Abstract
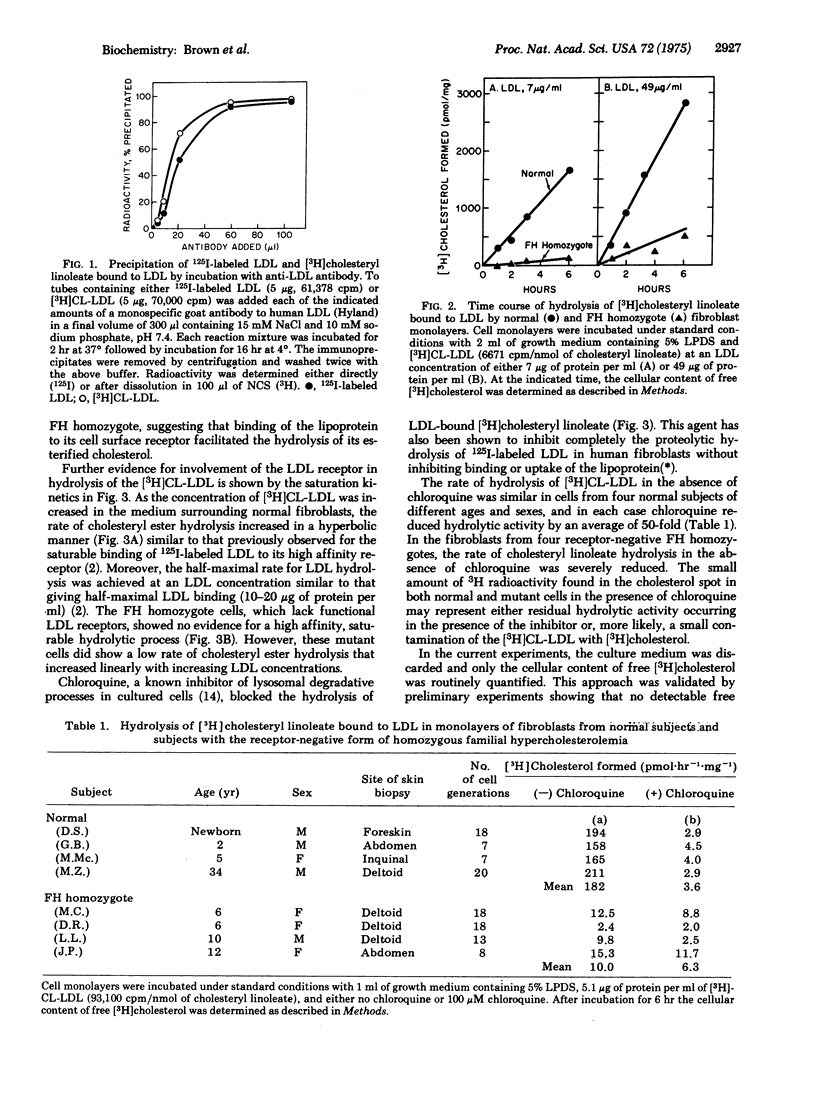
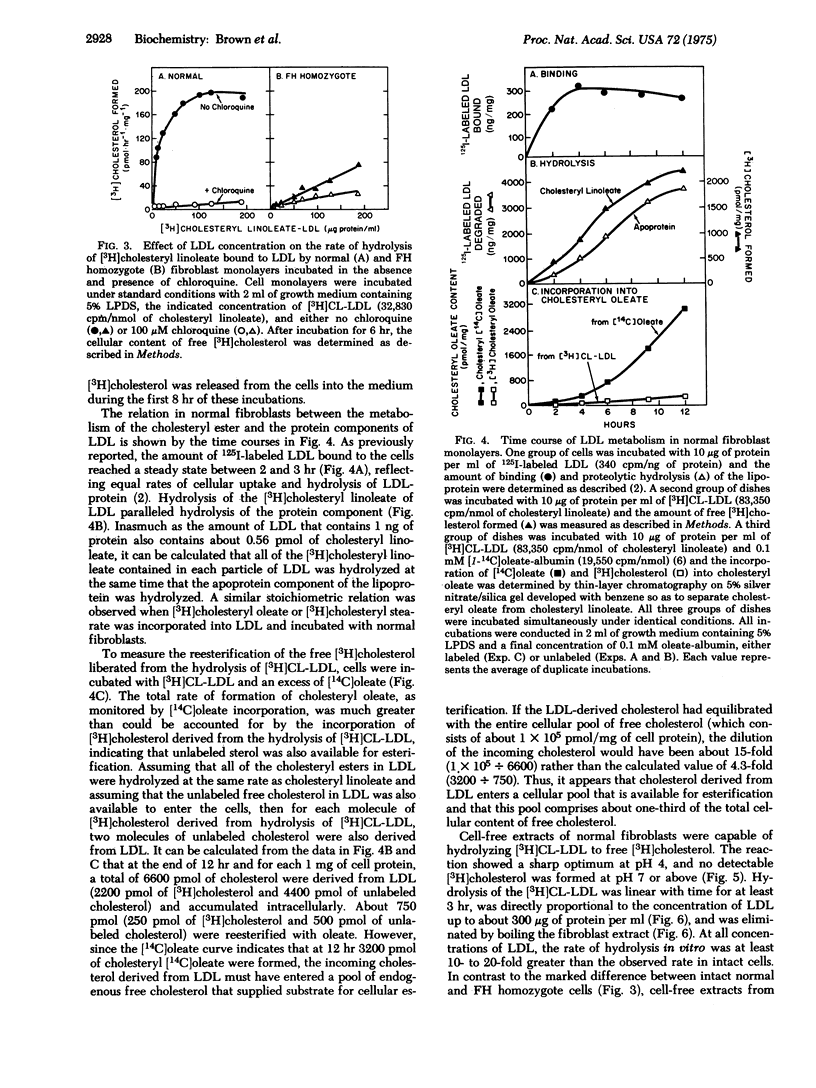
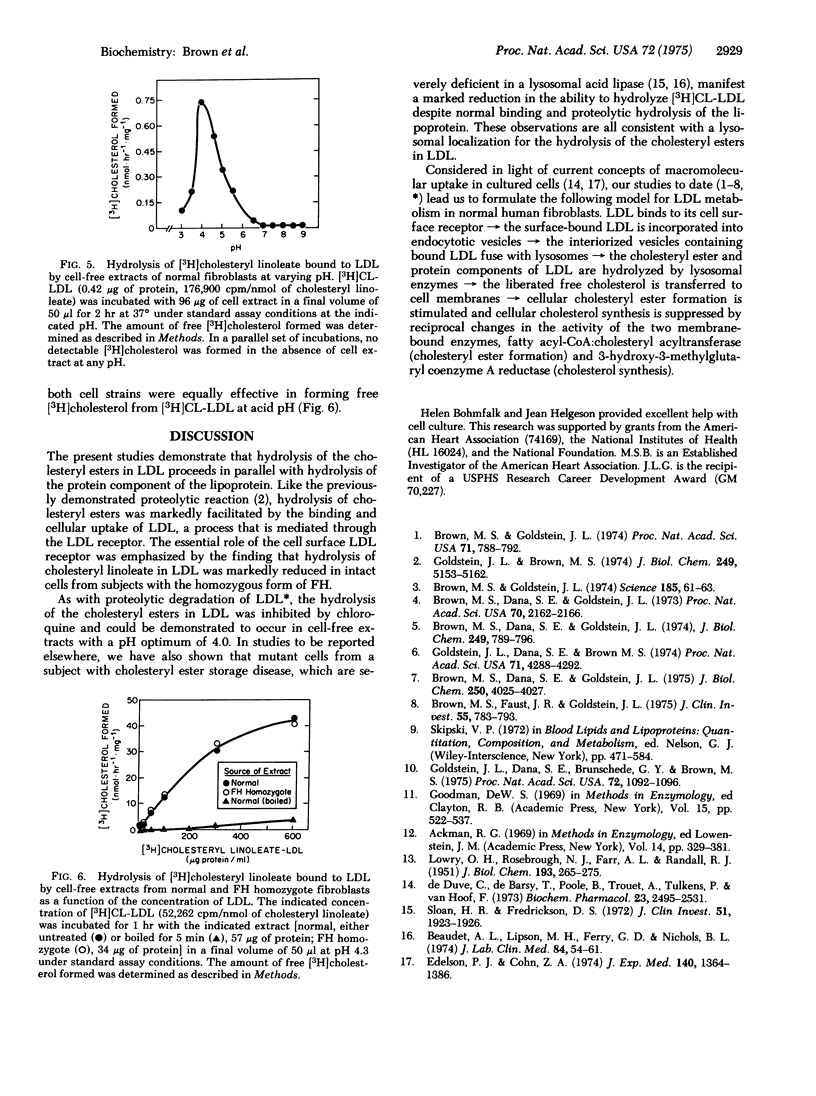
Selective radioactive labeling of the cholesteryl esters contained within human plasma low density lipoprotein has allowed the study of the metabolism of these molecules in monolayers and extracts of cultured human fibroblasts. In monolayers of normal cells, binding of low density lipoprotein to its cell surface receptor was followed by rapid hydrolysis of the [3H]cholesteryl linoleate contained within the lipoprotein and accumulation of the liberated [3H]cholesterol within the cell. The stoichiometry of the degradative process suggested that the protein and cholesteryl ester components of the lipoprotein were hydrolyzed in parallel. Incubation of intact fibroblasts with chloroquine, a known inhibitor of lysosomal degradative processes, inhibited the hydrolysis of the lipoprotein-bound cholesteryl esters. Fibroblasts from subjects with the homozygous form of familial hypercholesterolemia, which lack functional low density lipoprotein receptors and thus are unable to take up this lipoprotein when it is present in the culture medium at low concentrations, were therefore unable to hydrolyze the lipoprotein-bound [3H]cholesteryl linoleate. However, cell-free extracts from these mutant cells were capable of hydrolyzing the lipoprotein-bound [3H]cholesteryl linoleate at the same rapid rate as normal cells when incubated at acid pH. These data illustrate the essential role of the low density lipoprotein receptor and of lysosomal acid hydrolases in the metabolic utilization of low density lipoproteins by cultured human fibroblasts.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudet A. L., Lipson M. H., Ferry G. D., Nichols B. L., Jr Acid lipase in cultured fibroblasts: cholesterol ester storage disease. J Lab Clin Med. 1974 Jul;84(1):54–61. [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Cholesterol ester formation in cultured human fibroblasts. Stimulation by oxygenated sterols. J Biol Chem. 1975 May 25;250(10):4025–4027. [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Feb 10;249(3):789–796. [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2162–2166. doi: 10.1073/pnas.70.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L. Role of the low density lipoprotein receptor in regulating the content of free and esterified cholesterol in human fibroblasts. J Clin Invest. 1975 Apr;55(4):783–793. doi: 10.1172/JCI107989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Expression of the familial hypercholesterolemia gene in heterozygotes: mechanism for a dominant disorder in man. Science. 1974 Jul 5;185(4145):61–63. doi: 10.1126/science.185.4145.61. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Familial hypercholesterolemia: defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Proc Natl Acad Sci U S A. 1974 Mar;71(3):788–792. doi: 10.1073/pnas.71.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. Effects of concanavalin A on mouse peritoneal macrophages. I. Stimulation of endocytic activity and inhibition of phago-lysosome formation. J Exp Med. 1974 Nov 1;140(5):1364–1386. doi: 10.1084/jem.140.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Dana S. E., Brown M. S. Esterification of low density lipoprotein cholesterol in human fibroblasts and its absence in homozygous familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4288–4292. doi: 10.1073/pnas.71.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Dana S. E., Brunschede G. Y., Brown M. S. Genetic heterogeneity in familial hypercholesterolemia: evidence for two different mutations affecting functions of low-density lipoprotein receptor. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1092–1096. doi: 10.1073/pnas.72.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Sloan H. R., Fredrickson D. S. Enzyme deficiency in cholesteryl ester storage idisease. J Clin Invest. 1972 Jul;51(7):1923–1926. doi: 10.1172/JCI106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]



