Abstract
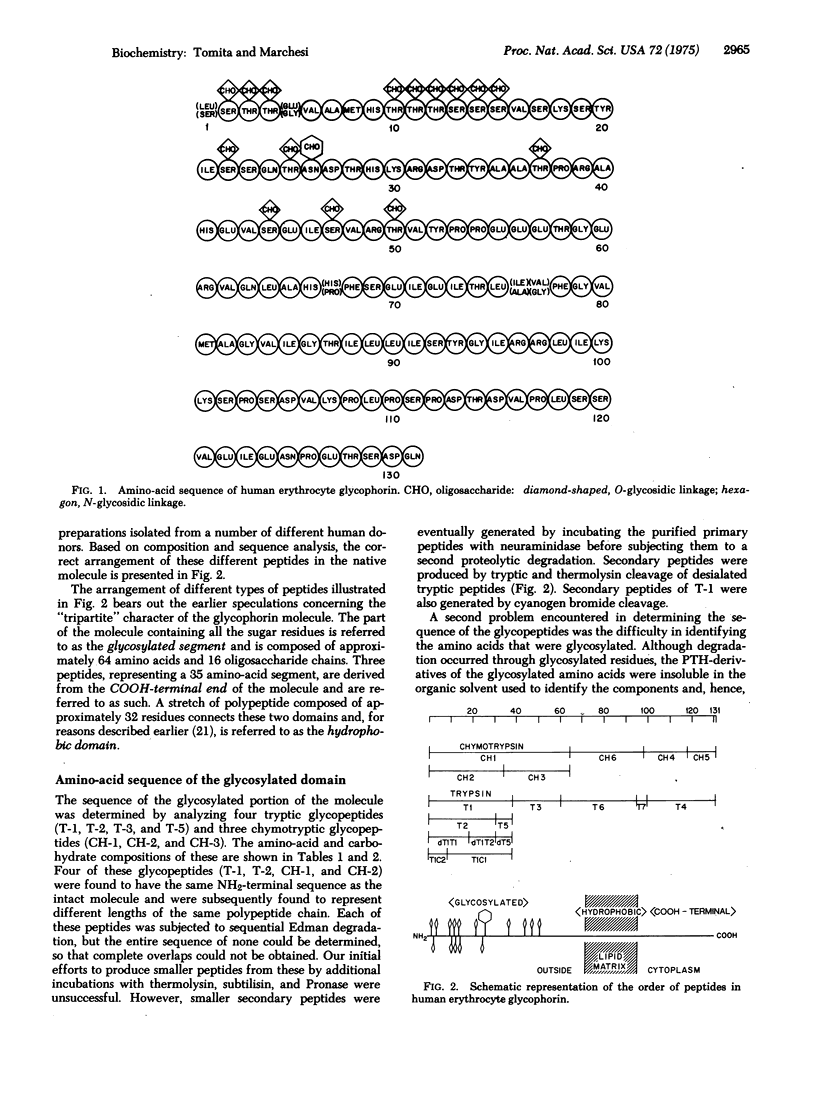
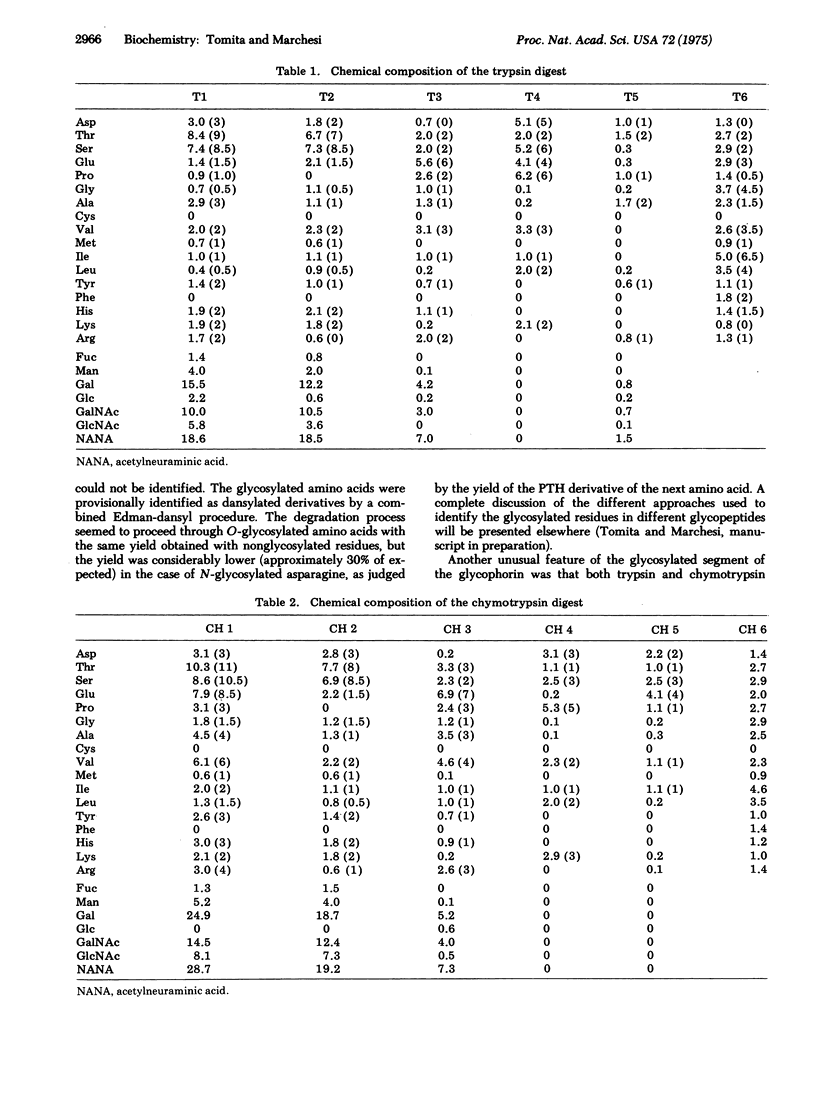
Glycophorin, the major sialoglycoprotein of the human erythrocyte membrane, is composed of 131 amino acids and an average of 16 oligosaccharide chains. Fifteen oligosaccharides are linked to threonine/serine residues via O-glycosidic bonds, and one more complex unit is attached to asparagine. The location of each of these oligosaccharides and the complete amino-acid sequence of this molecular have been determined by Edman degradation techniques. Glycophorin appears to be organized into three distinct "domains" on the basis of the locations of glycosylated amino acids and the clustering of residues of similar type. These include (i) a glycosylated segment composed of approximately 64 residues from the NH2-terminus, (ii) a "hydrophobic" segment of approximately 32 nonpolar residues, and (iii) a COOH-terminal segment, composed of approximately 35 residues, which has an unusual concentration of hydrophilic amino acids. This unique structure is consistent with the earlier suggestions that glycophorin is one of the major "intrinsic" membrane proteins which has a transmembrane orientation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretscher M. S. Major human erythrocyte glycoprotein spans the cell membrane. Nat New Biol. 1971 Jun 23;231(25):229–232. doi: 10.1038/newbio231229a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Illiano G. Purification of neuraminidases from Vibrio Cholerae, Clostridium Perfringens and influenza virus by affinity chromatography. Biochem Biophys Res Commun. 1971 Jul 2;44(1):178–184. doi: 10.1016/s0006-291x(71)80175-4. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Osawa T. Isolation and characterization of a glycoprotein from human group O erythrocyte membrane. J Biol Chem. 1973 Jul 25;248(14):5100–5105. [PubMed] [Google Scholar]
- Grefrath S. P., Reynolds J. A. The molecular weight of the major glycoprotein from the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3913–3916. doi: 10.1073/pnas.71.10.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. L., Segrest J. P., Kahane I., Marchesi V. T. Studies on the major sialoglycoprotein of the human red cell membrane. Isolation and characterization of tryptic glycopeptides. Biochemistry. 1973 Jul 31;12(16):3131–3138. doi: 10.1021/bi00740a600. [DOI] [PubMed] [Google Scholar]
- Janado M., Azuma J., Onodera K. Reversible aggregation of a human erythrocyte membrane glycoprotein. J Biochem. 1973 Nov;74(5):881–887. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. The structure of a phytohemagglutinin receptor site from human erythrocytes. J Biol Chem. 1970 May 25;245(10):2536–2545. [PubMed] [Google Scholar]
- Maddy A. H., Kelly P. G. The inadequacy of 6 M guanidine hydrochloride as a dispersive agent for membrane proteins. Biochim Biophys Acta. 1971 Jul 6;241(1):114–116. doi: 10.1016/0005-2736(71)90309-9. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Tillack T. W., Jackson R. L., Segrest J. P., Scott R. E. Chemical characterization and surface orientation of the major glycoprotein of the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1445–1449. doi: 10.1073/pnas.69.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. D., Nehrlich S., Oyer P. E., Steiner D. F. Determination of the amino acid sequence of the monkey, sheep, and dog proinsulin C-peptides by a semi-micro Edman degradation procedure. J Biol Chem. 1972 Aug 10;247(15):4866–4871. [PubMed] [Google Scholar]
- Sauer R. T., Niall H. D., Hogan M. L., Keutmann H. T., O'Riordan J. L., Potts J. T., Jr The amino acid sequence of porcine parathyroid hormone. Biochemistry. 1974 Apr 23;13(9):1994–1999. doi: 10.1021/bi00706a033. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Marchesi V. T., Guyer R. B., Terry W. Red cell membrane glycoprotein: amino acid sequence of an intramembranous region. Biochem Biophys Res Commun. 1972 Nov 15;49(4):964–969. doi: 10.1016/0006-291x(72)90306-3. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Kahane I., Jackson R. L., Marchesi V. T. Major glycoprotein of the human erythrocyte membrane: evidence for an amphipathic molecular structure. Arch Biochem Biophys. 1973 Mar;155(1):167–183. doi: 10.1016/s0003-9861(73)80019-0. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gibson D., Fanning E. M., Goodfliesh R. M., Gilman J. G., Ballantyne D. L. Quantitative procedures for use with the Edman-Begg sequenator. Partial sequences of two unusual immunoglobulin light chains, Rzf and Sac. Biochemistry. 1971 Dec 21;10(26):4912–4921. doi: 10.1021/bi00802a013. [DOI] [PubMed] [Google Scholar]
- Spiro R. G., Bhoyroo V. D. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974 Sep 25;249(18):5704–5717. [PubMed] [Google Scholar]
- Springer G. F., Nagai Y., Tegtmeyer H. Isolation and properties of human blood-group NN and meconium-Vg antigens. Biochemistry. 1966 Oct;5(10):3254–3272. doi: 10.1021/bi00874a028. [DOI] [PubMed] [Google Scholar]
- Summers M. R., Smythers G. W., Oroszlan S. Thin-layer chromatography of sub-nanomole amounts of phenylthiohydantoin (PTH) amino acids on polyamide sheets. Anal Biochem. 1973 Jun;53(2):624–628. doi: 10.1016/0003-2697(73)90114-0. [DOI] [PubMed] [Google Scholar]
- Thomas D. B., Winzler R. J. Structural studies on human erythrocyte glycoproteins. Alkali-labile oligosaccharides. J Biol Chem. 1969 Nov 10;244(21):5943–5946. [PubMed] [Google Scholar]



