activity, however, the simultaneous expression of icaA and icaD promotes a significant increase in the amount of polysaccharide [8].
However, recent studies have begun to highlight the existence of PIA/PNAG-independent biofilm mechanisms in both species [9]. Accumulation-associated protein (Aap) independently or toghether with ica operon, has also been suggested to be important in coagulase-negative staphylococci [10]. In S. aureus and S. epidermidis more additional surface proteins such as SasG, extracellular matrix binding protein (Embp), biofilm-associated protein (Bap) and the fibronectin-binding proteins FnbpA and B, were implicated in matrix formation. These findings suggest that other surface proteins may also be involved in biofilm development. In some strains, biofilm formation may be mediated additionally or exclusively by specific surface proteins (Bap/Bhp and Aap) [6].
While several studies have extensively described the distribution of genes involved in biofilm formation and virulence in Staphylococcal strains causing orthopaedic peri-implant infections, to our knowledge, this is the first study characterizing isolates causing breast peri-implant infections.
Results
Antimicrobial susceptibility and mecA presence
All S. aureus isolates were susceptible to, oxacillin, co-trimoxazole, daptomycin, gentamicin, linezolid, rifampin, tetracycline and vancomycin. Ciprofloxacin- and erythromycin resistance was detected in one (1/13, 7.7%) and three (3/13, 23.1%) strains, respectively (Table 1). Two out of the three erythromycin-resistant S. aureus showed inducible clindamycin resistance.
Table 1.
Susceptibility of 20 Staphylococci isolated from breast implant infections
| Range | MIC50 | MIC90 | %S | ||
|---|---|---|---|---|---|
| S. aureus (13) | ciprofloxacin | ≤0.12-4 | ≤0.12 | 1 | 92.3 |
| cotrimoxazole | ≤2/38 | ≤2/38 | ≤2/38 | 100 | |
| clindamycin | ≤0.25 | ≤0.25 | ≤0.25 | 100* | |
| erythromycin | ≤0.25- ≥ 8 | ≤0.25 | ≥8 | 76.9 | |
| tetracycline | ≤1 | ≤1 | ≤1 | 100 | |
| rifampicin | ≤0.5 | ≤0.5 | ≤0.5 | 100 | |
| linezolid | 1 | 1 | 1 | 100 | |
| vancomycin | ≤0.5-1 | 1 | 1 | 100 | |
| oxacillin | ≤0.25-1 | 0.5 | 1 | 100 | |
| gentamicin | ≤0.25-0.5 | 0.5 | 0.5 | 100 | |
| daptomycin | 0.12-1 | 0.25 | 1 | 100 | |
| S. epidermidis (7) | ciprofloxacin | ≤0.12 | 100 | ||
| cotrimoxazole | ≤2/38-4 | 85.7 | |||
| clindamycin | ≤0.25 | 100 | |||
| erythromycin | ≤0.25- ≥ 8 | 28.6 | |||
| tetracycline | ≤1 | 100 | |||
| rifampicin | ≤0.5 | 100 | |||
| linezolid | 1 | 100 | |||
| vancomycin | 1 | 100 | |||
| oxacillin | ≤0.25-4 | 71.4 | |||
| gentamicin | ≤0.25- > 16 | 71.4 | |||
| daptomycin | 0.25-0.5 | 100 |
*Two strains showed inducible clindamycin resistance.
All S. epidermidis were susceptible to, ciprofloxacin, clindamycin, daptomycin, linezolid, rifampin, tetracycline and vancomycin. Oxacillin, erythromycin, gentamicin and co-trimoxazole resistance was detected in 2/7, 5/7, 2/7 and 1/7 strains, respectively.
The oxacillin susceptibility results were confirmed by cefoxitin disk test and mecA PCR.
Biofilm assays
All S. aureus isolates, and only one out of seven S. epidermidis isolates were classified as slime producers by the Congo Red Agar (CRA) plate method, developing brown-black (S. aureus) or black (S. epidermidis) colonies.
All isolates were classified as biofilm-producing strains by the polystyrene microtiter plates (MtP) method.
When grown in brain heart infusion (BHI) supplemented with 1% glucose, biofilm development was significantly induced in twelve S. aureus and six S. epidermidis strains while 4% NaCl activated biofilm development in five S. aureus and one S. epidermidis strain (Figure 1). For these six strains NaCl was a stronger biofilm inducer than glucose.
Figure 1.
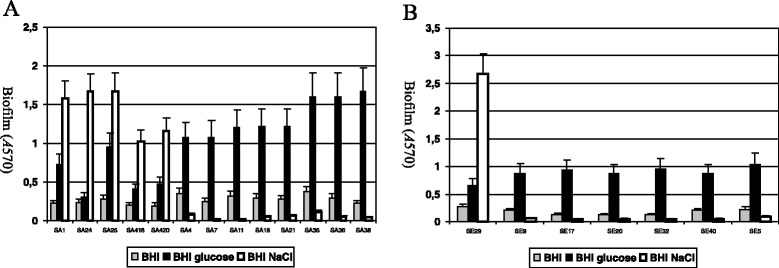
Biofilm formation in 13 S. aureus (A) and 7 S. epidermidis (B) strains studied. The strains were grown in BHI medium or in BHI supplemented with 1% glucose or 4% NaCl at 37°C for 24 h. Biofilm formation in tissue culture-treated 96-well plates was measured three times, and standard deviations, which were less than 20%, are indicated.
Addition of DNase I did not affect bacterial biofilm stability. Although not statistically significant the lowest percentages of reduction of primary attachment (<10%) were observed for those strains producing NaCl inducible biofilms (Figures 2 and 3).
Figure 2.
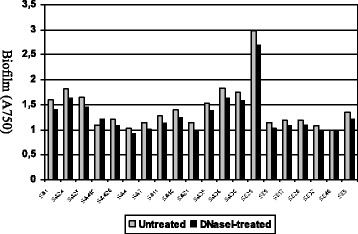
Susceptibility of S. aureus and S. epidermidis biofilms to treatment with DNase I. Strains SA 1, SA 24, SA 25, SA 418, SA 420 and SE 29 were grown in BHI supplemented with 4% NaCl at 37°C for 24 h prior to treatment. The remaining strains were grown in BHI supplemented with 1% glucose at 37°C for 24 h prior to treatment.
Figure 3.
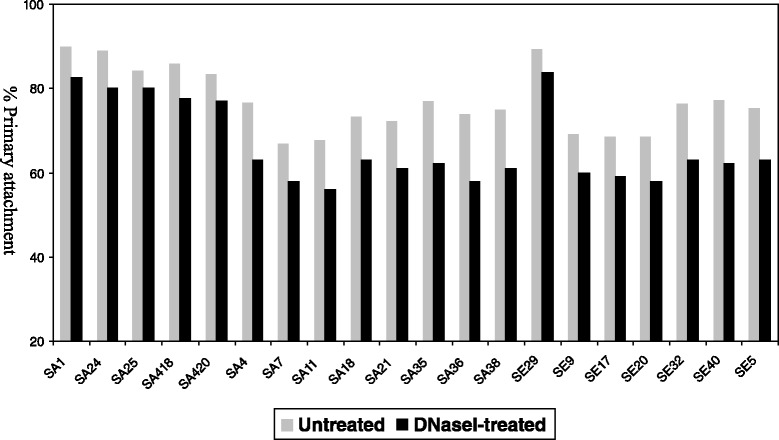
Effect of DNase I on S. aureus and S. epidermidis primary attachment. Strains SA 1, SA 24, SA 25, SA 418, SA 420 and SE 29 were grown in BHI supplemented with 4% NaCl at 37°C for 24 h prior to treatment. The remaining strains were grown in BHI supplemented with 1% glucose at 37°C for 24 h prior to dilution and treatment.
All NaCl-induced biofilms were susceptibile to sodium metaperiodate (>70% reduction in optical density (OD)) and resistant to proteinase K (Figure 4). All the remaining biofilms (significantly or only slightly glucose-induced) were resistant to sodium metaperiodate and substantially dispersed by treatment with proteinase K (>60% reduction in OD), with the exception of those produced by two S. aureus strains (both proteinase K and sodium metaperiodate resistant).
Figure 4.
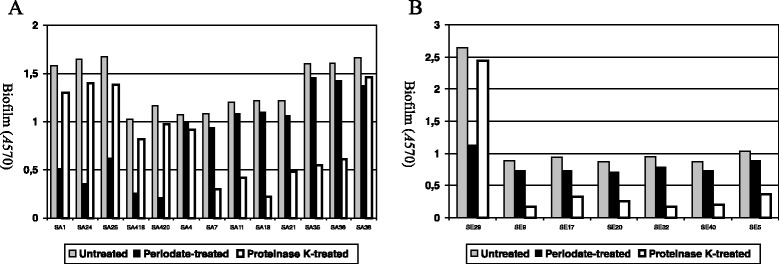
Susceptibility of S. aureus (A) and S. epidermidis (B) biofilms to treatment with sodium metaperiodate and proteinase K. Strains SA 1, SA 24, SA 25, SA 418, SA 420 and SE 29 were grown in BHI supplemented with 4% NaCl at 37°C for 24 h prior to treatment. The remaining strains were grown in BHI supplemented with 1% glucose at 37°C for 24 h prior to treatment.
The different behaviours observed suggested different chemical compositions in the biofilm extracellular matrices.
Detection of MSCRAMM and biofilm genes
All S. aureus harboured the icaA/D, atlA, clfA, FnA, eno and cna genes and the great majority carried sasG (10/13), ebpS (10/13) and fib (9/13), while only two strains carried FnB (Table 2). No strain harboured the bap and bbp genes.
Table 2.
Distribution of MSCRAMM and biofilm genes among S. aureus and S. epidermidis
| ebpS | eno | AtlA/E | aae | aap | bap/bhp | sasG | cna | bbp | fnbA | fnbB | clfA | clfB | fbe | sdrF | embp | fib | icaA/D | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus (13) | 10 | 13 | 13 | n. t. | n.t. | 0 | 10 | 13 | 0 | 13 | 2 | 13 | 8 | n.t | n.t | n.t | 9 | 13 |
| S. epidermidis (7) | n.t. | n.t. | 7 | 7 | 6 | 0 | n.t. | n.t. | n.t. | 0 | 0 | n.t. | n.t. | 6 | 4 | 7 | n.t. | 2* |
*1 strain was icaA negative and icaD positive.
Two S. epidermidis carried ica genes. One S. epidermidis (classified as a slime producer according to the CRA plate method) carried both the icaA and icaD genes and another strain (classified as a non slime producer, by CRA plate method) was icaD positive.
All S. epidermidis harboured the atlE, aae, embp genes, the aap and the fbe genes were carried by six strains, while four strains carried the SdrF gene (Table 2). No strains harboured the bhp gene (Additional files 1 and 2).
Expression of MSCRAMM and biofilm genes
Under the experimental conditions used all strains expressed icaA/D genes with the exception of one S. epidermidis strain which did not express the icaD gene (Additional file 3).
For all S. aureus strains the RT-PCR amplicons showed positive expression signals for all the remaining genes tested with the exception of fib. All S. epidermidis strains expressed the atlE, aae, embp genes, and six strains out of seven expressed the fbe, aap genes.
Detection of S. aureus capsular and leukotoxin genes
Six strains were PCR-positive for capsular type 8 and seven for capsular type 5 genes (Additional file 1).
Seven out of thirteen S. aureus strains carried the LukE/LukD leukotoxin genes, while the PVL and LukM leukotoxins genes were not detected.
Randomly amplified polymorphic DNA (RAPD) polymerase chain reaction (PCR) profiles
To rule out the possibility of infections due to one epidemic S. aureus or S. epidermidis strain, all isolates (13 S. aureus and 7 S. epidermidis) underwent RAPD PCR with three and two different primers respectively. All S. aureus and all S. epidermidis had different RAPD profiles (Additional files 4 and 5).
S. aureus MLST and eBURST analysis
As shown in Table 3, a total of 8 sequence types (STs) were revealed, which were further grouped into 6 clonal complexes (CCs).
Table 3.
Clonal relatedness of 13 S. aureus isolates and their agr groups
| Strains | agr group | Sequence type | Clonal complex* |
|---|---|---|---|
| SA1 | I | 45 | 45 |
| SA4 | III | 30 | 30 |
| SA7 | I | 45 | 45 |
| SA 11 | II | 15 | 5 |
| SA18 | II | 1162 | 10 |
| SA21 | I | 20 | 20 |
| SA24 | II | 5 | 5 |
| SA 25 | I | 22 | 22 |
| SA35 | II | 194 | 5 |
| SA 36 | II | 5 | 5 |
| SA 38 | I | 45 | 45 |
| SA 418 | I | 22 | 22 |
| SA 420 | I | 22 | 22 |
*CCs as defined by eBurst analysis with a stringent group definition with six of seven loci.
S. aureus agr typing
The distribution of agr alleles among the 13 S. aureus strains is provided in Table 3.
The agr type I was predominant (7/13; 53,8%), followed by agr type II (5/13; 38,5%). Only one S. aureus belonged to agr type III.
Discussion
Staphylococcal infection represents a major concern when associated with breast reconstruction, as it may necessitate additional hospitalization, antibiotic treatment, and in more serious cases removal of the device making subsequent reinsertion and re-expansion more difficult [5].
Theoretically, both endogenous and hospital-acquired staphylococci may gain access to breast implants during or following placement. Our data (susceptible antibiotypes, heterogeneity of the STs and the RAPD profiles) supports a community origin of the infecting microorganisms, in most cases. After accession to surgical implants, the essential factor in the evolution and persistence of infections is the formation of bacterial biofilm around implanted devices [11]. All isolates studied were able to form biofilm on polystyrene surfaces but, in contrast to S. aureus, the great majority of S. epidermidis isolates did not carry the icaA or icaD genes. Our results are in agreement with previous studies showing that the ica-operon is present and expressed in almost all S. aureus isolates, although the absence of ica-operon activity does not necessarily impact on the ability of S. epidermidis to cause clinical biofilm disease. In this study, only one out of seven S. epidermidis strains carried both icaA and icaD. In a recent letter, Persichetti and colleagues [12], described 10 S. epidermidis causing periprosthetic breast infections and capsular contracture, yet they reported only three icaA and icaD positive strains. Although the number of cases analysed in both studies is limited, it seems that ica-independent biofilm formation plays a major role compared to ica-dependent mechanisms in S. epidermidis breast peri-implant infections. In contrast, all S. epidermidis studied carried and expressed the gene for Aap protein, a protein that has been shown to mediate biofilm formation in strains lacking the ica genes. Biofilm induction by glucose or NaCl (a known activator of ica operon transcription) had different effects on our strains and revealed that our strains had different mechanisms of biofilm development, irrespective of ica operon carriage. Proteinase K and sodium metaperiodate treatment confirmed that strains causing breast peri-implant infection can produce different types of biofilm extracellular matrix (involving polysaccharides, or proteins or both). Although not statistically significant DNase I appeared less effective in reducing bacterial attachment in those strains producing NaCl-induced biofilm, suggesting a minor role of extracellular DNA in these isolates. Our data adds further evidence that, in the context of peri-implant infections, biofilm formation is a complex, phenomenon involving distinct molecules, whose relative importance may vary depending on the different clinical settings. Addition of DNase I did not affect bacterial biofilm stability as already reported [13].
Genes coding for biofilm-associated molecules have been thoroughly studied [14] in S. aureus and S. epidermidis isolated from prosthetic joint infections (PJI).
The most striking difference observed between S. aureus strains isolated from PJI and breast implant infections was the rate of surface adhesin (sasG) and collagen adhesin (cna) genes found. The isolation of a cna-positive S. aureus strain was more probable in isolates from breast implant infections than from PJI (100% vs 22-29%) indicating that cna is of major importance in the specific context of breast implant infections. Other Authors [15-17] have shown the prevalence of the cna gene to be much higher in methicillin-resistant S. aureus (MRSA) (93%) than methicillin-sensible S. aureus (MSSA) clones (46.6%) and that cna positive strains appeared to be associated with capsular type 8. However, our strains were all methicillin sensible (MS) and 7 out of 13 strains carried the cps5 gene for capsular type 5 and the remaining cps8, suggesting that the site of infection may have been the driving force in selecting a subpopulation of cna positive S. aureus strains irrespective of other characteristics such as methicillin resistance and capsular type.
An elevated breast collagen concentration has been shown to be one of the greatest risk factors for developing breast carcinoma [18].
Breast implants, especially in oncologic patients, due to the increased collagen deposition, could be easily covered by a more dense collagen matrix and thus become more prone to adhesion of S. aureus strains able to bind collagen.
In contrast to S. aureus causing PJI, the great majority of our strains carried and expressed the sasG gene (a gene coding for a surface protein that has been shown to promote biofilm formation and adherence to nasal epithelial cells).
This difference in distribution according to the site of infection, is remarkable as its S. epidermidis homologue aap (accumulation-associated protein) appears to be almost ubiquitously present. Further studies are needed to better characterize this protein, its function/s and to understand its role in different types of infections.
The gene coding for Bap, a cell wall protein found in bovine mastitis S. aureus isolates, involved in intercellular adhesion and biofilm formation was absent in all S. aureus isolates studied. Our results support the suggestion of other authors, that the role of Bap in human infections is doubtful [14].
Besides the ability to form biofilms, other authors have investigated the incidence of genes coding for leukocidal toxins in S. aureus isolated from implant orthopaedic infections. Panton-Valentine, LukE/LukD and LukM leukocidins are known to be associated with necrotic skin and soft tissue infections and could promote damage to peri-implant tissues. lukE/lukD was the only gene found in strains from breast implant infections (53.8%). Leukotoxin genes displayed a similar prevalence in strains from breast implants and PJIs, suggesting that LukE/LukD could be the only relevant leukocidin in both types of implant-related infections.
The great majority of S. epidermidis analysed carried AtlE, aap, aae, embp and fbe genes, a condition already described for S. epidermidis isolated from catheter-related bloodstream infections and PJIs as well as for commensal S. epidermidis, indicating that these proteins are valuable during both infection and colonization.
In contrast to S. aureus only three S. epidermidis strains carried a gene (SdrF) coding for a surface protein binding collagen. This is not surprising, since adhesins that recognize host proteins coating the device seem to be the primary mechanism of adhesion in S. aureus, while S. epidermidis initial adherence is probably multifactorial [19].
Breast reconstruction has become a standard of care for almost all women requiring mastectomy for breast cancer. Reduction of mutilation greatly improves the psychological status of women without interfering with oncological treatment, However, the reconstructive approach may be compromised by early or late implant infection in 8% of patients in prospective series [20]. Further studies are needed to confirm our observation and to verify if patients colonized by cna positive strains are at higher risk of breast peri-implant infections than those colonized by cna negative S. aureus. If this holds true, screening of patients before and after reconstructive surgery at regular intervals could be extremely useful to identify those colonized and thus concentrate management, and resources. Should colonization with cna-positive strains confirm to be predictive for higher risk of implant infection, effective prophylactic strategies/treatments could be devised to reduce the risk of infection of reconstruction breast implants.
Conclusions
S. aureus and S. epidermidis breast peri-implant infections are caused by heterogeneous strains with different biofilm development mechanisms. Since the collagen adhesin (cna) gene is not ubiquitously distributed among S. aureus, this protein could have an important role in the cause of breast peri-implant infections. Further studies are needed to confirm our observation and eventually recommend screening of patients before and after reconstructive surgery to identify those colonized by cna-positive S. aureus and at higher risk of peri-implant infections.
Methods
Twenty staphylococci (13 S. aureus isolates and 7 S. epidermidis isolates) were studied.
The isolates represented non repetitive strains causing periprosthetic infection in patients who underwent breast implantation (silicone mammary prosthesis) after mastectomy for breast cancer at the Oncology Institute (IST) of Genoa, Italy, between January 2011 and January 2013.
Clinical data and isolates were taken as part of standard patient care and according to this condition the study was exempt from specific ethics commitee scrutiny and approval. Informed consent for the use of clinical data has been obtained by patients.
Infection was defined as a complication occurring after breast implant surgery that was characterized by three or more of the following findings: pain, local swelling, erythema, pus, fever, seroma, wound dehiescence, or perforation of the skin, as previously described [20].
All isolates were identified using a commercial biochemical tests (API Staph identification strip; bioMérieux, Marcy-l’Etoile, France).
Strains were isolated from implants (8), peri-implant fluids (6), capsular tissues (4), a tissue expander (1) and a pus sample (1).
A sonication procedure was adopted to detach adherent bacteria from the expander, implants and capsular tissues [21].
Susceptibility tests
The minimal inhibitory concentrations (MICs) of oxacillin, ciprofloxacin, gentamicin, erythromycin, clindamycin, co-trimoxazole, tetracycline, rifampin, vancomycin and linezolid were determined by the broth microdilution method following the European committee for Antimicrobial Susceptibility testing (EUCAST) (Version 4.0, 2014) guidelines and interpretative criteria [22,23]. Daptomycin-MICs were determined by E-test (bioMérieux, Marcy-l’Etoile, France) on Muller-Hinton agar (Biolife, Milan, Italy) supplemented with 50 mg/L calcium. S. aureus ATCC-29213 was included for quality control of antimicrobial susceptibility patterns. Cefoxitin disk diffusion test was used to confirm oxacillin resistance (EUCAST, version 4.0, 2014) [23].
Inducible clindamycin resistance was evaluated using the D-zone test as recommended by EUCAST (Version 4.0, 2014) [23].
Detection of S. epidermidis mecA gene
The presence of the mecA gene in S. epidermidis strains was investigated by PCR using primers and conditions described by Vannuffel et al. [24].
Characterization of slime-producing ability
The phenotypic characterization of slime-producing ability by culture of the strains on CRA plates, prepared by adding 0.8 g of Congo red (Sigma, Missouri) and 36 g of saccharose (Sigma, Missouri) to 1 L of brain heart infusion agar (Oxoid, Basingstoke, Hampshire, England) as described by Freeman et al. [25]. S. aureus colonies on CRA were kept under observation for up to 72 h, as described by Arciola et al. [26].
Biofilm assays
The presence and extent of biofilm structures, produced by staphylococci was quantified spectrophotometrically at 570 nm using MtP plates following the indications described by Christensen et al., [27] and as reported previously by this group [28]. In accordance with the original method, strains with OD values < 0.120 were considered as negative, those with ODs >0.120 and <0.240 were regarded as weak biofilm-producers. An OD value > 0.240 was indicative of biofilm-producing bacterial strains. For each isolate the MtP test was repeated in triplicate.
In order to assess glucose- and NaCl-induced biofilm formation, bacteria were grown in 96-well polystyrene microtitre plates in BHI medium or BHI supplemented with 1% glucose or 4% NaCl at 37°C for 24 h. Biofilms were quantified spectrophotometrically as described by O’Neill et al. [29].
Biofilm stability against proteinase K, sodium metaperiodate and DNaseI treatment was tested for both species as described previously [29]. Effect of DNaseI on biofilm development was assessed as described by Houston et al. [30].
Two-tailed, two sample equal variance Student’s t-tests (Microsoft Excel 2007) were used to determine statistically significant differences in biofilm-forming capacity and biofilm stability.
PCR-method for the detection of MSCRAMM and biofilm genes
The presence of icaA and icaD genes was detected by PCR using primers and conditions described by O’Neill et al., Rohde et al., Arciola et al. and de Silva et al. [26,29,31,32].
The genes encoding the autolysins/adhesins responsible for primary attachment to abiotic surfaces (atlA/ atlE and aae), the components causing intercellular aggregation (bap, sasG, bhp, aap) and microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) bbp, cna, fib, fnbA, fnbB, clfA, clfB, fbe, ebpS, eno, sdrF and embp, were amplified by PCR using the primers and conditions summarized in (Additional file 6: Table S3) [33-45].
Expression of MSCRAMM and biofilm genes
Expression of MSCRAMM and biofilm genes was assessed by Reverse transcription (RT)-PCR for all staphylococcal strains.
RNA was purified from cultures grown at 37°C in BHI medium supplemented with 1% glucose (strains producing glucose-induced biofilms) or 4% NaCl (strains producing NaCl-induced biofilms) to an A600 of 2.0. Total bacterial RNA was isolated using the High Pure RNA Isolation Kit (Roche, Mannheim, Germany) according to the manufacturer's instructions. After purification, contaminating DNA was removed with DNase I recombinant RNase-free (10 U/40 μg of total bacterial RNA) at 37°C for 20 min. The RNA was then repurified using RNeasy Mini columns (Qiagen, Inc.). The amount of RNA recovered was determined spectrophotometrically, and the absence of DNA was verified by PCR using primers described in the Multilocus sequence typing (MLST) database (http://www.mlst.net) corresponding to the constitutively expressed phosphate acetyltransferase gene (pta) for S. aureus and the carbamate kinase gene (arcC) for S. epidermidis. Samples were then stored at −80°C.
RT-PCR was performed using the Transcriptor First Strand cDNA synthesis kit (Roche, Mannheim, Germany) and the cDNA was directly used as a template to amplify selected MSCRAMM and biofilm genes using the same primers and PCR conditions mentioned earlier (Additional file 6: Table S3). PCR products were separated by electrophoresis in a 1.2% agarose gel and visualized with ethidium bromide under UV light.
Detection of S. aureus capsular and leukotoxin genes
PCR amplification of S. aureus cap5 (to assess for genes encoding capsular type 5), and cap8 (to assess for genes encoding capsular type 8), was performed using primers and conditions described by Sau et al., [46].
Detection of the Panton-Valentine leukocidin (PVL) and LukE/LukD and LukM leukotoxin genes was performed for all S. aureus isolates by PCR as previously reported [47,48] (Additional file 6: Table S3).
RAPD
RAPD analysis was performed on S. aureus strains using three separate primers, AP-PCR1 (5′-ggTTgggTgAgAATTgcAcg) AP-PCR7 (5′-gTggATgcgA) and AP-PCR ERIC-2 (5′-AAgTAAgTgAcTGGGGTgAgCg), with PCR conditions described by Van Belkum et al. [49].
For S. epidermidis strains RAPD analysis was performed using primers AP-PCR ERIC-2 and AP-PCR7.
The RAPD patterns were considered to be different when the profiles differed by at least one band.
S. aureus MLST and eBURST analysis
MLST was performed for all S. aureus isolates according to the protocol described on the S. aureus MLST website (http://saureus.mlst.net). The allele types and the resulting sequence types were determined by submitting the allelic profile of representative alleles to the S. aureus MLST database via the Internet. Sequence types were clustered into groups by eBURST analysis (v3.0 software) with a stringent group definition with six of seven loci to determine the clonal relationship of the isolates.
S. aureus agr typing
For all S. aureus the agr locus was typed by a multiplex PCR as previously described [50].
Acknowledgements
We would like to thank Jennifer McDermott (Genoa, Italy) for the English revision of the manuscript. The authors wish also to thank Dr. Michaela Adami and Dr. Maria Stella Leone for heir help in clinical samples collection. Preliminary results of this work were presented as a poster at the 23nd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Berlin, 2013.
Abbreviations
- MSCRAMMs
Microbial surface components recognizing adhesive matrix molecules
- (PIA)
Extracellular polysaccharide promoting intercellular adhesion
- PNAG
Polymeric N-acetyl-glucosamine
- CRA
Congo red agar
- MtP
Polystyrene microtiter plates
- BHI
Brain heart infusion
- OD
Optical density
- RAPD
Randomly amplified polymorphic DNA
- PCR
Polymerase chain reaction
- PJI
Prosthetic joint infections
- MRSA
Methicillin-resistant S. aureus
- MSSA
Methicillin-susceptible S. aureus
- MS
Methicillin-sensible
- aap
Accumulation-associated protein
- MIC
Minimum inhibitory concentration
- EUCAST
European committee for antimicrobial susceptibility testing
- RT
Reverse transcription
- MLST
Multi locus sequencing typing
- PVL
Panton-Valentine leukocidin
- CC
Clonal complex
Additional files
Genetic and phenotypic characteristics of 13 S. aureus strains studied.
Genetic and phenotypic characteristics of 7 S. epidermidis strains studied.
Comparison of ica operon expression in S. aureus and S. epidermidis grown in BHI medium or in BHI supplemented with 4% NaCl or 1% glucose (Glu).
RAPD fingerprinting of the 13 S. aureus strains studied. RAPD profiles generated by primer AP-PCR1 (A), primer AP-PCR7 (B) and primer AP-PCR ERIC-2 (C). The DNA molecular weight marker XIV (Roche, Mannheim, Germany) is in lanes 1 and 15.
RAPD fingerprinting of the 7 S. epidermidis strains studied. RAPD profiles generated by primer AP-PCR7 (A) and primer AP-PCR ERIC-2 (B). The DNA molecular weight marker XIV (Roche, Mannheim, Germany) is in lanes 1 and 9.
Primers used for PCR-based detection of staphylococcal factors involved in the pathogenesis of foreign-body associated infections.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RB carry out the experiments, PM, FS and BI contributed to collect clinical samples and data and revised the manuscript, ADM designed the study and revised the manuscript, AM designed the study, carry out the experiments and wrote the article. All authors read and approved the final version for publication.
Authors’ information
Ramona Barbieri is a Microbiology PhD student working at the Microbiology Unit, DISC, University of Genoa, Italy. Marianna Pesce, Simonetta Franchelli and Ilaria Baldelli work at the Reconstruction Plastic Surgery Unit, San Martino IST, Genoa and their main research topics are plastic surgery and breast cancer. Andrea De Maria, is Associate Professor in Infectious Diseases at the University of Genoa, Genoa, Italy. His research fields include: immunopathogenesis of chronic and persistent infections (HIV, HCV, HPV, HTLV and Mycobacteria) and control and therapy of infectious diseases in oncologic patients. Anna Marchese is Associate Professor of Clinical Microbiology at the University of Genoa, Genoa, Italy. Her research fields include: epidemiology of mechanisms of antibiotic resistance, antimicrobial susceptibility testing, antimicrobial profile of new drugs, bacterial genetics. She has published more than 80 international papers.
Contributor Information
Ramona Barbieri, Email: ramona.barbieri@unige.it.
Marianna Pesce, Email: S2894245@studenti.unige.it.
Simonetta Franchelli, Email: simonetta.franchelli@istge.it.
Ilaria Baldelli, Email: ilaria.baldelli@unige.it.
Andrea De Maria, Email: de-maria@unige.it.
Anna Marchese, Email: anna.marchese@unige.it.
References
- 1.Spiegel AJ, Butler CE. Recurrence following treatment of ductal carcinoma in situ with skin-sparing mastectomy and immediate breast reconstruction. Plast Reconstr Surg. 2003;111(Suppl 2):706–11. doi: 10.1097/01.PRS.0000041440.12442.05. [DOI] [PubMed] [Google Scholar]
- 2.Mustonen P, Lepistö J, Papp A, Berg M, Pietiläinen T, Kataja V, et al. The surgical and oncological safety of immediate breast reconstruction. Eur J Surg Oncol. 2004;30(Suppl 8):817–23. doi: 10.1016/j.ejso.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Radovanovic Z, Radovanovic D, Golubovic A, Ivkovic-Kapicl T, Bokorov B, Mandic A. Early complications after nipple-sparing mastectomy and immediate breast reconstruction with silicone prosthesis: results of 214 procedures. Scand J Surg. 2010;99(Suppl 3):115–8. doi: 10.1177/145749691009900302. [DOI] [PubMed] [Google Scholar]
- 4.Phillips BT, Bishawi M, Dagum AB, Khan SU, Bui DT. A systematic review of antibiotic use and infection in breast reconstruction: what is the evidence? Plast Reconstr Surg. 2013;131(Suppl 1):1–13. doi: 10.1097/PRS.0b013e3182729c39. [DOI] [PubMed] [Google Scholar]
- 5.Kendrick AS, Chase CW. Salvage of an infected breast tissue expander with an implant sizer and negative pressure wound management. Plast Reconstr Surg. 2008;121(Suppl 3):138e–9. doi: 10.1097/01.prs.0000300196.75587.bf. [DOI] [PubMed] [Google Scholar]
- 6.Otto M. Molecular basis of Staphylococcus epidermidis infections. Semin Immunopathol. 2012;34(Suppl 2):201–14. doi: 10.1007/s00281-011-0296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jabbouri S, Sadovskaya I. Characteristics of the biofilm matrix and its role as a possible target for the detection and eradication of Staphylococcus epidermidis associated with medical implant infections. FEMS Immunol Med Microbiol. 2010;59(Suppl 3):280–91. doi: 10.1111/j.1574-695X.2010.00695.x. [DOI] [PubMed] [Google Scholar]
- 8.Götz F. Staphylococcus and biofilms. Mol Microbiol. 2002;43(6):1367–78. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 9.O'Gara JP. ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol Lett. 2007;270(Suppl 2):179–88. doi: 10.1111/j.1574-6968.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 10.Fredheim EG, Klingenberg C, Rohde H, Frankenberger S, Gaustad P, Flaegstad T, et al. Biofilm formation by Staphylococcus haemolyticus. J Clin Microbiol. 2009;47(4):1172–80. doi: 10.1128/JCM.01891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(Suppl 14):1422–9. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 12.Persichetti P, Giovanni Lombardo GA, Marangi GF, Gherardi G, Dicuonzo G. Capsular contracture and genetic profile of ica genes among Staphylococcus epidermidis isolates from subclinical periprosthetic infections. Plast Reconstr Surg. 2011;127(Suppl 4):1747–8. doi: 10.1097/PRS.0b013e31820a6592. [DOI] [PubMed] [Google Scholar]
- 13.Montanaro L, Poggi A, Visai L, Ravaioli S, Campoccia D, Speziale P, et al. Extracellular DNA biofilms. Int J Artif Organs. 2011;34:824–31. doi: 10.5301/ijao.5000051. [DOI] [PubMed] [Google Scholar]
- 14.Rohde H, Burandt EC, Siemssen N, Frommelt L, Burdelski C, Wurster S, et al. Polysaccharide intercellular adhesin or protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials. 2007;28(Suppl 9):1711–20. doi: 10.1016/j.biomaterials.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 15.Atshan SS, Nor Shamsudin M, Sekawi Z, Lung LT, Hamat RA, Karunanidhi A, Mateg Ali A, Ghaznavi-Rad E, Ghasemzadeh-Moghaddam H, Chong Seng JS, Nathan JJ, Pei CP: Prevalence of adhesion and regulation of biofilm-related genes in different clones of Staphylococcus aureus. J Biomed Biotechnol 2012; 2012:976972. http://dx.doi.org/10.1155/2012/976972. [DOI] [PMC free article] [PubMed]
- 16.Cassat JE, Dunman PM, McAleese F, Murphy E, Projan SJ, Smeltzer MS. Comparative genomics of Staphylococcus aureus musculoskeletal isolates. J Bacteriol. 2005;187(Suppl 2):576–92. doi: 10.1128/JB.187.2.576-592.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryding U, Flock JI, Flock M, Söderquist B, Christensson B. Expression of collagen-binding protein and types 5 and 8 capsular polysaccharide in clinical isolates of Staphylococcus aureus. J Infect Dis. 1997;176(Suppl 4):1096–9. doi: 10.1086/516520. [DOI] [PubMed] [Google Scholar]
- 18.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;15(Suppl 6):1159–69. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 19.Fluckiger U, Ulrich M, Steinhuber A, Döring G, Mack D, Landmann R, et al. Biofilm formation, icaADBC transcription, and polysaccharide intercellular adhesin synthesis by staphylococci in a device-related infection model. Infect Immun. 2005;73(Suppl 3):1811–9. doi: 10.1128/IAI.73.3.1811-1819.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franchelli S, Vassallo F, Porzio C, Mannucci M, Priano V, Schenone E, et al. Breast implant infections after surgical reconstruction in patients with breast cancer: assessment of risk factors and pathogens over extended post-operative observation. Surg Infect (Larchmt) 2012;13(Suppl 3):154–8. doi: 10.1089/sur.2011.004. [DOI] [PubMed] [Google Scholar]
- 21.Rieger UM, Pierer G, Lüscher NJ, Trampuz A. Sonication of removed breast implants for improved detection of subclinical infection. Aesthetic Plast Surg. 2009;33(Suppl 3):404–8. doi: 10.1007/s00266-009-9333-0. [DOI] [PubMed] [Google Scholar]
- 22.European Committee on Antimicrobial Susceptibility Testing (EUCAST) (2014) MIC determination of non-fastidious and fastidious organisms, version 4.0. http://www.eucast.org/ast_of_bacteria/mic_determination.
- 23.European Committee on Antimicrobial Susceptibility Testing (EUCAST) (2014) Breakpoint tables for interpretation of MICs and zone diameters, version 4.0. http://www.eucast.org/clinical_breakpoints/.
- 24.Vannuffel P, Gigi J, Ezzedine H, Vandercam B, Delmee M, Wauters G, et al. Specific detection of methicillin-resistant Staphylococcus species by multiplex PCR. J Clin Microbiol. 1995;33(Suppl 11):2864–7. doi: 10.1128/jcm.33.11.2864-2867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman DJ, Falkiner FR, Keane CT. New method for detecting slime production by coagulase-negative staphylococci. J Clin Pathol. 1989;42:872–4. doi: 10.1136/jcp.42.8.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arciola CR, Baldassarri L, Montanaro L. Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. J Clin Microbiol. 2001;39(Suppl 6):2151–6. doi: 10.1128/JCM.39.6.2151-2156.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roveta S, Marchese A, Schito GC. Activity of daptomycin on biofilms produced on a plastic support by Staphylococcus spp. Int J Antimicrob Agents. 2008;31(Suppl 4):321–8. doi: 10.1016/j.ijantimicag.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 29.O'Neill E, Pozzi C, Houston P, Smyth D, Humphreys H, Robinson DA, et al. Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolates from device-related infections. J Clin Microbiol. 2007;45(Suppl 5):1379–88. doi: 10.1128/JCM.02280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houston P, Rowe SE, Pozzi C, Waters EM, O'Gara JP. Essential role for the major autolysin in the fibronectin-binding protein mediated Staphylococcus aureus biofilm phenotype. Infect Immun. 2011;79:1153–65. doi: 10.1128/IAI.00364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rohde H, Knobloch JK, Horstkotte MA, Mack D. Correlation of Staphylococcus aureus icaADBC genotype and biofilm expression phenotype. J Clin Microbiol. 2001;39(Suppl 12):4595–6. doi: 10.1128/JCM.39.12.4595-4596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Silva GD, Kantzanou M, Justice A, Massey RC, Wilkinson AR, Day NP, et al. The ica operon and biofilm production in coagulase-negative Staphylococci associated with carriage and disease in a neonatal intensive care unit. J Clin Microbiol. 2002;40:382–8. doi: 10.1128/JCM.40.02.382-388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wootton M, Bennett PM, MacGowan AP, Walsh TR. Reduced expression of the atl autolysin gene and susceptibility to autolysis in clinical heterogeneous glycopeptide-intermediate Staphylococcus aureus(hGISA) and GISA strains. J Antimicrob Chemother. 2005;56:944–7. doi: 10.1093/jac/dki289. [DOI] [PubMed] [Google Scholar]
- 34.Vandecasteele SJ, Peetermans WE, Merckx R, Van Eldere J. Expression of biofilm-associated genes in Staphylococcus epidermidis during in vitro and in vivo foreign body infections. J Infect Dis. 2003;188(Suppl 5):730–7. doi: 10.1086/377452. [DOI] [PubMed] [Google Scholar]
- 35.Lou Q, Zhu T, Hu J, Ben H, Yang J, Yu F, et al. Role of the SaeRS two-component regulatory system in Staphylococcus epidermidis autolysis and biofilm formation. BMC Microbiol. 2011;11:146. doi: 10.1186/1471-2180-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tormo MA, Knecht E, Götz F, Lasa I, Penadés JR. Bap-dependent biofilm formation by pathogenic species of Staphylococcus: evidence of horizontal gene transfer? Microbiology. 2005;151(Pt 7):2465–75. doi: 10.1099/mic.0.27865-0. [DOI] [PubMed] [Google Scholar]
- 37.Abraham NM, Jefferson KK. A low molecular weight component of serum inhibits biofilm formation inStaphylococcus aureus. Microb Pathog. 2010;49(Suppl 6):388–91. doi: 10.1016/j.micpath.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu J, Li H, Li M, Vuong C, Otto M, Wen Y, et al. Bacterial insertion sequence IS256 as a potential molecular marker to discriminate invasive strains from commensal strains of Staphylococcus epidermidis. J Hosp Infect. 2005;61(Suppl 4):342–8. doi: 10.1016/j.jhin.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Monk AB, Archer GL. Use of outer surface protein repeat regions for improved genotyping of Staphylococcus epidermidis. J. Clin Microbiol. 2007;45(Suppl 3):730–5. doi: 10.1128/JCM.02317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tristan A, Ying L, Bes M, Etienne J, Vandenesch F, Lina G. Use of multiplex PCR to identify Staphylococcus aureus adhesins involved in human hematogenous infections. J Clin Microbiol. 2003;41:4465–7. doi: 10.1128/JCM.41.9.4465-4467.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montanaro L, Arciola CR, Baldassarri L, Borsetti E. Presence and expression of collagen adhesin gene (cna) and slime production in Staphylococcus aureus strains from orthopaedic prosthesis infections. Biomaterials. 1999;20:1945–9. doi: 10.1016/S0142-9612(99)00099-X. [DOI] [PubMed] [Google Scholar]
- 42.Vancraeynest D, Hermans K, Haesebrouck F. Genotypic and phenotypic screening of high and low virulence Staphylococcus aureus isolates from rabbits for biofilm formation and MSCRAMMs. Vet Microbiol. 2004;103(Suppl 3–4):241–7. doi: 10.1016/j.vetmic.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Ugolotti E, Bandettini R, Marchese A, Gualco L, Vanni I, Borzi L, et al. Molecular characterization of hospital-acquired methicillin-resistant Staphylococcus aureus strains in pediatric outbreaks using variable tandem repeat analysis with spa and ClfB typing. Diagn Microbiol Infect Dis. 2011;69(Suppl 2):213–7. doi: 10.1016/j.diagmicrobio.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 44.de Araujo GL, Coelho LR, de Carvalho CB, Maciel RM, Coronado AZ, Rozenbaum R, et al. Commensal isolates of methicillin-resistant Staphylococcus epidermidis are also well equipped to produce biofilm on polystyrene surfaces. J Antimicrob Chemother. 2006;57:855–64. doi: 10.1093/jac/dkl071. [DOI] [PubMed] [Google Scholar]
- 45.Nilsson M, Frykberg L, Flock JI, Pei L, Lindberg M, Guss B. A fibrinogen-binding protein of Staphylococcus epidermidis. Infect Immun. 1998;66:2666–73. doi: 10.1128/iai.66.6.2666-2673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sau S, Bhasin N, Wann ER, Lee JC, Foster TJ, Lee CY. The Staphylococcus aureus allelic genetic loci for serotype 5 and 8 capsule expression contain the type-specific genes flanked by common genes. Microbiology. 1997;143:2395–405. doi: 10.1099/00221287-143-7-2395. [DOI] [PubMed] [Google Scholar]
- 47.Lina G, Piémont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, et al. Involvement of Panton–Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–32. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 48.Jarraud S, Mougel C, Thioulouse J, Lina G, Meugnier H, Forey F, et al. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect Immun. 2002;70:631–41. doi: 10.1128/IAI.70.2.631-641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Belkum A, Kluytmans J, van Leeuwen W, Bax R, Quint W, Peters E, et al. Multicenter evaluation of arbitrarily primed PCR for typing of Staphylococcus aureus strains. J Clin Microbiol. 1995;33:1537–47. doi: 10.1128/jcm.33.6.1537-1547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lina G, Boutite F, Tristan A, Bes M, Etienne J, Vandenesch F. Bacterial competition for human nasal cavity colonization: role of Staphylococcal agr alleles. Appl Environ Microbiol. 2003;69:18–23. doi: 10.1128/AEM.69.1.18-23.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


