Abstract
Treatment of silkmoth chorion mRNAs with calf thymus RNase H (EC 3.1.4.34; RNA-DNA-hybrid ribonucleotidohydrolase) in the presence of oligo(dT) specifically and effectively removes the 3'-terminal poly(A) sequences. Excision of non-poly(A) fragments cannot be detected. Under these conditions, RNase H leads to increased electrophoretic homogeneity of rabbit globin mRNA, presumably as a result of removal of poly(A) sequences that are inherently variable in length. Treatment with RNase H converts the three diffuse zones of messages for the several chorion proteins into multiple sharp bands.
Full text
PDF
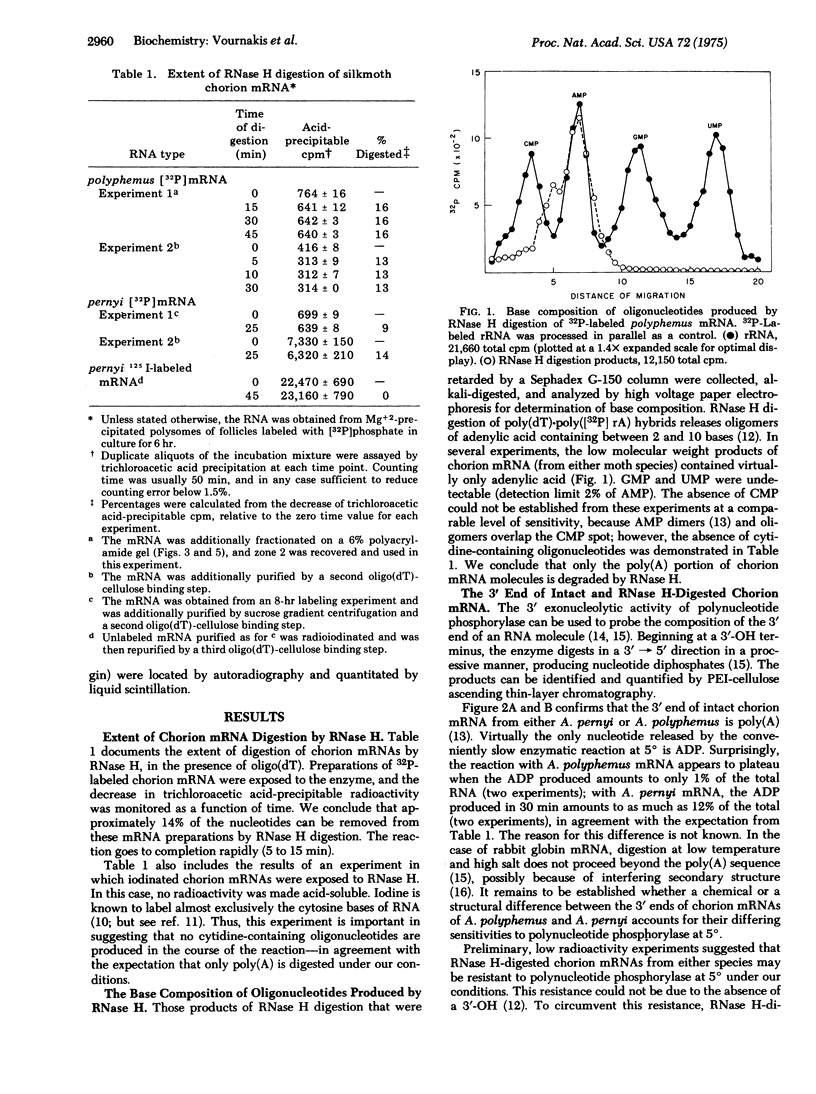
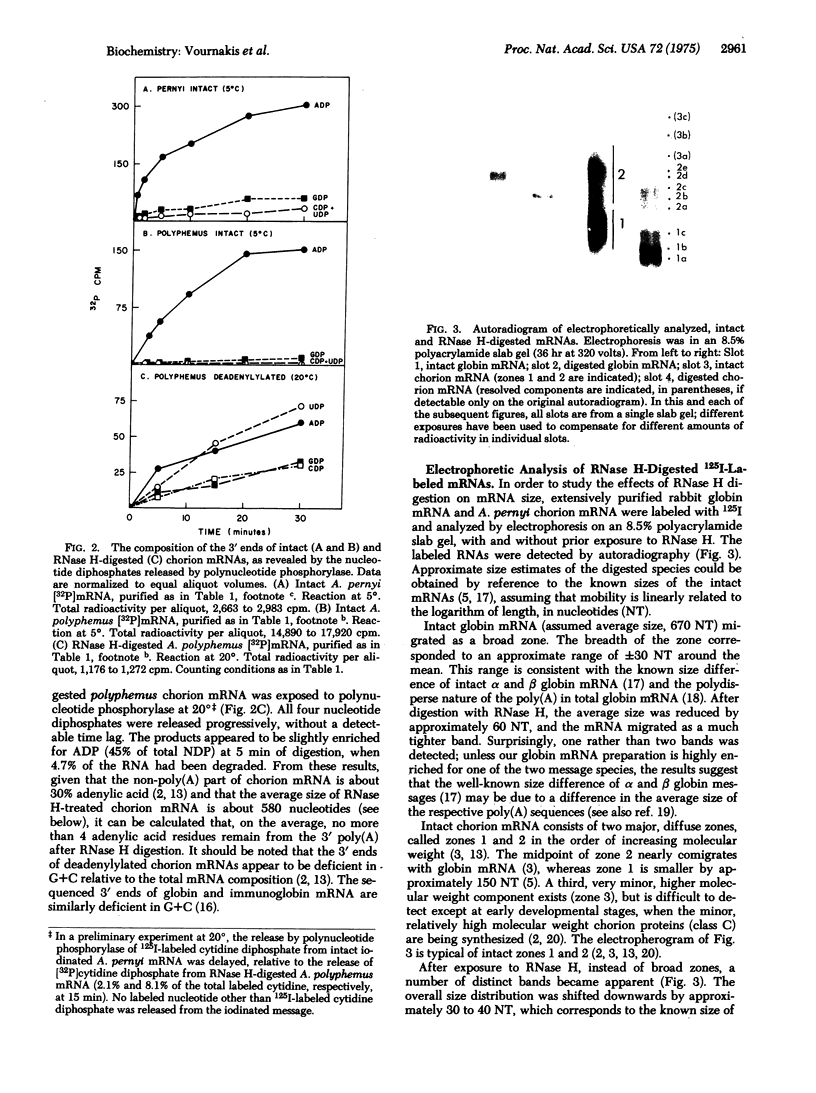


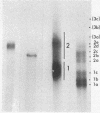
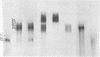
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berns A., Jansen P., Bloemendal H. The separation of alpha- and beta-rabbit globin mRNA by polyacrylamide gel electrophoresis. FEBS Lett. 1974 Oct 15;47(2):343–347. doi: 10.1016/0014-5793(74)81044-6. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Organization, transcription, and regulation in the animal genome. Q Rev Biol. 1973 Dec;48(4):565–613. doi: 10.1086/407817. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Maniatis T., Kafatos F. C., Jeffrey A., Vournakis J. N. Full length and discrete partial reverse transcripts of globin and chorion mRNAs. Cell. 1975 Apr;4(4):367–378. doi: 10.1016/0092-8674(75)90157-9. [DOI] [PubMed] [Google Scholar]
- Gelinas R. E., Kafatos F. C. Purification of a family of specific messenger ribonucleic acids from moth follicular cells. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3764–3768. doi: 10.1073/pnas.70.12.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Maxam A. The nucleotide sequence of the lac operator. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3581–3584. doi: 10.1073/pnas.70.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Morrison M. R., Merkel C. G., Lingrel J. B. Size heterogeneity of polyadenylate sequences in mouse globin messenger RNA. J Mol Biol. 1974 Jun 25;86(2):363–371. doi: 10.1016/0022-2836(74)90025-4. [DOI] [PubMed] [Google Scholar]
- Gould H. J., Hamlyn P. H. The molecular weight of rabbit globin messenger RNA's. FEBS Lett. 1973 Mar 15;30(3):301–304. doi: 10.1016/0014-5793(73)80674-x. [DOI] [PubMed] [Google Scholar]
- Keller W., Crouch R. Degradation of DNA RNA hybrids by ribonuclease H and DNA polymerases of cellular and viral origin. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3360–3364. doi: 10.1073/pnas.69.11.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S., Wood P., Grunstein M., Kedes L. Individual histone messenger RNAs: identification by template activity. Cell. 1975 Mar;4(3):239–248. doi: 10.1016/0092-8674(75)90171-3. [DOI] [PubMed] [Google Scholar]
- Paul M., Goldsmith M. R., Hunsley J. R., Kafatos F. C. Specific protein synthesis in cellular differentiation. Production of eggshell proteins by silkmoth follicular cells. J Cell Biol. 1972 Dec;55(3):653–680. doi: 10.1083/jcb.55.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M., Kafatos F. C., Regier J. C. A comparative study of eggshell proteins in lepidoptera. J Supramol Struct. 1972;1(1):60–65. doi: 10.1002/jss.400010109. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. Sequence at the 3' end of globin mRNA shows homology with immunoglobulin light chain mRNA. Nature. 1974 Nov 29;252(5482):359–362. doi: 10.1038/252359a0. [DOI] [PubMed] [Google Scholar]
- Scherberg N. H., Refetoff S. The radioiodination of ribopolymers for use in hybridizational and molecular analyses. J Biol Chem. 1974 Apr 10;249(7):2143–2150. [PubMed] [Google Scholar]
- Sheiness D., Puckett L., Darnell J. E. Possible relationship of poly(A) shortening to mRNA turnover. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1077–1081. doi: 10.1073/pnas.72.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A. E., Stavrianopoulos J. G., Schutz G., Feigelson P. Translational properties of rabbit globin mRNA after specific removal of poly(A) with ribonuclease H. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4635–4639. doi: 10.1073/pnas.71.11.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H., Nudel U., Salomon R., Revel M., Littauer U. Z. In vitro translation of polyadenylic acid-free rabbit globin messenger RNA. J Mol Biol. 1974 Sep 5;88(1):233–245. doi: 10.1016/0022-2836(74)90307-6. [DOI] [PubMed] [Google Scholar]
- Stavrianopoulos J. G., Chargaff E. Purification and properties of ribonuclease H of calf thymus. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1959–1963. doi: 10.1073/pnas.70.7.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vournakis J. N., Gelinas R. E., Kafatos F. C. Short polyadenylic acid sequences in insect chorion messenger RNA. Cell. 1974 Nov;3(3):265–273. doi: 10.1016/0092-8674(74)90141-x. [DOI] [PubMed] [Google Scholar]
- Williamson R., Crossley J., Humphries S. Translation of mouse globin messenger ribonucleic acid from which the poly(adenylic acid) sequence has been removed. Biochemistry. 1974 Feb 12;13(4):703–707. doi: 10.1021/bi00701a011. [DOI] [PubMed] [Google Scholar]