Abstract
Vismodegib, a highly selective inhibitor of hedgehog (Hh) pathway, is an approved treatment for basal-cell carcinoma. Patients on treatment with vismodegib often report profound alterations in taste sensation. The cellular mechanisms underlying the alterations have not been studied. Sonic Hh (Shh) signaling is required for cell growth and differentiation. In taste buds, Shh is exclusively expressed in type IV taste cells, which are undifferentiated basal cells and the precursors of the three types of taste sensing cells. Thus, we investigated if vismodegib has an inhibitory effect on taste cell turnover because of its known effects on Hh signaling. We gavaged C57BL/6J male mice daily with either vehicle or 30 mg/kg vismodegib for 15 weeks. The gustatory behavior and immunohistochemical profile of taste cells were examined. Vismodegib-treated mice showed decreased growth rate and behavioral responsivity to sweet and bitter stimuli, compared to vehicle-treated mice. We found that vismodegib-treated mice had significant reductions in taste bud size and numbers of taste cells per taste bud. Additionally, vismodegib treatment resulted in decreased numbers of Ki67- and Shh-expressing cells in taste buds. The numbers of phospholipase Cβ2- and α-gustducin-expressing cells, which contain biochemical machinery for sweet and bitter sensing, were reduced in vismodegib-treated mice. Furthermore, vismodegib treatment resulted in reduction in numbers of T1R3, glucagon-like peptide-1, and glucagon-expressing cells, which are known to modulate sweet taste sensitivity. These results suggest that inhibition of Shh signaling by vismodegib treatment directly results in alteration of taste due to local effects in taste buds.
Keywords: Basal-cell carcinoma, sonic hedgehog, taste buds, taste perception, Vismodegib
Introduction
Basal-cell carcinoma (BCC) is the most common nonmelanoma skin cancer in the United States, with an annual incidence rate of approximately 1.5% that continues to increase 1. BCC is associated with mutations in components of the hedgehog (Hh) signaling pathway. Hh is a key regulator of cell growth and differentiation during development and controls epithelial and mesenchymal interactions in many tissues during embryogenesis. Hh binds to its receptor patched homologue 1 (PTCH1) and prevents PTCH1-mediated inhibition of signaling by smoothened homologue (SMO). Signaling by SMO results in the activation of transcription factors encoded by GLI family zinc finger (GLI) and consequent induction of Hh target genes, including GLI1 2. Basal-cell tumors have mutations in the Hh signaling pathway that inactivate PTCH1 (loss-of-function mutation) or, less commonly, constitutively activate SMO (gain-of function mutation) 3–5. These mutations cause constitutive activation of Hh signaling that may result in uncontrolled proliferation of basal cells. Thus, blocking the Hh pathway is a therapeutic option in patients with BCC 6,7.
Vismodegib (Erivedge®, Genentech-Curis) is the first oral medicine approved by the US Food and Drug Administration for the treatment of adults with advanced BCC (refer to both locally advanced and distantly metastatic BCCs) that has recurred after surgery or cannot be resected or irradiated. It selectively inhibits SMO, a central mediator of Hh signaling 8–12.
Intriguingly, patients on treatment with vismodegib often report alterations in taste sensation (dysgeusia) 9–11. In a trial conducted by Von Hoff et al., dysgeusia as a grade 2 adverse event occurred in six percent of patients 9. Sekulic et al. reported dysgeusia in 51% of recipients, which was mild to moderate in severity 10. Dysgeusia is also a class effect because it has been reported to occur with other SMO inhibitors, such as sonidegib 13–15: the therefore is likely to be mechanism-related 16–18. However, to date, there is no evidence for direct effects of systemic vismodegib administration in taste buds.
Taste cells are organized within onion-shaped taste buds that reside in three types of papillae in the tongue; fungiform, foliate, and circumvallate papillae. Fungiform and foliate papillae are present on the anterior two-thirds and the posterior sides of the tongue, respectively, while circumvallate papillae, containing the greatest numbers of taste buds, are located under the uvula 19. The taste cells contain the signaling molecules to detect all five prototypic types of taste: sweet, umami, bitter, salty, and sour 20. Taste cells are maintained by continuous cell renewal and the average taste cell lifespan is approximately 10–16 days 21,22. Taste cells are categorized into four types (types I–IV) 20. Type I cells have chemosensing (for salt taste) and supporting functions 23. Type II cells are primary chemosensing that contain the molecules for detecting sweet, umami, and bitter tastes: α-gustducin, T1 receptors (T1R) that detect sweet, including natural sweet tasting food, sweeteners and umami, and T2R that detect bitter 24,25. Type III cells have direct afferent contacts, considered to be the neuronal output cells, and they contain many neurotransmitters as well as the molecular machinery for detecting sour taste 26,27. Type IV cells are nonpolarized, undifferentiated cells located at the base of taste buds, in which sonic Hh (Shh) is exclusively expressed. They are the precursors of the remaining three types (types I–III) of taste cells in taste buds 28. Additionally, taste cells contain many hormones, cholecystokinin, glucagon, glucagon-like peptide-1 (GLP-1), vasoactive intestinal peptide, neuropeptide Y, and ghrelin, that modulate the perception of prototypic tastes 29–32.
In addition to its role in proliferation of type IV cells, Shh plays a critical role in development and patterning of taste papillae in rodents 33. The steroidal alkaloid, cyclopamine, which is a selective disruptor of Shh signaling pathway, or a Shh-blocking antibody altered fungiform papilla induction and distribution in embryonic rat tongue cultures 33. Here we hypothesized that vismodegib inhibits Shh signaling in taste cells, leading to disruption of taste cell turnover, that results, over time, in taste disturbance. We show that vismodegib treatment causes alteration in taste bud morphology and expression of taste sensing machinery in taste cells. Our results suggest that inhibition of Shh signaling by vismodegib treatment directly results in alteration of taste due to local effects in taste buds.
Materials and Methods
Animal and tissue processing
All animal care and experimental procedures followed U.S. National Institutes of Health guidelines and were approved by the Animal Care and Use Committee of the National Institute on Aging. Male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were administered by daily oral gavage of 30 mg/kg vismodegib (LC Laboratories, Woburn, MA) in 0.5% methylcellulose and 0.2% Tween 80 (MCT) 34. Animals of both vehicle and vismodegib groups were euthanized after 15 weeks of treatment using isoflurane overdose and tongues were collected from each animal. The length of the study was to allow for at least four taste cell turnovers. Tongues were fixed in 10% neutral-buffered formalin (Sigma-Aldrich, St Louis, MO) for 1 h and then cryoprotected with 20% sucrose in 0.1 mol/L phosphate buffer overnight at 4°C. Serial sections (8–10 μm thickness) were cut through circumvallate papillae using a cryostat (HM 500M, MICRON, GmbH, Germany). In order to obtain a systematic appreciation without bias of the entire papillae, each papilla was sectioned and every 10th section was saved onto a slide. As taste buds are approximately 80–100 μm in length, sampling every 10th section ensured that no two sections were from the same taste bud.
Taste behavioral tests
Two-bottle taste test was carried out as described previously 35,36. All tastants were prepared with purified water from the National Institute on Aging animal facility and reagent-grade chemicals were presented to the animals at room temperature. Two different testing protocols were used: one for normally preferred stimulus (sucrose; Sigma-Aldrich) and one for normally avoided stimulus (denatonium benzoate, DB; Sigma-Aldrich). Preference was characterized by calculating the ratio of tastant intake to water intake over 24 h.
RNA isolation and real-time PCR of taste buds
Real-time RT-PCR experiments were performed on total RNA isolated from taste buds of foliate papillae and nontaste epithelial tissue devoid of taste cells as described previously 36. The reverse-transcriptase reactions were performed using qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD). Reverse-transcribed cDNAs were amplified using PerfeCTa SYBR Green SuperMix, UNG (Quanta Biosciences). Primer sequences were described in Table1. The data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.
Table 1.
Sequence of primers for real-time PCR
| Gene | Forward primer | Reverse primer | Amplicon size (bp) |
|---|---|---|---|
| Gli1 | ATGAGTGTCTTGCTGGGGTCT | ATCTGCTTGGGGTTCCTTACC | 84 |
| GAPDH | AACTTTGGCATTGTGGAAGG | GGATGCAGGGATGATGTTCT | 132 |
Immunohistochemistry
Following antigen retrieval with 10 mmol/L sodium citrate buffer (pH 6.0) at 98°C for 20 min, immunofluorescence analyses were performed as described previously 37. Sources and dilutions of the applied primary antibodies are listed in Table2. 4′,6-diamidino-2-phenylindole (DAPI, 1:5000 dilution; Sigma-Aldrich) was used for nuclear staining. No fluorescent staining was observed in any sections when the primary antibodies were omitted.
Table 2.
Primary antibodies used in immunofluorescence analyses
| Antigen | Host | Vender | Dilution |
|---|---|---|---|
| Ki67 | Rat | eBioscience, San Diego, CA | 1:100 |
| Shh | Rabbit | Santa-Cruz, Santa Cruz, CA | 1:100 |
| α-Gustducin | Rabbit | Santa-Cruz, Santa Cruz, CA | 1:200 |
| PLCβ2 | Rabbit | Santa-Cruz, Santa Cruz, CA | 1:200 |
| T1R3 | Goat | Santa-Cruz, Santa Cruz, CA | 1:100 |
| GLP-1 | Mouse | US biological, Swampscott, MA | 1:100 |
| Glucagon | Mouse | Sigma-Aldrich | 1:200 |
Quantification of immunoreactive taste cells
Mouse taste bud images were collected using an LSM-710 confocal microscope (Carl Zeiss MicroImaging, Thornwood, NY) in single planes. Approximately 100–120 taste buds per group were analyzed as described previously 31,36,37. Cells were scored as immunopositive only if a nuclear profile was present within the cell. The total number of cells in the section was determined by counting the number of DAPI-stained nuclei present in each taste bud. Finally, the percentage of immunoreactive taste cells was calculated by dividing the number of immunopositive taste cells by the total number of the taste cells in each taste bud. Both image capture and data analysis was performed by trained researchers who were blind to the experimental and control conditions.
Quantification of taste bud size and taste cell numbers per taste bud
Taste bud sizes were calculated in accordance with our previous methods 37. In brief, the perimeters of the taste bud from every 10th section were outlined and the corresponding area was computed by the Zeiss LSM Image Browser software (Carl Zeiss MicroImaging). Simultaneously, 20 taste buds were randomly selected at different regions of each tongue to count the number of cells in a single taste bud, where one nucleus corresponded to one cell on the section.
Statistical analyses
All data represent means ± SEM from at least three independent experimental replicates. Error bars on graphs represent the ±95% confidence interval. One-way analysis of variance (ANOVA) with the Bonferroni post-hoc test was performed by GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA) as appropriate. P < 0.05 was considered statistically significant throughout the study.
Results
Vismodegib attenuates body growth rate and taste responsivity
In patients received vismodegib for treatment of BCC, weight loss and taste disturbance were commonly reported 10,11. We monitored the body weight of vehicle- and vismodegib-treated mice during treatment for 15 weeks. After 4–5 weeks of treatment, the body weights of the two groups began to diverge and by 10 weeks of treatment, the weights of vismodegib-treated mice were significantly different from vehicle-treated mice (Fig.1A) and continued to diverge. By 15 weeks, vismodegib-treated mice were 9% lighter compared with vehicle-treated mice (P < 0.05).
Figure 1.
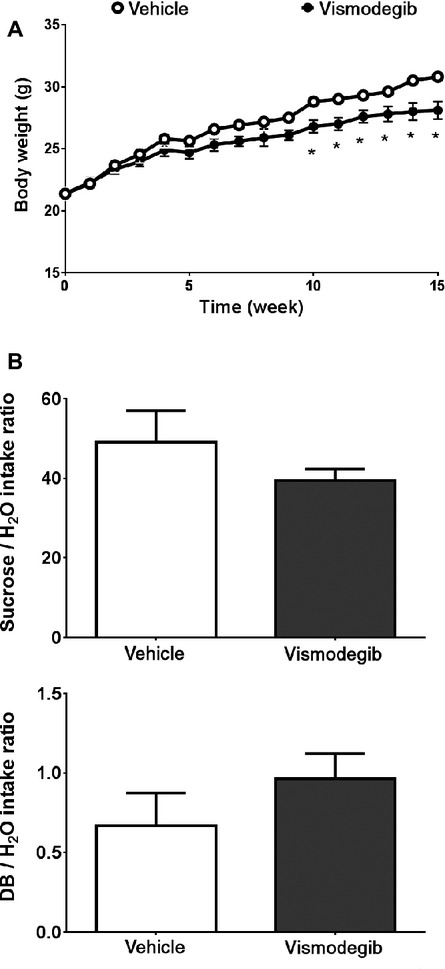
Effect of oral vismodegib and vehicle administration on body weight and hedgehog signaling in mice. (A) Growth curve for vehicle (open circle, n = 8) and vismodegib (closed circle, n = 8) during 15 weeks of treatment. Value are expressed as means ± SEM. *P < 0.05 versus vehicle-treated group. (B) Two-bottle taste testing for modalities of sweet (600 mmol/L sucrose; top) and bitter taste (50 nmol/L denatonium benzoate, DB, bottom) in vehicle- and vismodegib-treated mice.
Next, we investigated potential differences in their taste perception. We tested the ability of both vehicle- and vismodegib-treated mice to detect sweet (sucrose) and bitter (DB, Fig.1B) by two-bottle taste test. We found that vismodegib-treated animals showed trends of reduced taste responsivity compared with vehicle-treated animals for sucrose and DB (Fig.1B; sucrose [top], P = 0.371; DB [bottom], P = 0.296).
Vismodegib-GLI1 relationship
To determine whether vismodegib treatment suppresses Hh signaling in taste buds, we measured Gli1 transcription, which is a downstream target and transcription factor of Hh signaling pathway, in foliate papillae from vehicle- and vismodegib-treated animals. As expected, vismodegib significantly decreased the Hh signaling, as shown by a 40% decrease in Gli1 mRNA expression (Fig.2).
Figure 2.
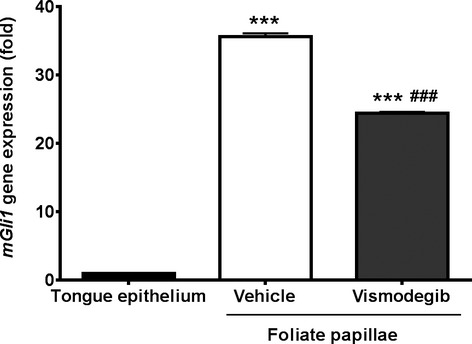
mRNA expression of the hedgehog target gene glioma-associated oncogene homolog 1 (GLI1) in a pool of taste buds from foliate papillae removed from tongues of vehicle- and vismodegib-treated mice. Tongue epithelium, devoid of taste buds, was used as a negative control. Values are expressed as means ± SEM. ***P < 0.001 versus tongue epithelium, ###P < 0.001 versus vehicle-treated group.
Vismodegib alters taste bud size and number of taste cells per taste bud
To investigate whether the alteration in taste behavior and reduction in Hh signaling in taste buds from vismodegib-treated mice causes any alterations in taste bud morphology, we determined taste bud size and taste cell numbers per taste bud. We found that vismodegib-treated mice had significantly smaller taste buds (Fig.3A), compared to vehicle-treated mice and this was likely because there was a significant reduction in the numbers of taste cells within each taste bud in vismodegib-treated mice (Fig.3B).
Figure 3.
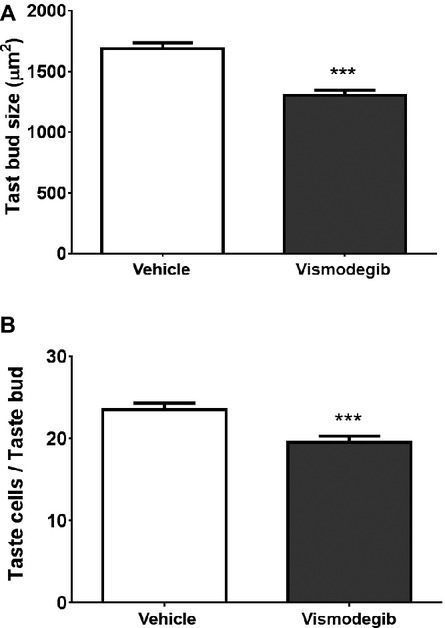
Taste bud size (A) and taste cell numbers per taste bud (B) in vehicle- and vismodegib-treated mice. Value are expressed as means ± SEM. ***P < 0.001 versus vehicle-treated group.
Vismodegib alters potential of taste cell renewal in taste bud
Adult taste buds are maintained by differentiation of type IV cells to any of the other three taste cell types 38,39. To investigate whether the alteration in taste bud size and taste cell numbers is associated with delayed cell renewal, we examined cell proliferation in taste buds by labeling with Ki67 as a marker of cell turnover. We found that vismodegib treatment resulted in decreased numbers of Ki67-positive cells in taste bud (Fig.4A). Additionally, there was a significant reduction in the number of Shh-expressing taste cells in vismodegib-treated animals compared with vehicle-treated animals (Fig.4B).
Figure 4.
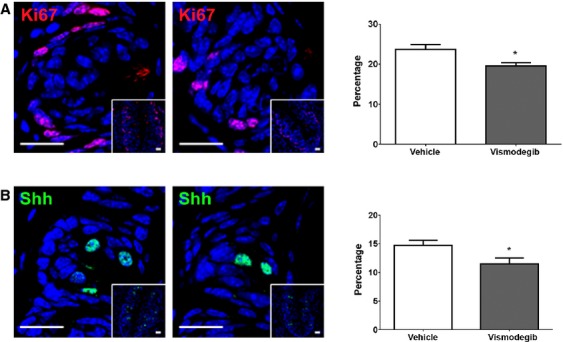
Expression of taste cell proliferation marker Ki67 (A) and Shh (B) in circumvallate papillae taste cells of vehicle- and vismodegib-treated mice. The histograms associated with each taste bud represent the percentage of immunoreactive taste cells containing each marker out of the total number of taste cells in each taste bud. All scale bars are 20 μm. Blue is DAPI nuclear stain. Value are expressed as means ± SEM. *P < 0.05 versus vehicle-treated group.
Vismodegib alters taste sensing machinery and hormone expression in taste buds
As we found alteration in taste modality and total taste cell number, we next investigated the expression of multiple taste-modulatory factors in the taste cells of vehicle- and vismodegib-treated mice. α-Gustducin and phospholipase Cβ2 (PLCβ2) are essential chemosensing molecules in type II cells 24,40–42. α-Gustducin is the alpha subunit of the G-protein coupled to T1R and T2R and α-gustducin null mice have significantly reduced behavioral and/or nerve responses to bitter, sweet, and umami stimuli 40,43. PLCβ2 is a key enzyme that is necessary for sweet-, umami-, and bitter-signal transduction. It is activated by βγ subunits of trimeric G proteins and it produces the 2-sec messengers, diacylglycerol, and inositol triphosphate, that connect taste receptor signals to downstream components of taste signal transduction ultimately to the brain 44. We found that the numbers of α-gustducin- and PLCβ2-expressing cells were significantly reduced in vismodegib-treated animals compared with vehicle-treated mice (Fig.5A and B). T1R3 (the G protein-coupled receptor necessary for detecting sweet and umami) null mice have the greatly reduced gustatory nerve and behavioral responses to sugars and artificial sweeteners 45. We also found a significant reduction in the number of T1R3 immunoreactive cells in vismodegib-treated mice compared with vehicle-treated mice (Fig.5C). We previously reported that GLP-1 and glucagon are expressed in taste cells where they enhance sweet taste responsivity 30,46. As shown in Figure5D and E, there were reductions in the numbers of GLP-1- and glucagon-expressing cells as well as severely reduced GLP-1 and glucagon expression in vismodegib-treated animals compared with vehicle-treated animals.
Figure 5.
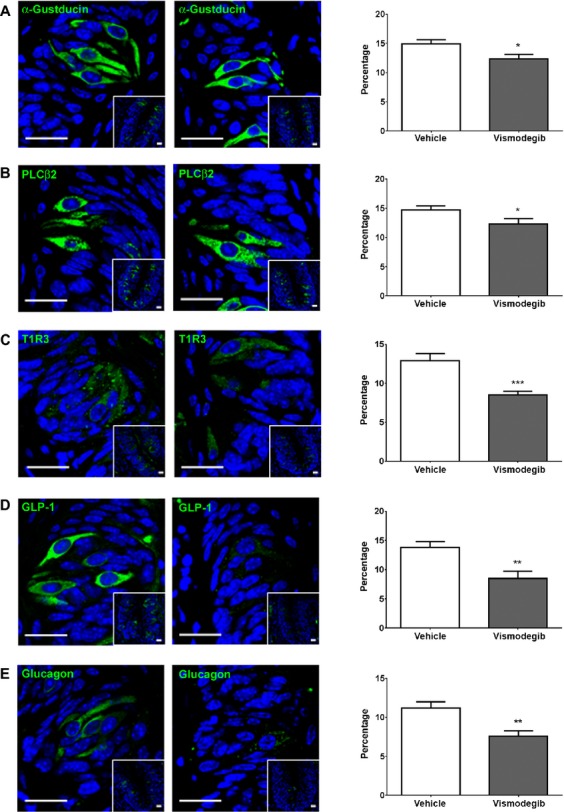
Expression of taste-modulating factors, α-gustducin (A), PLCβ2 (B), T1R3 (C), GLP-1 (D), and glucagon (E), in circumvallate papillae taste cells of vehicle- and vismodegib-treated mice. The histograms associated with each taste bud represent the percentage of immunoreactive taste cells containing each marker out of the total number of taste cells in each taste bud. All scale bars are 20 μm. Blue is DAPI nuclear stain. Value are expressed as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle-treated group.
Discussion
In this study, we investigated the effects of vismodegib, given orally to mice so as to mimic how it is administered to patients, in order to provide a potential explanation for the distortion in taste perception that is reported to occur in up to 55% of patients taking vismodegib for BCC. In mice, oral vismodegib administration leads to decreased body weight, and reduction in the number of cells expressing sweet taste receptors and downstream signaling molecules. We also found a lower expression of hormones known to enhance sweet taste as well as a decrease in the numbers of cells expressing those hormones.
Taste cells have a limited lifespan, die by apoptosis and so must undergo replacement throughout life. Developmentally, they arise from Shh-expressing cells that have the properties of taste cell progenitors and that undergo continuous division 38,39. Either innervating nerves supply signals to the type IV cells to become specific ‘newborn’ taste cells or they are fate-committed cells that require signals from the nerves to become specifically terminally differentiated taste cells 47. These conclusions regarding neuronal input to taste bud maintenance derive from elegant experiments showing that denervation leads to disappearance of taste buds in the first few weeks after denervation followed by their reappearance once nerve regrowth has occurred [38]. It is also known that the type IV cells continuously undergo replication, as shown by Ki67, Shh and BrdU staining, so as to maintain sufficient precursor cell numbers continuously available to receive neuronal signals 38,39.
It appears that vismodegib causes a decrease in the numbers of type IV cells because of deceleration of their replication rate, as shown by a significant reduction in the number of cells positive for Ki67, a well-accepted marker of replicating cells, and Shh. And this, in turn, leads to a decrease in the numbers of cells capable of being in the ‘on’ mode so as to differentiate, on an as-needed basis, in order to refill the population of cells that undergoes death. The cells most affected by the reduction in Shh-positive numbers are the sweet taste receptor T1R3-expressing cells, in addition to GLP-1-and glucagon-containing cells; the receptor is necessary for sweet tasting and the hormones, both active fragments of the proglucagon molecule, locally enhance sweet taste responsivity.
Vismodegib is a competitive antagonist of SMO, a part of the Hh signaling pathway. Total SMO inhibition causes the transcription factor GLI1 to remain inactive, that in turn depresses expression of genes regulated by the Hh signaling pathway 2. In the present study GLI1 expression in taste buds was decreased, but not fully inhibited, by vismodegib treatment in the mice. Even though not fully suppressed, this still resulted in a severe phenotype in taste cells. Therefore, complete suppression is not required to cause alterations in taste molecules.
To our knowledge, the current study is the first to demonstrate that vismodegib affects taste cell turnover and taste functional integrity. These findings provide an explanation for the changes in vismodegib-induced human taste perception because vismodegib most likely results in similar alterations in human taste buds.
Conflict of Interest
None declared.
References
- Wu S, Han J, Li WQ, Li T. Qureshi AA. Basal-cell carcinoma incidence and associated risk factors in US women and men. Am. J. Epidemiol. 2013;178:890–897. doi: 10.1093/aje/kwt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW. McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3097. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, et al. Mutations of the human homolog of Drosophila patched in the nenoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- Aszterbaum M, Rothman A, Johnson RL, Fisher M, Xie J, Bonifas JM, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J. Invest. Dermatol. 1998;110:885–888. doi: 10.1046/j.1523-1747.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, et al. Activating smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- Rubin LL. de Sauvage FJ. Targeting the hedgehog pathway in cancer. Nat. Rev. Drug Discov. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat. Rev. Cancer. 2008;8:743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robarge KD, Brunton SA, Castanedo GM, Cui Y, Dina MS, Goldsmith R, et al. GDC-0449-A potent inhibitor of the hedgehog pathway. Bioorg. Med. Chem. Lett. 2009;19:5576–5581. doi: 10.1016/j.bmcl.2009.08.049. [DOI] [PubMed] [Google Scholar]
- Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N. Engl. J. Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JY, Mackay-Wiggan JM, Aszterbaum M, Yauch RL, Lindgren J, Chang K, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N. Engl. J. Med. 2012;366:2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrone F. Harris CS. Vismodegib and the hedgehog pathway: a new treatment for basal cell carcinoma clinical therapeutics. Clin. Ther. 2012;34:2039–2050. doi: 10.1016/j.clinthera.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Rodon J, Tawbi HA, Thomas AL, Stoller RG, Turtschi CP, Baselga J, et al. A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LED225) in patients with advanced solid tumors. Clin. Cancer Res. 2014;20:1900–1999. doi: 10.1158/1078-0432.CCR-13-1710. [DOI] [PubMed] [Google Scholar]
- Bendell J, Weiss G, Infante J, Ramanathan R, Jones S, Korn R, et al. 594 A phase I dose-escalation, pharmacokinetic (PK) and pharmacodynamic (PD) evaluation of LY2940680, an oral Smo inhibitor. Eur. J. Cancer. 2012;48:182. [Google Scholar]
- Amakye D, Jagani Z. Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat. Med. 2013;19:1410–1422. doi: 10.1038/nm.3389. [DOI] [PubMed] [Google Scholar]
- Hall JMH, Bell ML. Fingera TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev. Biol. 2003;255:263–277. doi: 10.1016/s0012-1606(02)00048-9. [DOI] [PubMed] [Google Scholar]
- Liu HX, MacCallum DK, Edwards C, Gaffield W. Mistretta CM. Sonic hedgehog exerts distinct, stage-specific effects on tongue and taste papilla development. Dev. Biol. 2004;276:280–300. doi: 10.1016/j.ydbio.2004.07.042. [DOI] [PubMed] [Google Scholar]
- Liu HX, Ermilov A, Grachtchouk M, Li L, Gumucio DL, Dlugosz AA, et al. Multiple Shh signaling centers participate in fungiform papilla and taste bud formation and maintenance. Dev. Biol. 2013;382:82–97. doi: 10.1016/j.ydbio.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ. Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- Roper SD. Cell communication in taste buds. Cell. Mol. Life Sci. 2006;63:1494–1500. doi: 10.1007/s00018-006-6112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beidler LM. Smallman RL. Renewal of cells within taste buds. J. Cell Biol. 1965;27:263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farbman AI. Renewal of taste bud cells in rat circumvallate papillae. Cell Tissue Kinet. 1980;13:349–357. doi: 10.1111/j.1365-2184.1980.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Vandenbeuch A, Clapp TR. Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008;9:1. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Chaudhari N. Roper SD. The cell biology of taste. J. Cell Biol. 2010;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee CL, Yang R, Böttger B, Finger TE. Kinnamon JC. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J. Comp. Neurol. 2001;440:97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Stimac R. Roper SD. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J. Physiol. 2008;586:2903–2912. doi: 10.1113/jphysiol.2008.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura H, Kusakabe Y. Harada S. Cell lineage and differentiation in taste buds. Arch. Histol. Cytol. 2006;69:209–225. doi: 10.1679/aohc.69.209. [DOI] [PubMed] [Google Scholar]
- Martin B, Maudsley S, White CM. Egan JM. Hormones in the naso-oropharynx: endocrine modulation of taste and smell. Trends Endocrinol. Metab. 2009;20:163–170. doi: 10.1016/j.tem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson AET, Dotson CD, Egan JM. Munger SD. Glucagon signaling modulates sweet taste responsiveness. FASEB J. 2010;24:3960–3969. doi: 10.1096/fj.10-158105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YK, Martin B, Kim W, White CM, Ji S, Sun Y, et al. Ghrelin is produced in taste cells and ghrelin receptor null mice show reduced taste responsivity to salty (NaCl) and sour (citric acid) tastants. PLoS ONE. 2010;5:e12729. doi: 10.1371/journal.pone.0012729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YK. Egan JM. Roles of hormones in taste signaling. Results Probl. Cell Differ. 2010;52:115–137. doi: 10.1007/978-3-642-14426-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistretta CM, Liu HX, Gaffield W. MacCallum DK. Cyclopamine and jervine in embryonic rat tongue cultures demonstrate a role for Shh signaling in taste papilla development and patterning: fungiform papillae double in number and form in novel location in dorsal lingual epithelium. Dev. Biol. 2003;254:1–18. doi: 10.1016/s0012-1606(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Wong H, Alicke B, West KA, Pacheco P, La H, Januario T, et al. Pharmacokinetic-pharmacodynamic analysis of vismodegib in preclinical model of mutational and ligand-dependent hedgehog pathway activation. Clin. Cancer Res. 2011;17:4682–4692. doi: 10.1158/1078-0432.CCR-11-0975. [DOI] [PubMed] [Google Scholar]
- Zukerman S, Glendinning JI, Margolskee RF. Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R866–R876. doi: 10.1152/ajpregu.90870.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Cong WN, Daimon CM, Wang R, Tschöp MH, Sévigny J, et al. Altered lipid and salt taste responsivity in ghrelin and GOAT null mice. PLoS ONE. 2013;8:e76553. doi: 10.1371/journal.pone.0076553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YK, Cong WN, Cai H, Kim W, Maudsley S, Egan JM, et al. Age-related changes in mouse taste bud morphology, hormone expression, and taste responsivity. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:336–344. doi: 10.1093/gerona/glr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura H, Kato H, Kusakabe Y, Tagami M, Miura-Ohnuma J, Ninomiya Y, et al. A strong nerve dependence of sonic hedgehog expression in basal cells in mouse taste bud and an autonomous transcriptional control of genes in differentiated taste cells. Chem. Senses. 2004;29:823–831. doi: 10.1093/chemse/bjh248. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Borecki AA. Oleskevich S. Stem and progenitor cell compartments within adult mouse taste buds. Eur. J. Neurosci. 2010;31:1549–1560. doi: 10.1111/j.1460-9568.2010.07184.x. [DOI] [PubMed] [Google Scholar]
- Wong GT, Gannon KS. Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- Margolskee RF. Molecular mechanisms of bitter and sweet taste transduction. J. Biol. Chem. 2002;277:1–4. doi: 10.1074/jbc.R100054200. [DOI] [PubMed] [Google Scholar]
- He W, Yasumatsu K, Varadarajan V, Yamada A, Lem J, Ninomiya Y, et al. Umami taste responses are mediated by alpha-transducin and alpha-gustducin. J. Neurosci. 2004;24:7674–7680. doi: 10.1523/JNEUROSCI.2441-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz CJ, Wray K, Delay E, Margolskee RF. Kinnamon SC. Behavioral evidence for a role of alpha-gustducin in glutamate taste. Chem. Senses. 2003;28:573–579. doi: 10.1093/chemse/bjg049. [DOI] [PubMed] [Google Scholar]
- Kinnamon SC. Taste receptor signaling-from tongues to lungs. Acta Physiol. (Oxf) 2012;204:158–168. doi: 10.1111/j.1748-1716.2011.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- Shin YK, Martin B, Golden E, Dotson CD, Maudsley S, Kim W, et al. Modulation of taste sensitivity by GLP-1 signaling. J. Neurochem. 2008;106:455–463. doi: 10.1111/j.1471-4159.2008.05397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee KK, Li Y, Redding KM, Iwatsuki K, Margolskee RF. Jiang P. Lgr5-EGFP marks taste bud stem/progenitor cells in posterior tongue. Stem Cells. 2013;31:992–1000. doi: 10.1002/stem.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]


