Abstract
Purpose of review
We propose here that the dynamics rather than the structure of cellular and viral networks plays a determining role in chronic immune activation of HIV infected individuals. A number of novel avenues of experimental analysis and modeling strategies is discussed to conclusively address these network dynamics in the future.
Recent findings
Recent insights into the molecular dynamics of immune activation and its control following SIV infection in natural host primates has provided possible alternate interpretations of SIV and HIV pathogenesis. Concomitant with insights gained in other host-pathogen systems, as well as an increased understanding of innate immune activation mechanisms, these observations lead to a new model for the timing of innate HIV immune-responses and a possible primordial role of this timing in programming chronic immune activation.
Summary
Chronic immune activation is today considered the leading cause of AIDS in HIV-infected individuals. Systems biology has recently lent arguments for considering chronic immune activation a result of untimely innate immune responses by the host to the infection. Future strategies for the analysis, comprehension, and incorporation of the dynamic component of immune activation into HIV vaccination strategies are discussed.
Keywords: Human Immunodeficiency Virus (HIV), Simian Immunodeficiency Virus (SIV), Acquired Immune Deficiency Syndrome (AIDS), Systems Biology, Innate and Adaptive Immunity, Natural Host, Non-human primate (NHP), African Green Monkey (AGM), Rhesus Macaque (RM), AGM SIV (SIVagm), RM SIV (SIVmac), Epigenome
1. Introduction
Despite prodigious efforts, the definition of an effective HIV vaccine has only made modest progress [1, 2]. Currrent strategies aim at establishing and/or amplifying both innate and adaptive immune responses [3••]. The central idea being that HIV evades efficient, durable recognition by the human immune system and that therefore a successful vaccination strategy needs to provide for such increased recognition by presetting a humoral immune response [4, 5••]. Similarly, AIDS resistance in SIV natural host primates has been formerly believed to be caused by a lack of innate and adaptive immune activation (IA) contrary to Asian and New World non-human primates (NHPs) which develop AIDS following SIV or HIV infection. Recently, four comparative systems biology studies have shown independently and remarkably concordantly that just like AIDS progressors, natural hosts stage a comparable, in magnitude and composition, innate immune response to SIV infection [6••,7••,8••,9••, see also: 10•]. The major difference in SIV responses between AIDS progressors and non-progressors in NHPs is the duration of this innate response which is attenuated in natural hosts and becomes chronic in AIDS progressor species [11, 12•, 13, 14••, 15, 16]. It is hence the control of excessive immune activation, rather than absence of immune activation, which protects natural hosts from developing AIDS. Importantly, natural host primates tolerate comparable levels of SIV particles in their system which in progessor-species lead to AIDS [6••,7••,8••, 9••, 17]. It is thinkable that protection from AIDS in humans could be achieved by similarly establishing HIV tolerance as opposed to attempts to eradicate the virus from the body [10•, 14••, 15, 16, 18•, 19]. Hence, rather than boosting immune responses, vaccine strategies might want to explore shielding HIV from chronic immune recognition. Furthermore, it should be considered that humans, with the possible exception of longterm non-progressors (LTNPs) and elite controllers [20, 21], and similarly to Asian and New World non-human primates, also stage effective innate immune responses during the acute phase of HIV infection and that only the absence of attenuation/control of this innate response leads to chronic IA and thus AIDS. The current hypothesis of how attenuation or control of IA is established in natural hosts is the presence of active signaling to attenuate IA in natural hosts which would not be present/active in progressors species, or, alternatively, the presence of active signaling to promote continued AI in progressors and being absent in natural hosts [14••, 16]. The molecular, longitudinal profiles of transcriptional responses in AIDS progressors and non-progressors are currently being scrutinized in order to identify such signaling events, and it will be of outmost interest, also beyond the field of HIV research, to see whether candidate pathways to control or promote chronic IA can be identified [10•, 14••, 16, 22, 23]. Here, we discuss an alternate hypothesis for the absence of chronic IA in natural hosts which is based on a purely dynamic interpretation of the events following viral infection in general. While being, given the limited number of available studies, currently only modestly carried by direct experimental observations, this hypothesis not only has the benefit of simplicity, if true, it also would call for a very different approach for HIV vaccine development
2. The West Coast Model for IA
Rather than building the hypothesis we want to discuss here from different arguments, we put upfront a simplified model, and then discuss the evidences that are in support or argue against this proposition (Figure 1). Assume for the sake of argument that acute viral infection be a wave at the beach, let further immune activation be symbolized by mounting a surf-board. Mounting your board too late (case I) will lead to slow sinking; mounting too early (case II) will get you washed away; only the right timing will let you surf (case III). Note that case I can also lead to case II if a second wave approaches. We propose this model as indeed a relevant way to look at viral infections: SIV-infected NHPs and HIV-infected human AIDS progressors would thereby mount their innate immune response too slow or rather too late leading to an inconclusive situation and thus chronic ‘waiting’ for something already gone by. This unresolved innate IA wears down the system and leads consequently to ‘sinking’ (in AIDS: decline in CD4+). Other viruses such as Ebola family viruses elicit such rapid (early) and massive innate IA leading to devastating break-down of the entire immune system (case II) [24, 25, 26, 27, 28]. Finally, natural hosts for SIV, such as sooty mangabeys, African green monkeys, and mandrills display timely responses to infection leading to resolution of IA and long-term tolerance of the virus (surfing).
Figure 1.
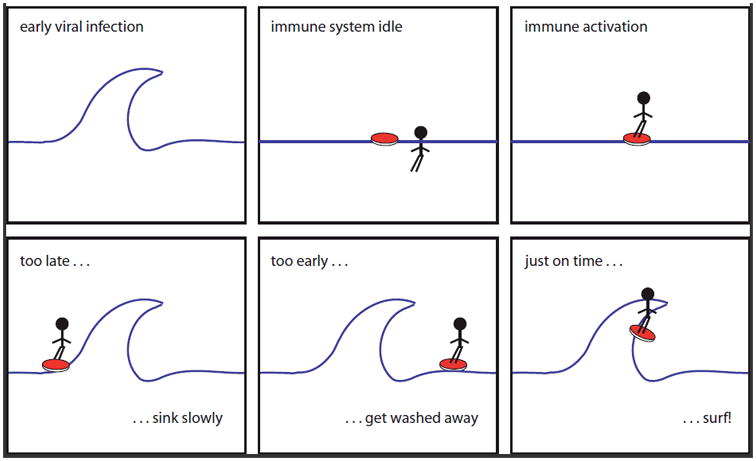
The West Coast Model for IA. From left to right, top to bottom. Let acute viral infection be a wave at the beach, let further mounting your surf-board be symbol for IA. Then, as every surfer knows, mounting your board too late (case I) will lead to slow sinking; mounting too early (case II) will get you washed away; only the right timing will let you surf (case III). Note that case I can also lead to case II if a second wave approaches. We propose this model as indeed a relevant way to look at viral infections: SIV-infected NHPs and HIV-infected human AIDS progressors would there by mount their innate immune response too late leading to an inconclusive, chronic situation and consequently sinking (here: decline in CD4+). Other viruses such as Ebola family viruses elicit such rapid (early) innate IA leading to devastating breakdown of the entire immune system (case II). Finally, natural hosts, such as sooty mangabeys, African green monkeys, and mandrills for SIV-infection display timely responses leading to tolerance of the virus (surfing).
Several, however yet inconclusive, arguments can be made in support of such an hypothesis: SIVagm is elite controlled in macaques. Despite triggering a strong innate IA apparently comparable to the IA in AGMs, SIVagm leads to adaptive immunity in RM demonstrating that acquired immunity against SIVagm is possible [29••]. Why does this not happen in AGMs themselves? Two options: (i) no can, (ii) no need. The latter is more likely and favored here. In AGMs the innate IA is resolved and the adaptive IA is likely not even triggered, which leads to persistent presence of the virus, however, without sufficiently significant consequences to the organism to be selected against. Another interesting question arises: Why are RMs capable of elite controlling SIVagm but not SIVmac? A possible explanation would be that SIVagm, unlike SIVmac, is not subjected to selective evolutionary pressure to evade adaptive immunity as its natural host does not stage an adaptive immune response. Comparative transcriptome profiling between SIV infected natural hosts and progressors has unrevealed a lag-time of IFNγ (as proxy for innate IA) signaling in progressors [6••]. This lag-time (≈6 days) might, however, be due to different effective doses of infection and different amplification kinetics of the SIVagm and SIVmac viruses in the respective species. On the other hand, only a ≈2 days lag can be justified when investigating the SIV amplification kinetics and measured viral titers. Hence, these experiments might provide the first direct evidence of differential activation kinetics for the innate IA fitting above model. Finally, a purely theoretical argument can be made in favor of the hypothesis. Timing of innate IA in this model would be an inherent property of the system and thus not require any dedicated signaling pathways or switches. In humans, it might thus turn out that the absence of an effective adaptive immune response is not per se a problem, but that rather inconclusive (case I) innate IA leads to chronic triggering as seems to be the case in non-human primate AIDS progressors. A mechanisms, where the rapid innate immune activation in AGMs and other natural hosts is presetting its proper attenuation to avoid chronic immune activation, is reminiscent of kinetic or conformational proofreading in molecular discrimination [30]. Interestingly, T-cell receptors use kinetic proofreading to enhance discrimination of bona fide ligands from other proteins to ensure correct signaling [31], and kinetic proofreading could well be at the basis of RIG-I or TLR mediated recognition of foreign in innate immunity [32••, 33, 34]. Kinetic proofreading allows, through expenditure of energy, to increase discrimination of bona fide ligands or interaction partners from closely related molecules with modestly different free energies of binding. It has been first proposed based on theoretical grounds and later demonstrated to be the mechanism by which aminoacyl tRNA synthetase operates [30, 35]. Proposing a mechanisms reminiscent of kinetic proofreading for the coupling between innate and adaptive immunity is appealing as it combines simplicity with fidelity. Thereby, innate IA, with its obvious role of identifying foreign from self, would in the same time serve as a guard against inappropriate initiation of adaptive immunity by being a signal amplifier or dampener where appropriate. In other words, the energy spent on the innate IA increases the capacity of adaptive immunity to increase selectivity. This would require strong coupling between both processes, which is increasingly recognized to be the case. In the case of HIV and SIV infection in AIDS progressors the West Coast Model would thus predict that despite an strong activation of innate immunity, the innate IA remains inconclusive with respect to the function of providing sufficient strong recognition as non-self and triggering adaptive immunity (Figure 2). While innate IA continues to be triggered through the presence of virus, it never reaches the threshold required to trigger subsequent adaptive IA leading to chronic, inconclusive activation. In natural hosts, SIV triggers innate IA and reaches an apparently conclusive situation of non-recognition as foreign and consequent auto-inhibition/attenuation. Despite stimulation of innate IA, SIV is thus not triggering a productive immune response and a equilibrium of co-existence between virus and host emerges. This model thus predicts either better discrimination between inoffensive and foreign by innate immunity in natural hosts. From this follows that for some recognition/binding event during innate IA the difference in free energy is increased when compared to AIDS progressors. A greater free energy will result in a more rapid and earlier activation kinetic of innate IA, and is thus in agreement with the hypothesis developed here.
Figure 2.
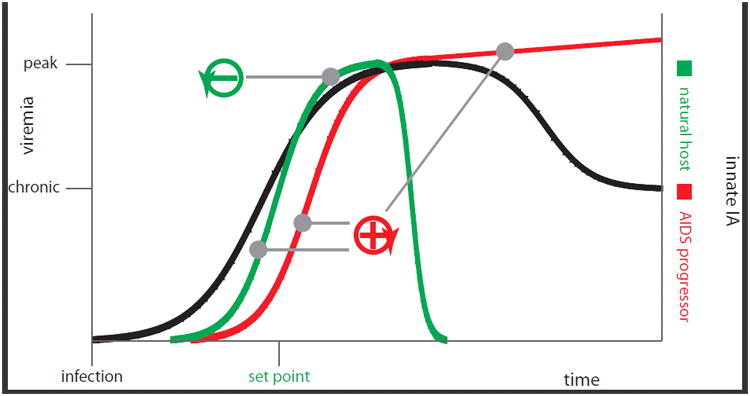
Is the coupling between innate and adaptive IA reminiscent of kinetic proofreading? Innate immune activation obligatorily follows SIV/HIV infection. It is not actively attenuated via a distinct signaling event in natural hosts, but sufficiently early to reach a kinetically encoded set-point and thus autoattenuated. AIDS progressors display too late (with respect to peak-viremia) innate IA to ever reach this set-point. Anything beyond IA set-point is without relevance to the system as it can no-longer reach the auto-attenuation signal
3. Propositions
If one considers the West Coast Model for IA a relevant hypothesis in the context of HIV/AIDS research, a new way of approaching HIV vaccine strategies needs to be considered. As a first measure, however, one would want to investigate some of the stronger hypotheses generated by the model experimentally [36•, 37, 38]. A much higher time-resolved investigation of the innate IA in progressors and non-progressors before peak-viremia using functional genomics and proteomics tools would allow to directly establish IA kinetics and compare them. The SIVagm infection of RMs thereby would be a key control system [29••], as the model would predict that elite control in this system requires rapid, conclusive innate IA compared to a RM response to SIVmac. Appropriate modeling tools would provide for a definitive answer on whether the time-lag is linked to the virus [39] or the host [19], the latter being favorable with respect to the model. Can one cause AIDS in natural hosts by delaying or dampening the innate IA following SIV infection? Current experimental vaccination strategies focus on developing protection in AIDS-progressors. It might be very difficult to find products that would accelerate rather than only amplify innate immune activation and thus very difficult to test present hypothesis this way. On the other hand, delaying innate IA in natural hosts would lead by current model to AIDS in these species, and thus would equally well proof the concept [40••, 41••]. Tampering with innate IA kinetics in non-human primates, however, might be out of reach in both scenarios as this might require reprogramming epigenetic control [42•]. The only viable strategy would hence rely on generating reassortant viruses between for instance SIVagm and SIVmac in order to identify the components of the virus that more efficiently trigger innate IA in natural hosts than in progressor species [39, 43, 44•]. As a corollary, it would be interesting to see whether or not SIVagm lost selective evolutionary pressure to evade adaptive immunity [44•]. As SIVagm is elite controlled in RMs such an analysis might provide additional insights into the model presented here [29••].
4. Conclusions
The works reviewed here have established that: (i) SIV infection is accompanied by innate IA in AIDS progressors and nonprogressors. (ii) Innate IA does not necessarily lead to adaptive immunity in AIDS progressors or non-progressors with the apparent exception of SIVagm infection in macaques. SIVagm might be a truly exceptional case where the virus is no longer under selective pressure to evade adaptive immunity as its natural host never triggers adaptive immunity. HIV and SIV other than SIVagm either evades successfully adaptive immunity, or adaptive immunity is never triggered in humans and AIDS progressor primates. (iii) Innate and adaptive immunity are tightly linked, the former being an activator of the latter [45•, 46, 47, 48•, 49••]. (iv) Innate IA continues until the trigger (here: virus) is cleared from the system or it is actively attenuated as must be the case in natural hosts and possibly in human LTNPs. (v) Viral load at peak viremia is correlated with outcome in AIDS progressors including human and natural hosts provided they were infected with low, more physiological doses of virus. It can furthermore reasonably be assumed that: (i) HIV infection will cause innate IA in humans. (ii) Humans other than LTNPs could similarly as LTNPs and natural hosts live with the high viral titers observed in chronically infected individuals provided innate IA is attenuated and chronic IA avoided. (iii) Innate immunity does not only serve as first line of defense but also is a driver of adaptive immunity and thereby could serve as a proofreading mechanism to avoid, together with regulatory T-cell signaling, IA for self or non-harmful agents. (iv) Attenuation of innate IA might result from active signaling (or discontinuation thereof), or, be an inherent feature of the activation process itself. Indeed, the easiest way of controlling activation is a direct feed-back circuit which likely would be coupled to viral amplification kinetics. Combining above we propose:
Innate immune activation obligatorily follows SIV/HIV infection.
Innate IA is not actively attenuated via a distinct signaling event in natural hosts, but sufficiently early to reach a kinetically encoded set-point, which probably, but not necessarily, is distinct from viral set-point. IA is thus autoattenuated following a mechanism likely reminiscent to kinetic proofreading.
AIDS progressors display too late (with respect to peakviremia) innate IA to ever reach this set-point.
Anything beyond set-point is without relevance to the system as AIDS non-progressors will not develop chronic IA and progressors can no longer avoid chronic IA [50••].
The question of whether an adaptive immune response can be mounted against HIV/SIV is irrelevant and should no longer be focus of vaccine research strategies [3••, 4, 48•, 51•].
Accelerating innate IA in AIDS-progressors will lead to protection from AIDS, decelerating innate IA in natural hosts will cause AIDS.
In conclusion, we might want to start to understand and manipulate the kinetics of innate immune activation. And yes, we all three do enjoy a good surf on the West Coast, whether that be in the U.S. or in France.
Key points.
Innate IA is not actively attenuated via a distinct signaling event in natural hosts, butsufficiently early to reach a kinetically encoded set-point leading to auto-attenuation.
Auto-attenuated thus follows a mechanism reminiscent to kinetic proofreading.
AIDS progressors display too late (with respect to peak-viremia) innate IA to ever reach this critical set-point.
Anything beyond set-point is without relevance to the system as AIDS non-progressors willnot develop chronic IA and progressors can no longer avoid chronic IA.
The question of whether an adaptive immune response can be mounted against HIV/SIV isirrelevant for the design of a preventive vaccine.
Acknowledgments
The authors are grateful to Guido Silvestri for critical reading of the manuscript and the members of their laboratories as well as their collaborators for many stimulating discussions on the matters treated here. Work in AB's laboratory was funded by the Agence Nationale de Recherches sur le SIDA et les h´epatites virales (ANRS), in MG's laboratory through the NIH, and in MGK's laboratory through NIH grants RR00166, RR016354, DA015625, and supplemental funds to RR00166.
References
- 1.Ross AL, Brave A, Scarlatti G, Manrique A, Buonaguro L. Progress towards development of an HIV vaccine: report of the AIDS Vaccine 2009 Conference. Lancet Infect Dis. 2010 May;10(5):305–16. doi: 10.1016/S1473-3099(10)70069-4. [DOI] [PubMed] [Google Scholar]
- 2.Belisle SE, Yin J, Shedlock DJ, Dai A, Yan J, Hirao L, Kutzler MA, Lewis MG, Andersen H, Lank SM, Karl JA, O'Connor DH, Khan A, Sardesai N, Chang J, Aicher L, Palermo RE, Weiner DB, Katze MG, Boyer J. Long-term programming of antigenspecific immunity from gene expression signatures in the PBMC of rhesus macaques immunized with an SIV DNA vaccine. PLoS One. 2011;6(6):e19681. doi: 10.1371/journal.pone.0019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3**.Borrow P, Shattock RJ, Vyakarnam A EUROPRISE Working Group. Innate immunity against HIV: a priority target for HIV prevention research. Retrovirology. 2010 Oct 11;7:84. doi: 10.1186/1742-4690-7-84. Important summary of reasons why to give more attention to innate immunity in vaccine research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katsikis PD, Mueller YM, Villinger F. The Cytokine Network of Acute HIV Infection: A Promising Target for Vaccines and Therapy to Reduce Viral Set-Point? PLoS Pathog. 2011 Aug;7(8):e1002055. doi: 10.1371/journal.ppat.1002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.*Palermo RE, Patterson LJ, Aicher LD, Korth MJ, Robert-Guroff M, Katze MG. Genomic analysis reveals pre- and postchallenge differences in a rhesus macaque AIDS vaccine trial: insights into mechanisms of vaccine efficacy. J Virol. 2011 Jan;85(2):1099–116. doi: 10.1128/JVI.01522-10. Important insight of reasons why to give more attention to innate immunity in vaccine research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, Silvestri G, Giavedoni LD, Lebon P, Barr-Sinoussi F, Benecke A, Müller-Trutwin MC. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009 Dec;119(12):3544–55. doi: 10.1172/JCI40093. Longitudinal, comparative transcriptome profiling of a natural host versus AIDS progresso. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, Zeng M, Masopust D, Carlis JV, Ran L, Vanderford TH, Paiardini M, Isett RB, Baldwin DA, Else JG, Staprans SI, Silvestri G, Haase AT, Kelvin DJ. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009 Dec;119(12):3556–72. doi: 10.1172/JCI40115. Longitudinal, comparative transcriptome profiling of a natural host versus AIDS progressor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8**.Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, Mold J, Swainson L, Barbour JD, Baskin CR, Palermo R, Pandrea I, Miller CJ, Katze MG, McCune JM. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009 Feb;5(2):e1000295. doi: 10.1371/journal.ppat.1000295. Longitudinal, comparative transcriptome profiling of a natural host versus AIDS progressor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Lederer S, Favre D, Walters KA, Proll S, Kanwar B, Kasakow Z, Baskin CR, Palermo R, McCune JM, Katze MG. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 2009 Feb;5(2):e1000296. doi: 10.1371/journal.ppat.1000296. Longitudinal, comparative transcriptome profiling of a natural host versus AIDS progressor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Rotger M, Dalmau J, Rauch A, McLaren P, Bosinger SE, Martinez R, Sandler NG, Roque A, Liebner J, Battegay M, Bernasconi E, Descombes P, Erkizia I, Fellay J, Hirschel B, Mir JM, Palou E, Ho_mann M, Massanella M, Blanco J, Woods M, Gnthard HF, de Bakker P, Douek DC, Silvestri G, Martinez-Picado J, Telenti A. Comparative transcriptomics of extreme phenotypes of human HIV-1 infection and SIV infection in sooty mangabey and rhesus macaque. J Clin Invest. 2011 Jun 1;121(6):2391–400. doi: 10.1172/JCI45235. Meta-analysis of non-human primate and human transcriptome profiles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosinger SE, Sodora DL, Silvestri G. Generalized immune activation and innate immune responses in simian immunodeficiency virus infection. Curr Opin HIV AIDS. 2011 Sep;6(5):411–8. doi: 10.1097/COH.0b013e3283499cf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Manches O, Bhardwaj N. Resolution of immune activation defines nonpathogenic SIV infection. J Clin Invest. 2009 Dec;119(12):3512–5. doi: 10.1172/JCI41509. Comprehensive review of the longitudinal, comparative transcriptome profiling of natural hosts versus AIDS progressors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mir KD, Gasper MA, Sundaravaradan V, Sodora DL. SIV infection in natural hosts: resolution of immune activation during the acute-to-chronic transition phase. Microbes Infect. 2011 Jan;13(1):14–24. doi: 10.1016/j.micinf.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.*Bosinger SE, Jacquelin B, Benecke A, Silvestri G, Müller-Trutwin M. Systems biology towards the understanding of nonpathogenic SIV infection in natural host 4 primate species. Curr Opin HIV AIDS. 2011 Oct 8; Review of the systems biology implications of SIV infection in natural hosts. [Google Scholar]
- 15.Brenchley JM, Silvestri G, Douek DC. Nonprogressive and progressive primate immunodeficiency lentivirus infections. Immunity. 2010 Jun 25;32(6):737–42. doi: 10.1016/j.immuni.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Harris LD, Tabb B, Sodora DL, Paiardini M, Klatt NR, Douek DC, Silvestri G, Müller-Trutwin M, Vasile-Pandrea I, Apetrei C, Hirsch V, Lifson J, Brenchley JM, Estes JD. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J Virol. 2010 Aug;84(15):7886–91. doi: 10.1128/JVI.02612-09. Discusses in detail the importance of control of chronic immune activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estes JD, Gordon SN, Zeng M, Chahroudi AM, Dunham RM, Staprans SI, Reilly CS, Silvestri G, Haase AT. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J Immunol. 2008 May 15;180(10):6798–807. doi: 10.4049/jimmunol.180.10.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Borrow P. Innate immunity in acute HIV-1 infection. Curr Opin HIV AIDS. 2011 Sep;6(5):353–63. doi: 10.1097/COH.0b013e3283495996. Current view of innate immune activation in HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fellay J, Shianna KV, Telenti A, Goldstein DB. Host genetics and HIV-1: the final phase? PLoS Pathog. 2010 Oct 14;6(10):e1001033. doi: 10.1371/journal.ppat.1001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fonseca SG, Procopio FA, Goulet JP, Yassine-Diab B, Ancuta P, Sékaly RP. Unique features of memory T cells in HIV elite controllers: a systems biology perspective. Curr Opin HIV AIDS. 2011 May;6(3):188–96. doi: 10.1097/COH.0b013e32834589a1. [DOI] [PubMed] [Google Scholar]
- 21.Shearer G, Clerici M. Historical perspective on HIV-exposed seronegative individuals: has nature done the experiment for us? J Infect Dis. 2010 Nov 1;202(Suppl 3):S329–32. doi: 10.1086/655974. [DOI] [PubMed] [Google Scholar]
- 22.Ye J, Maniatis T. Negative regulation of interferon-β gene expression during acute and persistent virus infections. PLoS One. 2011;6(6):e20681. doi: 10.1371/journal.pone.0020681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Lepelley A, Louis S, Sourisseau M, Law HK, Pothlichet J, Schilte C, Chaperot L, Plumas J, Randall RE, Si-Tahar M, Mammano F, Albert ML, Schwartz O. Innate sensing of HIV-infected cells. PLoS Pathog. 2011 Feb;7(2):e1001284. doi: 10.1371/journal.ppat.1001284. Exciting novel insights into innate immunity of HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Wit E, Feldmann H, Munster VJ. Tackling Ebola: new insights into prophylactic and therapeutic intervention strategies. Genome Med. 2011 Jan 27;3(1):5. doi: 10.1186/gm219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiropoulou CF, Ranjan P, Pearce MB, Sealy TK, Albario CG, Gangappa S, Fujita T, Rollin PE, Nichol ST, Ksiazek TG, Sambhara S. RIG-I activation inhibits ebolavirus replication. Virology. 2009 Sep 15;392(1):11–5. doi: 10.1016/j.virol.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 26.Bradfute SB, Warfield KL, Bavari S. Functional CD8+ T cell responses in lethal Ebola virus infection. J Immunol. 2008 Mar 15;180(6):4058–66. doi: 10.4049/jimmunol.180.6.4058. [DOI] [PubMed] [Google Scholar]
- 27.Kash JC. Applications of high-throughput genomics to antiviral research: evasion of antiviral responses and activation of inflammation during fulminant RNA virus infection. Antiviral Res. 2009 Jul;83(1):10–20. doi: 10.1016/j.antiviral.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandl JN, Akondy R, Lawson B, Kozyr N, Staprans SI, Ahmed R, Feinberg MB. Distinctive TLR7 signaling, type I IFN production, and attenuated innate and adaptive immune responses to yellow fever virus in a primate reservoir host. J Immunol. 2011 Jun 1;186(11):6406–16. doi: 10.4049/jimmunol.1001191. [DOI] [PubMed] [Google Scholar]
- 29**.Pandrea I, Gaufin T, Gautam R, Kristoff J, Mandell D, Montefiori D, Keele BF, Ribeiro RM, Veazey RS, Apetrei C. Functional cure of SIVagm infection in rhesus macaques results in complete recovery of CD4+ T cells and is reverted by CD8+ cell depletion. PLoS Pathog. 2011 Aug;7(8):e1002170. doi: 10.1371/journal.ppat.1002170. Provides solid evidence for SIVagm elite control in RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hopfield JJ. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4135–9. doi: 10.1073/pnas.71.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci U S A. 1995 May 23;92(11):5042–6. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Loo YM, Gale M., Jr Immune signaling by RIG-I-like receptors. Immunity. 2011 May 27;34(5):680–92. doi: 10.1016/j.immuni.2011.05.003. Detailed review of current state-of-the-art of RNA recognition by the innate system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu HM, Gale M. Hepatitis C Virus Evasion from RIG-I-Dependent Hepatic Innate Immunity. Gastroenterol Res Pract. 2010;2010:548390. doi: 10.1155/2010/548390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suthar MS, Ma DY, Thomas S, Lund JM, Zhang N, Da_s S, Rudensky AY, Bevan MJ, Clark EA, Kaja MK, Diamond MS, Gale M., Jr IPS-1 is essential for the control Of West Nile virus infection and immunity. PLoS Pathog. 2010 Feb 5;6(2):e1000757. doi: 10.1371/journal.ppat.1000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hopfield JJ, Yamane T, Yue V, Coutts SM. Direct experimental evidence for kinetic proofreading in amino acylation of tRNAIle. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1164–8. doi: 10.1073/pnas.73.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Aderem A, Adkins JN, Ansong C, Galagan J, Kaiser S, Korth MJ, Law GL, Mc-Dermott JG, Proll SC, Rosenberger C, Schoolnik G, Katze MG. A systems biology approach to infectious disease research: innovating the pathogen-host research paradigm. MBio. 2011 Mar;2(1):e00325–10. doi: 10.1128/mBio.00325-10. Propositions of how to move forward ‘systemically’. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tisoncik JR, Belisle SE, Diamond DL, Korth MJ, Katze MG. Is systems biology the key to preventing the next pandemic? Future Virol. 2009 Nov 1;4(6):553–561. doi: 10.2217/fvl.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tisoncik JR, Katze MG. What is systems biology? Future Microbiol. 2010 Feb;5(2):139–41. doi: 10.2217/fmb.09.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gnanadurai CW, Pandrea I, Parrish NF, Kraus MH, Learn GH, Salazar MG, Sauermann U, Tpfer K, Gautam R, Mnch J, Stahl-Hennig C, Apetrei C, Hahn BH, Kirchhoff F. Genetic identity and biological phenotype of a transmitted/founder virus representative of nonpathogenic simian immunodeficiency virus infection in African green monkeys. J Virol. 2010 Dec;84(23):12245–54. doi: 10.1128/JVI.01603-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40**.Schreiber F, Lynn DJ, Houston A, Peters J, Mwafulirwa G, Finlay BB, Brinkman FS, Hancock RE, Heyderman RS, Dougan G, Gordon MA. The Human Transcriptome During Nontyphoid Salmonella and HIV Coinfection Reveals Attenuated NFĸB-Mediated Inflammation and Persistent Cell Cycle Disruption. J Infect Dis. 2011 Oct;204(8):1237–45. doi: 10.1093/infdis/jir512. Example of retro-control in innate IA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.*Goodman AG, Tanner BC, Chang ST, Esteban M, Katze MG. Virus infection rapidly activates the P58(IPK) pathway, delaying peak kinase activation to enhance viral replication. Virology. 2011 Aug 15;417(1):27–36. doi: 10.1016/j.virol.2011.04.020. An example for kinetic control in viral infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Su RC, Sivro A, Kimani J, Jaoko W, Plummer FA, Ball TB. Epigenetic control of IRF1 responses in HIV-exposed seronegative versus HIV-susceptible individuals. Blood. 2011 Mar 3;117(9):2649–57. doi: 10.1182/blood-2010-10-312462. Epigenetic control might well be key to different kinetics of innate IA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Telenti A, McLaren P. Genomic approaches to the study of HIV-1 acquisition. J Infect Dis. 2010 Nov 1;202(Suppl 3):S382–6. doi: 10.1086/655969. [DOI] [PubMed] [Google Scholar]
- 44**.Riddick NE, Hermann EA, Loftin LM, Elliott ST, Wey WC, Cervasi B, Taaffe J, Engram JC, Li B, Else JG, Li Y, Hahn BH, Derdeyn CA, Sodora DL, Apetrei C, Paiardini M, Silvestri G, Collman RG. A novel CCR5 mutation common in sooty mangabeys reveals SIVsmm infection of CCR5-null natural hosts and efficient alternative coreceptor use in vivo. PLoS Pathog. 2010 Aug 26;6(8):e1001064. doi: 10.1371/journal.ppat.1001064. Beautiful example of genetic host-pathogen interaction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Boulware DR, Meya DB, Bergemann TL, Williams D, Vlasova-St Louis IA, Rhein J, Staddon J, Kambugu A, Janoff EN, Bohjanen PR. Antiretroviral therapy down-regulates innate antiviral response genes in patients with AIDS in sub-saharan Africa. J Acquir Immune Defic Syndr. 2010 Dec;55(4):428–38. doi: 10.1097/QAI.0b013e3181ef4963. As stimulating as disturbing in case our hypothesis would hold. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Catalfamo M, Wilhelm C, Tcheung L, Proschan M, Friesen T, Park JH, Adelsberger J, Baseler M, Maldarelli F, Davey R, Roby G, Rehm C, Lane C. CD4 and CD8 T cell immune activation during chronic HIV infection: roles of homeostasis, HIV, type I IFN, and IL-7. J Immunol. 2011 Feb 15;186(4):2106–16. doi: 10.4049/jimmunol.1002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalez VD, Landay AL, Sandberg JK. Innate immunity and chronic immune activation in HCV/HIV-1 co-infection. Clin Immunol. 2010 Apr;135(1):12–25. doi: 10.1016/j.clim.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 48*.Eller MA, Blom KG, Gonzalez VD, Eller LA, Naluyima P, Laeyendecker O, Quinn TC, Kiwanuka N, Serwadda D, Sewankambo NK, Tasseneetrithep B, Wawer MJ, Gray RH, Marovich MA, Michael NL, de Souza MS, Wabwire-Mangen F, Robb ML, Currier JR, Sandberg JK. Innate and adaptive immune responses both contribute to pathological CD4 T cell activation in HIV-1 infected Ugandans. PLoS One. 2011 Apr 19;6(4):e18779. doi: 10.1371/journal.pone.0018779. A spill-over effect from chronic innate IA to adaptive IA? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49**.Mogensen TH, Melchjorsen J, Larsen CS, Paludan SR. Innate immune recognition and activation during HIV infection. Retrovirology. 2010 Jun 22;7:54. doi: 10.1186/1742-4690-7-54. Comprehensive review of the interplay between recognition and activation of innate immunity by HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Meythaler M, Wang Z, Martinot A, Pryputniewicz S, Kasheta M, McClure HM, O'Neil SP, Kaur A. Early induction of polyfunctional simian immunodeficiency virus (SIV)-specific T lymphocytes and rapid disappearance of SIV from lymph nodes of sooty mangabeys during primary infection. J Immunol. 2011 May 1;186(9):5151–61. doi: 10.4049/jimmunol.1004110. Strongly supports the hypothesis of rapid innate IA followed by rapid attenuation due to resolution. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51*.Chang JJ, Altfeld M. Innate immune activation in primary HIV-1 infection. J Infect Dis. 2010 Oct 15;202(Suppl 2):S297–301. doi: 10.1086/655657. Important review of innate immune signaling in HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]


