Abstract
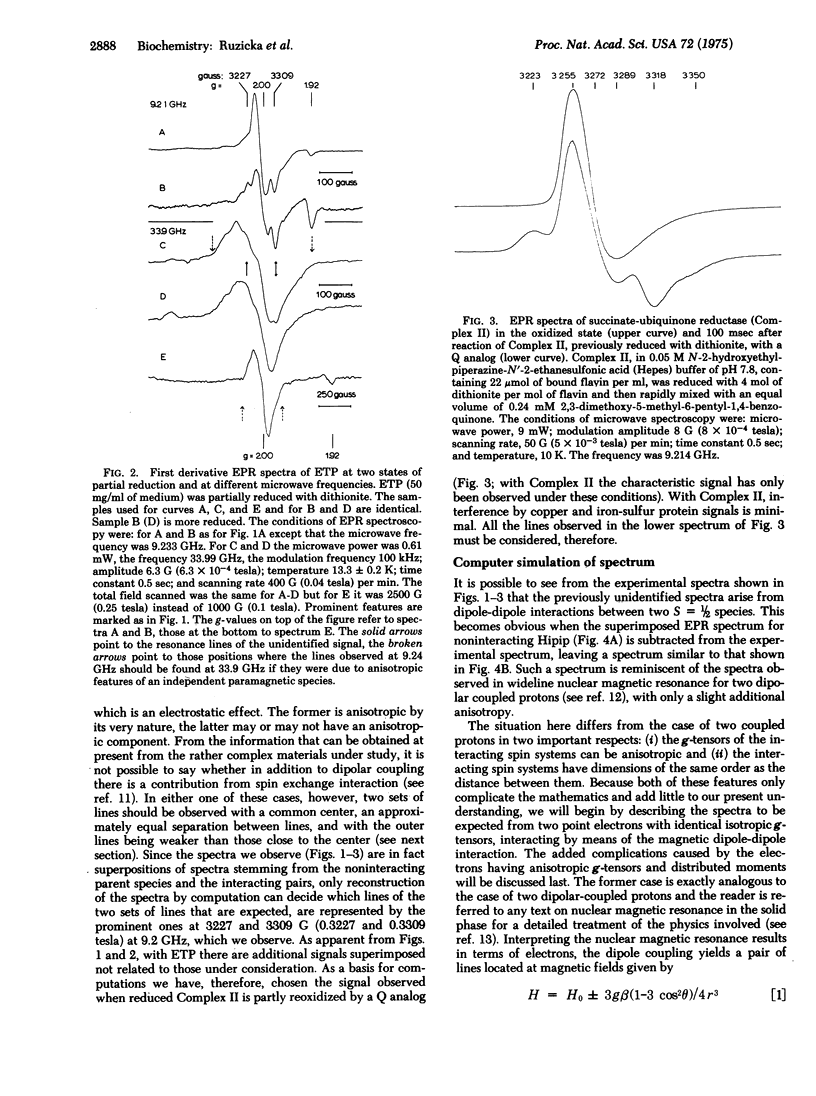
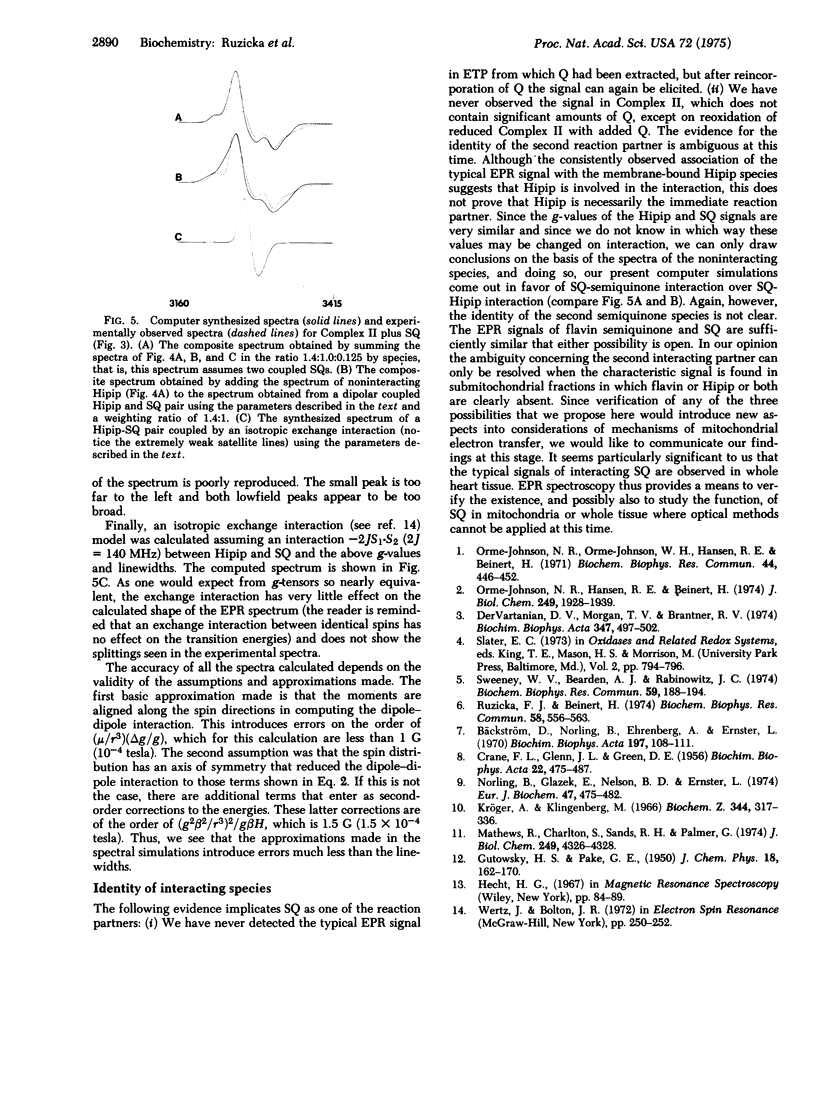
The origin of most of the electron paramagnetic resonances obtained at low temperature and low microwave power from heart tissue and subcellular fractions derived therefrom is now understood. A signal that emerges on partial reduction with characteristic lines at 3227 G (0.3227 tesla) and 3309 G (0.3309 tesla) (at 9.2 GHz) and disappears again on full reduction has remained unidentified. According to its behavior on oxidation-reduction, the substance giving rise to this signal has the properties of a two-electron acceptor. The signal is strongly dependent on temperature and can only be well resolved at less than 20 degrees K. It is readily elicited in submitochondrial particles by partial reduction, but has not been observed in submitochondrial particles from which ubiquinone has been removed by pentane extraction. When ubiquinone is reincorporated into extracted submitochondrial particles, the signal is again easily produced by partial reduction. Electron paramagnetic resonance spectra of partially reduced submitochondrial particles recorded at 34 GHz show lines centered about g approximately 2 with the same separation (approximately 82 G; approximately 0.0082 tesla) as do 9.2 GHz spectra, whereas no lines are detected with a separation of approximately 82 X 34/9.2 G (0.0082 X 34/9.2 tesla). We suggest, on the basis of these observations, that the unidentified signal arises from an interaction of ubisemiquinone and a second paramagnetic species. Three obvious choices exist concerning this second species: ubisemiquinone, flavin semiquinone, or an iorn-sulfur center. It is not possible without much additional information to decide between these possibilities. Since we have never observed the signal in the absence of the membrane-bound, high-potential type iron-sulfur protein, we have considered involvement of this species in the interaction. However, according to computer simulations of the observed electron paramagnetic resonance spectra, which yield best fits for semiquinone-semiquinone interaction, the possibility that ubi- or flavin semiquinone is the interaction partner appears more likely at this time. The interaction appears to be of the magnetic dipole-dipole type, but it is not certain whether there is also a contribution from spin exchange coupling. If it is assumed that the signal is due to magnetic dipole-dipole interaction, the distance of the partners is less than or equal to 7.7 A.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bäckström D., Norling B., Ehrenberg A., Ernster L. Electron spin resonance measurement on ubiquinone-depleted and ubiquinone-replenished submitochondrial particles. Biochim Biophys Acta. 1970 Jan 13;197(1):108–111. doi: 10.1016/0005-2728(70)90018-6. [DOI] [PubMed] [Google Scholar]
- CRANE F. L., GLENN J. L., GREEN D. E. Studies on the electron transfer system. IV. The electron transfer particle. Biochim Biophys Acta. 1956 Dec;22(3):475–487. doi: 10.1016/0006-3002(56)90058-0. [DOI] [PubMed] [Google Scholar]
- Dervartanian D. V., Morgan T. V., Brantner R. V. EPR studies by 57Fe isotopic substitution on the nature of an unknown electron acceptor in Azotobacter vinelandii phosphorylating particles. Biochim Biophys Acta. 1974 Jun 28;347(3):497–502. doi: 10.1016/0005-2728(74)90088-7. [DOI] [PubMed] [Google Scholar]
- Kröger A., Klingenberg M. On the role of ubiquinone in mitochondria. II. Redox reactions of ubiquinone under the control of oxidative phosphorylation. Biochem Z. 1966 Jun 7;344(4):317–336. [PubMed] [Google Scholar]
- Mathews R., Charlton S., Sands R. H., Palmer G. On the nature of the spin coupling between the iron-sulfur clusters in the eight-iron ferredoxins. J Biol Chem. 1974 Jul 10;249(13):4326–4328. [PubMed] [Google Scholar]
- Norling B., Glazek E., Nelson B. D., Ernster L. Studies with ubiquinone-depleted submitochondrial particles. Quantitative incorporation of small amounts of ubiquinone and its effects on the NADH and succinate oxidase activities. Eur J Biochem. 1974 Sep 16;47(3):475–482. doi: 10.1111/j.1432-1033.1974.tb03715.x. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson N. R., Hansen R. E., Beinert H. Electron paramagnetic resonance-detectable electron acceptors in beef heart mitochondria. Ubihydroquinone-cytochrome c reductase segment of the electron transfer system and complex mitochondrial fragments. J Biol Chem. 1974 Mar 25;249(6):1928–1939. [PubMed] [Google Scholar]
- Orme-Johnson N. R., Orme-Johnson W. H., Hansen R. E., Beinert H., Hatefi Y. EPR detectable electron acceptors in submitochondrial particles from beef heart with special reference to the iron-sulfur components of DPNH-ubiquinone reductase. Biochem Biophys Res Commun. 1971 Jul 16;44(2):446–452. doi: 10.1016/0006-291x(71)90621-8. [DOI] [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H. A mitochondrial iron protein with properties of a high-potential iron-sulfur protein. Biochem Biophys Res Commun. 1974 Jun 4;58(3):556–563. doi: 10.1016/s0006-291x(74)80456-0. [DOI] [PubMed] [Google Scholar]
- Sweeney W. V., Bearden A. J., Rabinowitz J. C. The electron paramagnetic resonance of oxidized clostridial ferredoxins. Biochem Biophys Res Commun. 1974 Jul 10;59(1):188–194. doi: 10.1016/s0006-291x(74)80192-0. [DOI] [PubMed] [Google Scholar]



