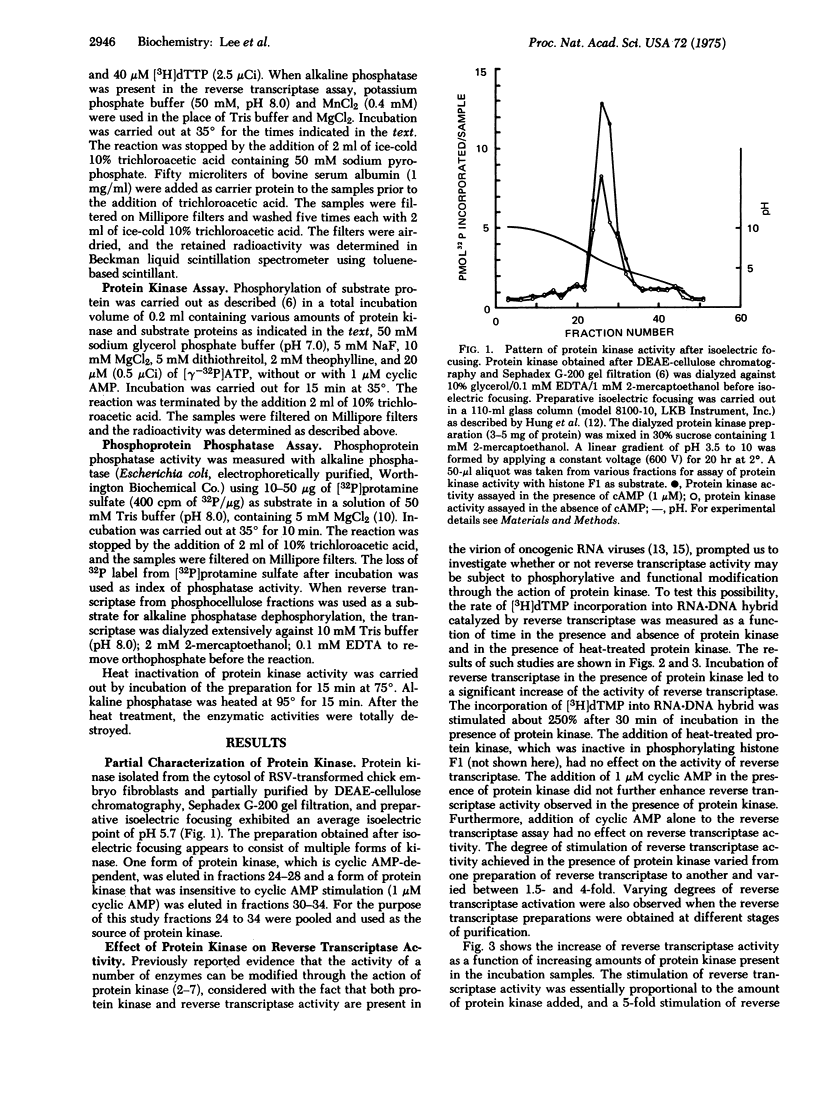
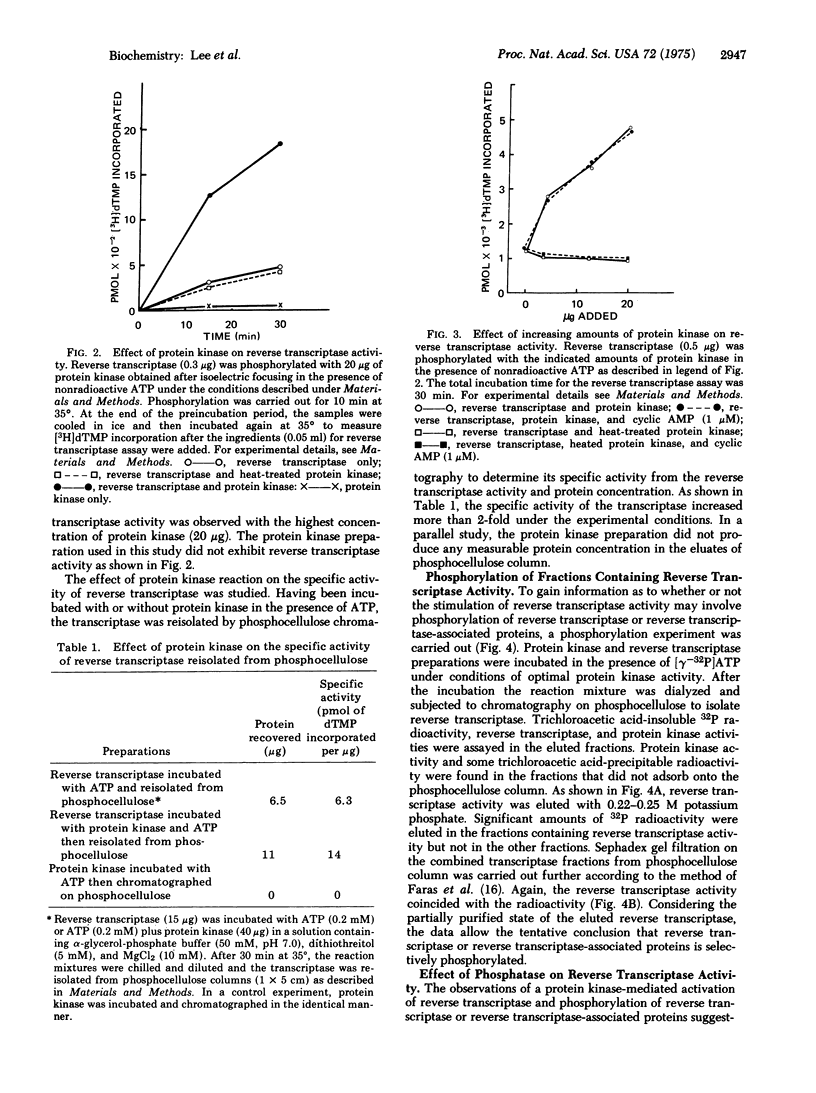
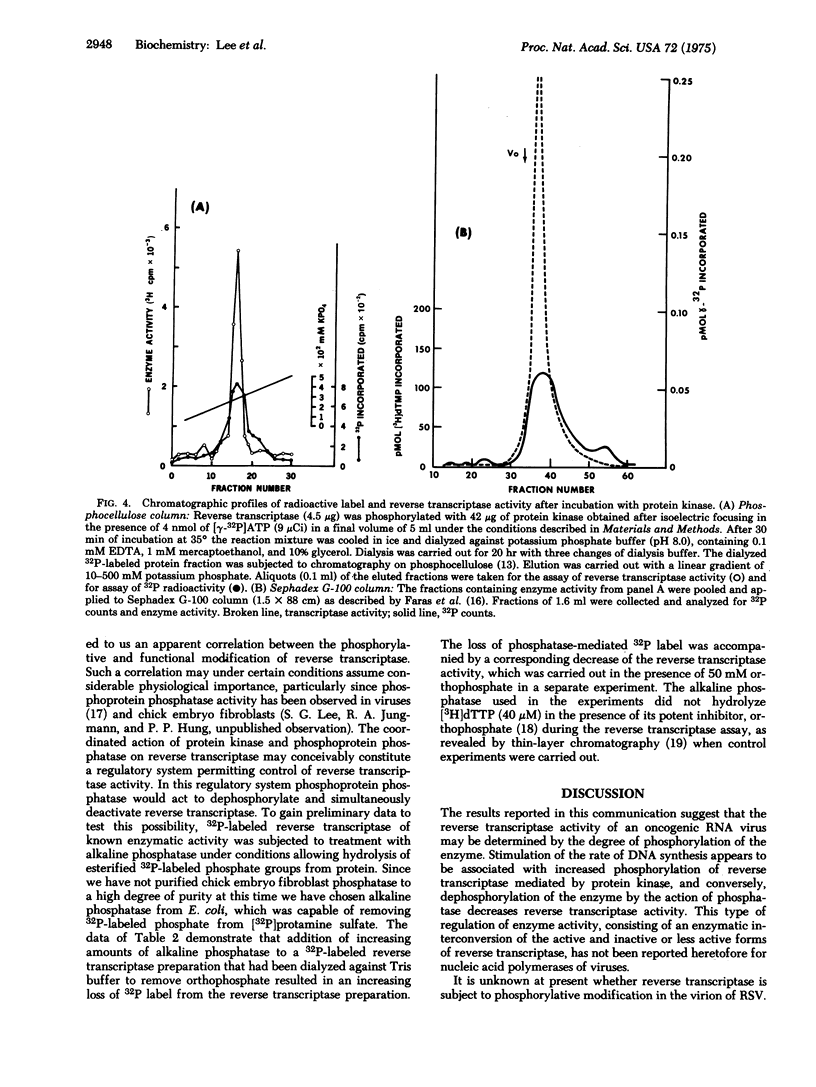
Abstract
We have studied the effect of protein phosphokinase (EC 2.7.1.37; ATP:protein phosphotransferase) and phosphoprotein phosphatase (EC 3.1.3.16; phosphoprotein phosphohydrolase) on reverse transcriptase (RNA-dependent DNA nucleotidyltransferase) activity of Rous sarcoma virus. Protein kinase from Rous sarcoma virus-transformed chick embryo fibroblasts was purified by DEAE-cellulose chromatography, Sephadex gel filtration, and isoelectric focusing. Purified reverse transcriptase from Rouse sarcoma virus was preincubated with protein kinase and ATP under conditions allowing incorporation of phosphate into substrate protein. After the preincubation, reverse transcriptase activity was assayed in the presence of poly(rA).oligo(dT) as template. A 2- to 5-fold increase of reverse transcriptase activity was found after the preincubation of reverse transcriptase with protein kinase and ATP. Incubation of reverse transcriptase with heat-treated, inactive protein kinase and ATP had no effect on transcriptase activity. When the transcriptase preparation was incubated with protein kinase and [gamma-32P]ATP and subsequently purified by chromatography on phosphocellulose and Sephadex gel filtration, significant amounts of 32P-labeled proteins were found in the fractions exhibiting reverse transcriptase activity, suggesting 32P incorporation into transcriptase or transcriptase-associated proteins. A 20-60% decrease of reverse transcriptase activity was observed after incubation of reverse transcriptase with phosphatase. The results suggest that phosphorylative modification of reverse transcriptase may be critical in the regulation of reverse transcriptase-catalyzed DNA synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Dahlberg J. E., Sawyer R. C., Taylor J. M., Faras A. J., Levinson W. E., Goodman H. M., Bishop J. M. Transcription of DNA from the 70S RNA of Rous sarcoma virus. I. Identification of a specific 4S RNA which serves as primer. J Virol. 1974 May;13(5):1126–1133. doi: 10.1128/jvi.13.5.1126-1133.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., McDonnell J. P., Levinson W. E., Bishop J. M. Purification and characterization of the deoxyribonucleic acid polymerase associated with Rous sarcoma virus. Biochemistry. 1972 Jun 6;11(12):2334–2342. doi: 10.1021/bi00762a020. [DOI] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. A single subunit from avian myeloblastosis virus with both RNA-directed DNA polymerase and ribonuclease H activity. Proc Natl Acad Sci U S A. 1973 Jan;70(1):230–234. doi: 10.1073/pnas.70.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEPPEL L. A., HARKNESS D. R., HILMOE R. J. A study of the substrate specificity and other properties of the alkaline phosphatase of Escherichia coli. J Biol Chem. 1962 Mar;237:841–846. [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Noninfectious RSV deficient in DNA polymerase. Virology. 1971 Jan;43(1):313–316. doi: 10.1016/0042-6822(71)90251-0. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Twiddy E., Gilden R. V. Protein kinase associated with RNA tumor viruses and other budding RNA viruses. Virology. 1972 Feb;47(2):536–538. doi: 10.1016/0042-6822(72)90297-8. [DOI] [PubMed] [Google Scholar]
- Hung P. P. Ribonucleases of Rous sarcoma virus. Virology. 1973 Feb;51(2):287–296. doi: 10.1016/0042-6822(73)90429-7. [DOI] [PubMed] [Google Scholar]
- Hung P. P., Robinson H. L., Robinson W. S. Isolation and characterization of proteins from Rous sarcoma virus. Virology. 1971 Jan;43(1):251–266. doi: 10.1016/0042-6822(71)90243-1. [DOI] [PubMed] [Google Scholar]
- Jungmann R. A., Hiestand P. C., Schweppe J. S. Adenosine 3':5'-monophosphate-dependent protein kinase and the stimulation of ovarian nuclear ribonucleic acid polymerase activities. J Biol Chem. 1974 Sep 10;249(17):5444–5451. [PubMed] [Google Scholar]
- Kato K., Bishop J. S. Glycogen synthetase-D phosphatase. I. Some new properties of the partially purified enzyme from rabbit skeletal muscle. J Biol Chem. 1972 Nov 25;247(22):7420–7429. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linial M., Mason W. S. Characterization of two conditional early mutants of Rous sarcoma virus. Virology. 1973 May;53(1):258–273. doi: 10.1016/0042-6822(73)90484-4. [DOI] [PubMed] [Google Scholar]
- Nakai C., Thomas J. A. Properties of a phosphoprotein phosphatase from bovine heart with activity on glycogen synthase, phosphorylase, and histone. J Biol Chem. 1974 Oct 25;249(20):6459–6467. [PubMed] [Google Scholar]
- Narumi S., Miyamoto E. Activation and phosphorylation of carbonic anhydrase by adenosine 3',5'-monophosphate-dependent protein kinases. Biochim Biophys Acta. 1974 May 20;350(1):215–224. doi: 10.1016/0005-2744(74)90219-8. [DOI] [PubMed] [Google Scholar]
- Segal H. L. Enzymatic interconversion of active and inactive forms of enzymes. Science. 1973 Apr 6;180(4081):25–32. doi: 10.1126/science.180.4081.25. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Protein kinase and phosphate acceptor proteins in Rauscher murine leukaemia virus. Nat New Biol. 1971 Sep 29;233(39):137–140. doi: 10.1038/newbio233137a0. [DOI] [PubMed] [Google Scholar]
- Trzeciak W. H., Boyd G. S. Activation of cholesteryl esterase in bovine adrenal cortex. Eur J Biochem. 1974 Jul 1;46(1):201–207. doi: 10.1111/j.1432-1033.1974.tb03612.x. [DOI] [PubMed] [Google Scholar]
- Verma I. M. Studies on reverse transcriptase of RNA tumor viruses. I. Localization of thermolabile DNA polymerase and RNase H activities on one polypeptide. J Virol. 1975 Jan;15(1):121–126. doi: 10.1128/jvi.15.1.121-126.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


