Abstract
Neurotransmission involves the exo-endocytic cycling of synaptic vesicle (SV) membranes. Endocytic membrane retrieval and clathrin-mediated SV reformation require curvature-sensing and membrane-bending BAR domain proteins such as endophilin A. While their ability to sense and stabilize curved membranes facilitates membrane recruitment of BAR domain proteins, the precise mechanisms by which they are targeted to specific sites of SV recycling has remained unclear. Here, we demonstrate that the multi-domain scaffold intersectin 1 directly associates with endophilin A to facilitate vesicle uncoating at synapses. Knockout mice deficient in intersectin 1 accumulate clathrin-coated vesicles at synapses, a phenotype akin to loss of endophilin function. Intersectin 1/endophilin A1 complex formation is mediated by direct binding of the SH3B domain of intersectin to a non-canonical site on the SH3 domain of endophilin A1. Consistent with this, intersectin-binding defective mutant endophilin A1 fails to rescue clathrin accumulation at neuronal synapses derived from endophilin A1-3 triple knockout (TKO) mice. Our data support a model in which intersectin aids endophilin A recruitment to sites of clathrin-mediated SV recycling, thereby facilitating vesicle uncoating.
Keywords: endophilin, intersectin, neurotransmission, SH3 domains, synaptic vesicle recycling
Introduction
Neurotransmission is mediated by the fusion of synaptic vesicles (SVs) with the presynaptic membrane, followed by compensatory endocytosis and clathrin-mediated SV reformation 1, 2, 3. Clathrin-mediated vesicle formation includes progressive membrane deformation by Bin/Amphiphysin-Rvs (BAR) domain superfamily proteins, in which BAR domains function as curvature sensors and/or inducers 4, 5, 6. Phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2) plays a key role in the recruitment and assembly of endocytic clathrin coats 7, but it has to be hydrolyzed rapidly via the PI(4,5)P2-phosphatase synaptojanin 1 8 to enable uncoating post-fission. The BAR domain superfamily protein endophilin A functions as the main recruiter of synaptojanin 1 via its C-terminal SH3 domain that also binds to proline-rich motifs of dynamin 9, to the vesicular glutamate transporter VGLUT1 10, and to the Ubl domain of parkin 11.
Many of the endocytic BAR domain proteins including FCHo, SNX9, and amphiphysins contain binding sites for clathrin/AP-2 5, 12; however, endophilin A is an exception. In contrast to other endocytic BAR domain proteins, no interaction of endophilin with clathrin coat components has been reported. This is particularly surprising as a major phenotype of endophilin A loss in vivo is the accumulation of free clathrin-coated vesicles (CCVs) 13, 14, 15, a phenotype resembling that of deletion of synaptojanin 8, 16 with which endophilin interacts physically and functionally 17. Data from retinal bipolar cells 18 and hippocampal neurons 19 indicate that endophilin may serve additional roles, for example, in clathrin-independent endocytosis at synapses. These data thus raise the question how endophilin is recruited to sites of clathrin-mediated SV reformation.
Here, we show that the multiple SH3 domain-containing scaffold protein intersectin directly associates with endophilin A and regulates its function. Intersectin is an early-acting evolutionary conserved endocytic protein that associates with multiple coat components such as AP-2, FCHo, and Eps15, in addition to dynamin 3. Intersectin 1 is overexpressed in Down syndrome, while intersectin loss of function in flies and mammals has been associated with defects in exo-endocytosis 20, 21, 22. We demonstrate that intersectin 1 knockout (KO) mice accumulate CCVs at synapses. Furthermore, we use NMR spectroscopy and biochemistry to delineate the determinants underlying endophilin/intersectin complex formation and show that the ability of endophilin to directly bind to intersectin is crucial for its role in vesicle uncoating at synapses.
Results and Discussion
Accumulation of clathrin-coated vesicles at synapses from intersectin 1 KO mice
Previous work has shown that Dap160, the fly ortholog of mammalian intersectins 1 and 2, is required to stabilize endocytic proteins, in particular endophilin A 20. To test whether intersectin 1 regulates endophilin A function in the mammalian nervous system, we analyzed the structural architecture of synapses within lamina IX of the lumbar spinal cord in wild-type (WT) and intersectin 1 knockout (KO) mice. Lumbar spinal cords from intersectin 1-KO were morphologically normal. Glutamatergic M-type and S-type asymmetric synapses from WT and KO mice displayed a similar morphology and contained compact clusters of SVs (Supplementary Fig S1). However, KO synapses showed a mild, yet, significant accumulation of apparently free CCVs (Fig1A–G), an observation confirmed by serial sectioning electron microscopy and quantitative morphometric analysis (Fig1H). This phenotype is similar, though clearly less severe than that observed in cortical synapses from endophilin A1-3 TKO mice 14, and suggests that intersectin 1 may directly or indirectly regulate endophilin function.
Figure 1.
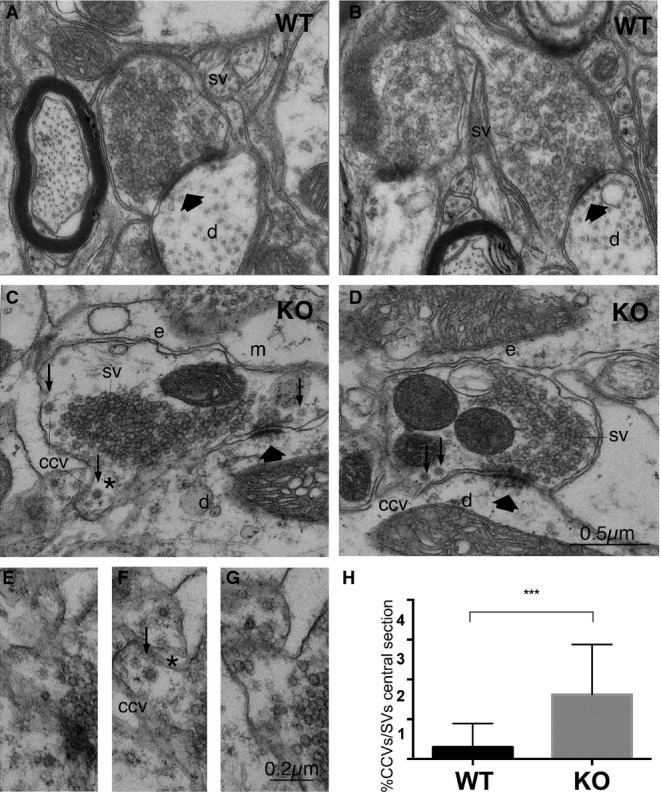
- Electron micrographs of S-boutons establishing synapses on dendritic shafts in lamina IX of the mouse lumbar spinal cord of WT (A, B) or intersectin 1 KO mice (C, D). Scale bar: (A–D) 0.5 μm.
- Serial section from area marked in (C) (asterisk); CCV, free clathrin-coated vesicle. Scale bar: (E–G) 0.2 μm.
- Percentage of CCVs/total number of SVs (***P < 0.001; two-tailed unpaired t-test; n = 30, control and 32, intersectin 1 KO synapses from three mice of each genotype). Data are given as mean ± SD. SV, synaptic vesicles; d, dendritic shafts; e, endosome-like structures; m, mitochondrion; thick arrows indicate active zones; small arrows, CCVs.
Direct SH3-SH3 domain-mediated interaction between endophilin A1 and intersectin 1
Given the phenotypic similarity between intersectin 1 KO and endophilin A1-3 TKO synapses, we probed for a possible interaction between both proteins. Antibodies against endophilin A1 efficiently immunoprecipitated not only endophilin A1 but also intersectin 1 from lysed synaptosomes, while both proteins were absent from control immunoprecipitates (Fig2A). Hsc70 taken as a control was not co-immunoprecipitated. Hence, endogenous intersectin 1 and endophilin A1 form a complex in native brain tissue. Affinity chromatography was used to delineate the site within intersectin 1 responsible for association with endophilin A1. We incubated GST-fused SH3 domains of intersectin 1 immobilized on beads with detergent-lysed rat brain homogenates and analyzed bound proteins by immunoblotting. Endophilin A1 was efficiently retained on the SH3B domain of intersectin 1, a domain that previously has not been assigned any specific ligand (Fig2B). All other SH3 domains were inactive with respect to endophilin binding, although intersectin 1-SH3s A, C, and E (and to a minor degree also B) avidly bound to dynamin 1. None of the intersectin 1-SH3 domains associated with actin (Fig2B). These data suggest that endophilin A1 specifically binds to the SH3B domain of intersectin 1.
Figure 2.
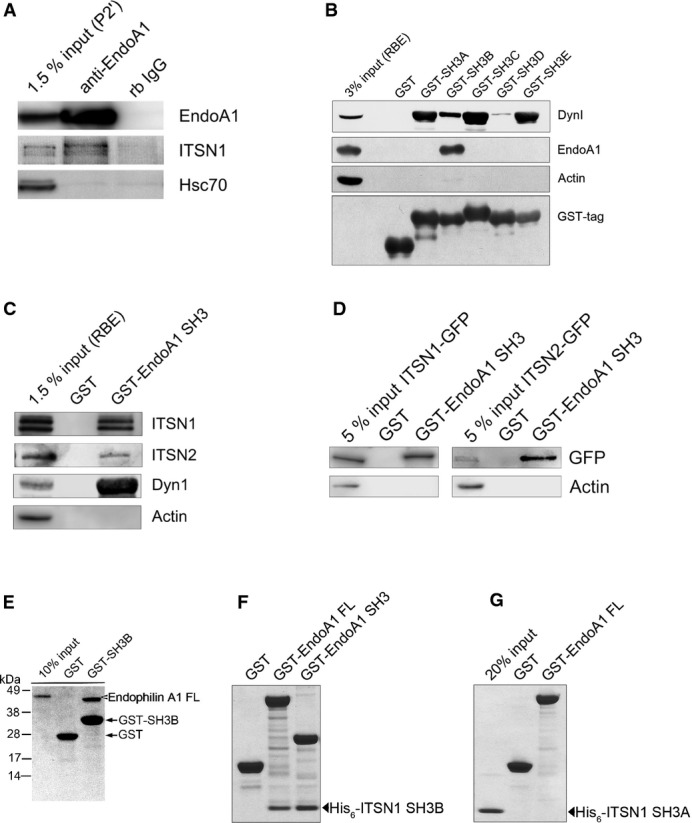
- Endogenous endophilin A1 and intersectin 1 form a complex in situ. Immunoprecipitation from detergent-extracted rat brain synaptosomal fractions (P2′) using control rabbit non-immune IgG (rb IgG) or anti-endophilin A1 antibodies. Samples were analyzed by immunoblotting for endophilin A1 (EndoA1), intersectin 1 (ITSN1), and Hsc70.
- GST-intersectin 1-SH3 associates with endophilin A1 present in detergent-lysed rat brain extract (RBE). Samples were analyzed by immunoblotting for endophilin A1 (EndoA1), dynamin 1 (DynI), actin, and GST.
- Endophilin A1 associates with intersectin 2. (C) GST or GST-endophilin A1-SH3 fusion proteins were incubated with detergent-lysed rat brain extract (RBE). Samples were analyzed by immunoblotting for intersectin 1 (ITSN1), intersectin 2 (ITSN2; note: antibody specificity was verified by samples from intersectin 2 knockout mice), dynamin 1 (DynI), or actin. (D) Same as in (C) but using detergent extracts from HEK293 cells expressing intersectin 1-eGFP (ITSN1-GFP) or intersectin 2-eGFP (ITSN2-GFP). Samples were analyzed by immunoblotting for eGFP or actin.
- Direct binding of endophilin A1-SH3 to intersectin 1-SH3B. Indicated GST-fusion proteins were incubated with full-length (FL) His6-endophilin A1 (E), His6-intersectin 1-SH3B (F), or His6-intersectin 1-SH3A (G). Samples were analyzed by SDS–PAGE and staining with Coomassie blue.
Mammals in addition to intersectin 1 express the closely related isoform intersectin 2 3. We therefore tested whether endophilin A1 also binds to intersectin 2. Native endogenous (Fig2C) or GFP-tagged intersectin 2 expressed in HEK293 cells (Fig2D) efficiently bound to GST-endophilin A1-SH3 in affinity chromatography experiments, indicating that endophilin A1 associates with both mammalian intersectins. Binding of intersectin-SH3B to endophilin was also observed in lamprey (Supplementary Fig S2B), suggesting that complex formation between endophilin and intersectin is evolutionary conserved.
The association between intersectin 1 and endophilin A1 was direct as purified full-length His6-endophilin A1 avidly bound to intersectin 1-SH3B immobilized on beads (Fig2E). Conversely, full-length GST-endophilin A1 or a truncated mutant version lacking its BAR domain efficiently associated with soluble recombinant His6-intersectin 1-SH3B (Fig2F), but not with His6-intersectin 1-SH3A (Fig2G). Thus, intersectin and endophilin A1 directly bind to one another via association of intersectin-SH3B with the C-terminal region of endophilin A1 encompassing its SH3 domain.
Structural basis for endophilin A1-SH3 association with intersectin 1-SH3B
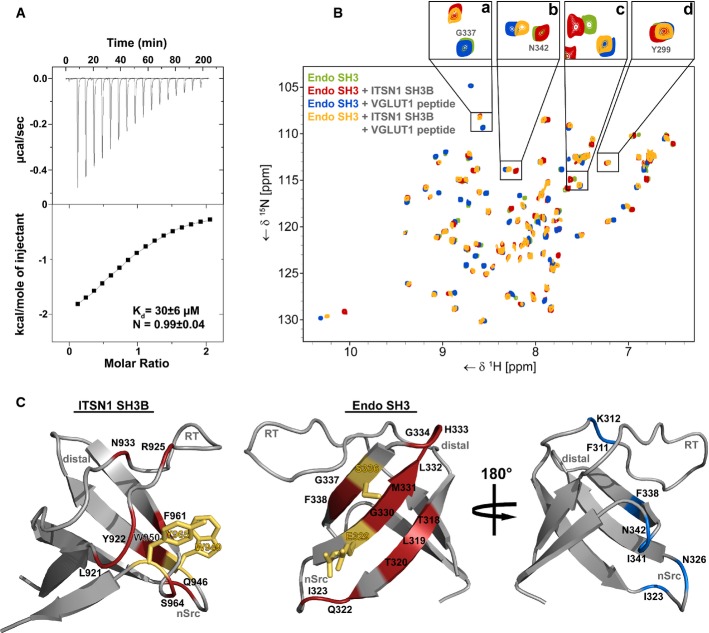
We quantitatively analyzed the interaction between purified recombinant intersectin 1-SH3B and the SH3 domain of endophilin A1 by isothermal titration calorimetry (ITC). This analysis revealed a KD of about 30 μM and a 1:1 stoichiometry of complex formation (Fig3A).
Figure 3.
- Isothermal titration calorimetry (ITC) profile of intersectin 1-SH3B titrated against endophilin A1-SH3. Top panel, heat changes upon ligand injection; the bottom panel, integrated power peaks fitted with a 1:1 model.
- 15N-HSQC spectrum of endophilin A1-SH3 (green) overlaid with spectra recorded after supplementation with intersectin 1-SH3B (red), a proline-rich peptide derived from VGLUT1 (blue) or both (yellow). Enlarged sections above illustrate typical signal changes caused by addition of SH3B (a) or peptide (b), both (c), or none (d).
- Epitopes of the direct SH3-SH3 interaction mapped onto the structures of intersectin 1-SH3B (left) (PDB: 4IIM) and endophilin A1-SH3 (middle) (PDB:3IQL). Red, residues showing shift changes larger than the average shift distance (± SD) or disappearing due to line broadening upon SH3 domain binding. Blue, endophilin A1-SH3 residues that display significant shift perturbation after VGLUT1 peptide addition. Orange residues showed significant shift changes and were mutated in order to abrogate binding.
To probe the mode of interaction between the two SH3 domains at the atomic level, we turned to NMR spectroscopy. Resonance assignments for 15N-isotope-labeled endophilin A1-SH3 were obtained on the basis of 3D-triple-resonance spectra, which then allowed us to interpret the interaction properties by chemical shift analysis of the corresponding 15N-1H correlation spectra. To obtain insights into the molecular determinants of complex formation between endophilin A1-SH3 and intersectin 1-SH3B, we supplemented 15N-labeled endophilin A1-SH3 with either purified recombinant intersectin 1-SH3B or a proline-rich peptide derived from the vesicular glutamate transporter 1 (VGLUT1), a known endophilin A1-SH3 ligand 10. Complex formation with SH3B invoked chemical shift changes or disappearance of resonances due to ligand-induced line broadening of residues mostly located on the surface of the β-sheet of endophilin A1-SH3 (Fig3B (red spectrum), Fig3C, middle panel; Supplementary Fig S3C) including E329, M331, and S336 (Fig3C, right). This location is opposite to the canonical proline-rich ligand-binding site, an epitope engaged by the VGLUT1 peptide (Fig3B, blue spectrum and Fig3C, right panel). Correspondingly, the NMR spectrum showed two sets of largely independent resonance shifts that could be attributed to endophilin A1-SH3 complex formation either with SH3B (inset a in Fig3B) or with VGLUT1 (inset b in Fig3B). Only a few resonances displayed chemical shifts upon addition of either of the two ligands (inset c in Fig3B). Consistent with the presence of two largely independent binding sites on endophilin A1-SH3 for VGLUT1 and intersectin 1, we observed negligible effects of the VGLUT1-derived peptide on resonances affected by SH3B binding and vice versa (compare red and orange and green and orange spectra in Fig3B). These data, thus, identify a novel binding surface on the endophilin A1-SH3 domain, which selectively engages the SH3B domain of intersectin 1, independently of its association with proline-rich motifs within VGLUT1, synaptojanin, or dynamin.
To define the epitope of the intersectin 1-SH3B domain that binds to the β-sheet region in endophilin A1, we performed a similar epitope mapping approach using 15N-labeled intersectin 1-SH3B. This analysis showed that the association of SH3B with endophilin A1-SH3 encompasses the RT and the nSrc-loops of SH3B, including R925, W949, and Y965 (Fig3C, left; Supplementary Fig S3A and B), structural elements that often enable proline-rich peptide binding of canonical SH3 domains. Collectively, our data establish a firm structural basis for complex formation between endophilin A1-SH3 and intersectin 1-SH3B.
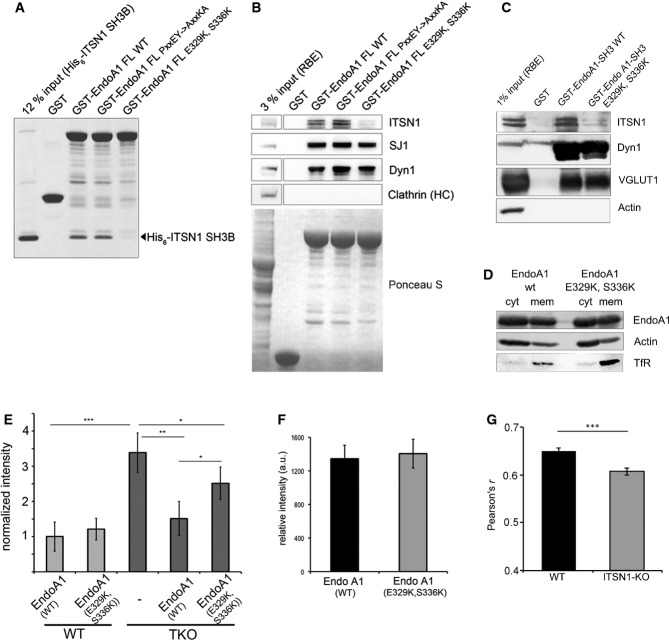
To probe whether the binding epitopes determined by NMR spectroscopy indeed are required for endophilin A1–intersectin 1 complex formation, we generated site-directed mutants of endophilin A1. Mutation of residues E329→K and S366→K within the SH3 domain of full-length endophilin A1 (Fig3C, right) resulted in failure of the mutant protein to bind His6-SH3B (Fig4A). Similar results were seen in affinity chromatography experiments from detergent-extracted rat brain lysates: WT endophilin A1 avidly bound to native endogenous intersectin 1, whereas only trace amounts of intersectin 1 were found in association with mutant endophilin A1 (E329K, S366K). Importantly, WT and mutant endophilin A1 bound indistinguishably to the proline-rich domain-containing proteins synaptojanin 1, dynamin 1, and VGLUT1 (Fig4B and C). Furthermore, when expressed in HEK293 cells, WT and mutant endophilin A1 partitioned with equal efficiencies between soluble cytoplasmic and membrane-bound fractions (Fig4D), suggesting that mutation of E329K, S366K has no apparent effect on the ability of endophilin to associate with membranes via its BAR domain. These data confirm that endophilin A1-SH3 association with intersectin 1 and with conventional proline-rich ligands including synaptojanin 1, dynamin 1, and VGLUT1 or with membrane lipids occurs independently at distinct sites, in line with our structural studies. We conclude that endophilin A1 associates with intersectin 1 via a novel non-canonical surface on its SH3 domain encompassing amino acids E329 and S366.
Figure 4.
- Endophilin A1 binding intersectin 1-SH3B binds via a non-canonical interface on its SH3 domain. GST-fused full-length (FL) endophilin A1 wild-type (WT), a proline-rich peptide binding defective mutant (PxxEY->AxxKA), or a mutant within the intersectin 1-SH3B binding interface (E329K, S336K) were incubated with purified recombinant intersectin 1-SH3B and analyzed by SDS–PAGE and staining with Coomassie blue.
- Mutant endophilin A1 fails to bind to intersectin 1 while retaining association with proline-rich ligands. (B) GST or GST-fused full-length (FL) endophilin A1 wild-type (WT), a proline-rich peptide binding defective mutant (PxxEY->AxxKA), or a mutant within the intersectin 1-SH3B binding interface (E329K, S336K) were incubated with RBE. Samples were analyzed by immunoblotting for synaptojanin 1 (SJ1), dynamin 1 (Dyn1), or clathrin heavy chain (HC). (C) GST or GST-endophilin A1-SH3 wild-type (WT) or mutant (E329K, S336K) were incubated with RBE. Samples were analyzed by immunoblotting for intersectin 1 (ITSN1), vesicular glutamate transporter 1 (VGLUT1), dynamin 1 (Dyn1), or actin as a negative control.
- Endophilin A1-mRFP WT and mutant (E329K, S336K) partition equally between membrane and soluble cytosolic fractions of HEK293 cells. Samples were immunoblotted for endophilin A1 (EndoA1), transferrin receptor (TfR), or actin.
- Endophilin A1 binding to intersectin 1 regulates clathrin uncoating. Quantification of clathrin clustering in cortical neurons (DIV 14–22) from WT or endophilin A1-3 TKO mice re-expressing endophilin A1 WT or mutant (E329K, S336K). Clustering was fully rescued by endophilin A1-mRFP WT, but only to a minor degree by mutant endophilin A1 (E329K, S336K); y-axis, fold increase of fluorescence puncta in mutant synapses normalized to WT. *P < 0.05, **P < 0.01, ***P < 0.001, t-test.
- Equal expression of endophilin 1 A1-mRFP wild-type (WT) or mutant (E329K, S336K) in primary cortical neurons (DIV 14–22; 68 random images from n = 4 independent experiments).
- Intersectin 1 regulates endophilin A1 targeting to sites of clathrin-mediated endocytosis in neurons. Colocalization between endogenous endophilin A1 and AP-2 in primary hippocampal neurons (DIV 14) from wild-type (WT) or intersectin 1 knockout (KO) mice assessed by Pearson's correlation (four independent experiments; 111 for WT and 110 random images for KO). Data represent mean ± SEM. ***P < 0.0001, two-tailed unpaired t-test.
Endophilin A1 binding to intersectin 1 regulates clathrin uncoating
A predominant phenotype of disruption of endophilin A function in a variety of organisms ranging from worms to lamprey and mice is an accumulation of CCVs, reflecting a role of endophilin A in vesicle uncoating 13, 14, 15. Neurons from endophilin A1–A3 TKO mice show a striking abundance of CCVs, reflected by enhanced punctate immunoreactivity for endocytic clathrin coat components, most notably clathrin itself 14. Hence, intersectin binding to endophilin A, which lacks the ability to directly associate with clathrin coat components, might facilitate endophilin function in vesicle uncoating at synapses. Primary cortical neurons derived from endophilin TKO mice displayed robust clustering of clathrin signal relative to WT neurons (Supplementary Fig S4A–C and Fig4E), reflecting the accumulation of CCVs as reported before 14. Re-expression of WT endophilin A1-mRFP rescued this phenotype as distribution of clathrin was diffuse and similar to that observed in WT neurons. In contrast, re-expression of intersectin-binding defective mutant endophilin A1 (E329K, S366K) (Fig4C) only resulted in a minor rescue reflected in significantly altered clathrin distribution compared to either WT neurons or endophilin TKO neurons expressing WT endophilin A1 (Fig4E, Supplementary Fig S4A–C). The inability of mutant endophilin A1 to fully rescue clathrin accumulation in endophilin TKO neurons was not due to protein instability as WT and mutant endophilin A1 were expressed to the same levels (Fig4D). Enrichment of CCVs and a corresponding reduction in the number of bona fide SVs was also observed in stimulated lamprey reticulospinal synapses following microinjection of mammalian intersectin 1-SH3B to acutely disrupt intersectin/endophilin complex formation in situ (Supplementary Fig S2A, C–H). These data demonstrate that endophilin A function in vesicle uncoating at synapses depends on its direct association with intersectin.
We demonstrate that endophilin A uses distinct surfaces on its SH3 domain to associate with intersectin and with proline-rich ligands including synaptojanin and dynamin. SH3-SH3 domain-based complex formation at least to our knowledge has rarely been observed in biology and has not been described for any other membrane trafficking protein so far.
Functional rescue analyses of neurons from endophilin A1-3 TKO mice show that SH3 domain-mediated endophilin–intersectin complex formation regulates SV uncoating mediated by endophilin A. Consistent with this observation, we detect that neurons from intersectin 1 KO mice display an accumulation of CCVs, although the phenotype of these animals is much weaker than that of endophilin A1-3 TKO mutants. This discrepancy likely is explained by functional overlap between intersectins 1 and 2 with respect to endophilin A binding (compare Fig2C and D) and recruitment. Consistent with this possibility, we find intersectin 2 levels to be significantly up-regulated by about 2-fold in brain or hippocampal lysates derived from intersectin 1 KO mice (Supplementary Fig S4D). Moreover, we cannot rule out that additional factors other than intersectins also contribute to this function.
Based on our data, we hypothesize that intersectin via its binding to endocytic clathrin adaptors (i.e. FCHo, AP-2) may regulate partitioning of endophilin A between pathways of clathrin-mediated SV reformation and clathrin-independent membrane retrieval at synapses and possibly in non-neuronal cells. In such a scenario, endophilin A binding to intersectin at sites of SV exo-endocytosis would facilitate its association with clathrin/AP-2-containing endocytic intermediates prior to vesicle uncoating, in spite of its inability to directly interact with clathrin coat components. Consistent with this, immuno-EM experiments have demonstrated that endophilin A is present early at the rim of forming coated pits 23 and accumulates at the CCP neck during late endocytic stages, presumably aided by its curvature inducing properties and the ability to self-assemble 24. Given that intersectin is recruited to clathrin-coated pits early, for example, prior to the peak of endophilin A 25, a likely function for SH3-SH3 domain-mediated intersectin/endophilin complex formation is to aid targeting of endophilin to CCPs to create a platform for efficient synaptojanin recruitment for uncoating. In support of this model, we find reduced co-localization of endophilin A1 with AP-2 in neurons lacking intersectin 1 (Fig4G, Supplementary Fig S4E). Our model is also consistent with the dominant-negative phenotype of intersectin 1 overexpression in Down syndrome and with recent studies in retinal bipolar cells and in mouse hippocampal neurons that have suggested an additional function of endophilin in fast membrane retrieval independent of clathrin/AP-2 coats 19. Future studies will need to address by which mechanisms other BAR domain proteins may be targeted to select pathways or membrane sites.
Materials and Methods
The following Supplementary Methods are available as Supplementary Information: plasmids, antibodies, primers and site-directed mutagenesis; lamprey microinjection experiments; electron microscopy; protein expression and purification; cell fractionation; immunofluorescence; isothermal titration calorimetry; 15N-HSQC NMR experiments and assignments; affinity chromatography and immunoprecipitations; and statistics.
NMR Spectroscopy
Protein samples were prepared in PBS pH 7.4 + 10% (v/v) D2O. Two-dimensional 15N-HSQC spectra were recorded on a Bruker Ultrashield 700 Plus (700 MHz spectrometer) equipped with 5-mm triple-resonance cryoprobes. Measurements of 15N-labeled ITSN1 SH3B or endophilin A1 SH3 in the absence or presence of the non-labeled ligands were performed at 298 K at protein concentrations of 100–250 μM. 1024 × 128 complex data points were acquired with eight scans in each HSQC experiment. See the supplementary information for details on NMR titration experiments and the backbone assignment of the two SH3 domains. Resonance assignments will be submitted to the BioMagResBank (BMRB).
Confocal microscopy analysis of primary cortical neurons in culture
Primary cortical neuronal cultures were prepared and transfected with chicken β-actin promoter-driven EGFP-clathrin light chain and WT or mutant endophilin A1-mRFP constructs using Amaxa system (Lonza), fixed after 14–18 day in-vitro with 4% paraformaldehyde/2% sucrose in phosphate-buffered saline (PBS) and subsequently imaged by spinning-disc confocal microscopy (Nikon/PerkinElmer), as described before 14. Fluorescent puncta were quantified with MetaMorph software version 7.2 (Molecular Devices) using the application Count Nuclei, as in 8. Data are presented as number of puncta per 100 μm2 and are normalized to controls. At least 15 images from four experiments were analyzed per genotype and statistically tested (t-test). See expanded view for further details.
Acknowledgments
We are indebted to Drs. Melanie A. Pritchard (Monash University, Victoria, Australia) and Pietro De Camilli for intersectin 1 KO and endophilin A1-3 TKO mice, and to Melanie König and Maria Mühlbauer for expert technical assistance. Supported by grants from the DFG (SFB958/A07 to V.H. & C.F., Emmy Noether Award to I.M.), the Swedish Research Council (13473), EU-FP7 (HEALTH-F2-2009-242167 (“SynSys-project”) to O.S.), and by an EMBO long-term fellowship to M.E.G.
Author contributions
Experiments were carried out by AP, FG, OV, MEG, JB, MJ, and TM (cell biology, biochemistry), FG and CF (protein purification, NMR), IM (endophilin A1-3 TKO analysis), and OS (lamprey microinjection experiments, EM). OS, CF, and VH wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting Information
Supplementary Information
Review Process File
References
- Dittman J, Ryan TA. Molecular circuitry of endocytosis at nerve terminals. Annu Rev Cell Dev Biol. 2009;25:133–160. doi: 10.1146/annurev.cellbio.042308.113302. [DOI] [PubMed] [Google Scholar]
- Saheki Y, De Camilli P. Synaptic vesicle endocytosis. Cold Spring Harbor Perspect Biol. 2012;4:a005645. doi: 10.1101/cshperspect.a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke V, Neher E, Sigrist SJ. Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat Rev Neurosci. 2011;12:127–138. doi: 10.1038/nrn2948. [DOI] [PubMed] [Google Scholar]
- Daumke O, Roux A, Haucke V. BAR domain scaffolds in dynamin-mediated membrane fission. Cell. 2014;156:882–892. doi: 10.1016/j.cell.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Frost A, Unger VM, De Camilli P. The BAR domain superfamily: membrane-molding macromolecules. Cell. 2009;137:191–196. doi: 10.1016/j.cell.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Raimondi A, O'Toole E, Paradise S, Collesi C, Cremona O, Ferguson SM, De Camilli P. Cell- and stimulus-dependent heterogeneity of synaptic vesicle endocytic recycling mechanisms revealed by studies of dynamin 1-null neurons. Proc Natl Acad Sci USA. 2008;105:2175–2180. doi: 10.1073/pnas.0712171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstad N, Gad H, Low P, Di Paolo G, Brodin L, Shupliakov O, De Camilli P. Endophilin/SH3p4 is required for the transition from early to late stages in clathrin-mediated synaptic vesicle endocytosis. Neuron. 1999;24:143–154. doi: 10.1016/s0896-6273(00)80828-4. [DOI] [PubMed] [Google Scholar]
- Voglmaier SM, Kam K, Yang H, Fortin DL, Hua Z, Nicoll RA, Edwards RH. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron. 2006;51:71–84. doi: 10.1016/j.neuron.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Trempe JF, Chen CX, Grenier K, Camacho EM, Kozlov G, McPherson PS, Gehring K, Fon EA. SH3 domains from a subset of BAR proteins define a Ubl-binding domain and implicate parkin in synaptic ubiquitination. Mol Cell. 2009;36:1034–1047. doi: 10.1016/j.molcel.2009.11.021. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- Gad H, Ringstad N, Löw P, Kjaerulff O, Gustafsson J, Wenk M, Di Paolo G, Nemoto Y, Crun J, Ellisman MH, et al. Fission and uncoating of synaptic clathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin. Neuron. 2000;27:301–312. doi: 10.1016/s0896-6273(00)00038-6. [DOI] [PubMed] [Google Scholar]
- Milosevic I, Giovedi S, Lou X, Raimondi A, Collesi C, Shen H, Paradise S, O'Toole E, Ferguson S, Cremona O, et al. Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron. 2011;72:587–601. doi: 10.1016/j.neuron.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Kjaerulff O, Lloyd TE, Atkinson R, Zhou Y, Meinertzhagen IA, Bellen HJ. Endophilin mutations block clathrin-mediated endocytosis but not neurotransmitter release. Cell. 2002;109:101–112. doi: 10.1016/s0092-8674(02)00688-8. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Koh TW, Schulze KL, Zhai RG, Hiesinger PR, Zhou Y, Mehta SQ, Cao Y, Roos J, Bellen HJ. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron. 2003;40:733–748. doi: 10.1016/s0896-6273(03)00644-5. [DOI] [PubMed] [Google Scholar]
- Chang-Ileto B, Frere SG, Chan RB, Voronov SV, Roux A, Di Paolo G. Synaptojanin 1-mediated PI(4,5) P2 hydrolysis is modulated by membrane curvature and facilitates membrane fission. Dev Cell. 2011;20:206–218. doi: 10.1016/j.devcel.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llobet A, Gallop JL, Burden JJ, Camdere G, Chandra P, Vallis Y, Hopkins CR, Lagnado L, McMahon HT. Endophilin drives the fast mode of vesicle retrieval in a ribbon synapse. J Neurosci. 2011;31:8512–8519. doi: 10.1523/JNEUROSCI.6223-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko NL, Puchkov D, Classen GA, Walter AM, Pechstein A, Sawade L, Kaempf N, Trimbuch T, Lorenz D, Rosenmund C, et al. Clathrin/AP-2 mediate synaptic vesicle reformation from endosome-like vacuoles but are not essential for membrane retrieval at central synapses. Neuron. 2014;82:981–988. doi: 10.1016/j.neuron.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Koh TW, Korolchuk VI, Wairkar YP, Jiao W, Evergren E, Pan H, Zhou Y, Venken KJ, Shupliakov O, Robinson IM, et al. Eps15 and Dap160 control synaptic vesicle membrane retrieval and synapse development. J Cell Biol. 2007;178:309–322. doi: 10.1083/jcb.200701030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Kononenko NL, Bacetic J, Pechstein A, Schmoranzer J, Yao L, Barth H, Shupliakov O, Kobler O, Aktories K, et al. Fast neurotransmitter release regulated by the endocytic scaffold intersectin. Proc Natl Acad Sci USA. 2013;110:8266–8271. doi: 10.1073/pnas.1219234110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Chu PY, Bowser DN, Keating DJ, Dubach D, Harper I, Tkalcevic J, Finkelstein DI, Pritchard MA. Mice deficient for the chromosome 21 ortholog Itsn1 exhibit vesicle-trafficking abnormalities. Hum Mol Genet. 2008;17:3281–3290. doi: 10.1093/hmg/ddn224. [DOI] [PubMed] [Google Scholar]
- Sundborger A, Soderblom C, Vorontsova O, Evergren E, Hinshaw JE, Shupliakov O. An endophilin-dynamin complex promotes budding of clathrin-coated vesicles during synaptic vesicle recycling. J Cell Sci. 2011;124:133–143. doi: 10.1242/jcs.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mim C, Cui H, Gawronski-Salerno JA, Frost A, Lyman E, Voth GA, Unger VM. Structural basis of membrane bending by the N-BAR protein endophilin. Cell. 2012;149:137–145. doi: 10.1016/j.cell.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Boucrot E, Meinecke M, Evergren E, Vallis Y, Mittal R, McMahon HT. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science. 2010;328:1281–1284. doi: 10.1126/science.1188462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Review Process File