Abstract
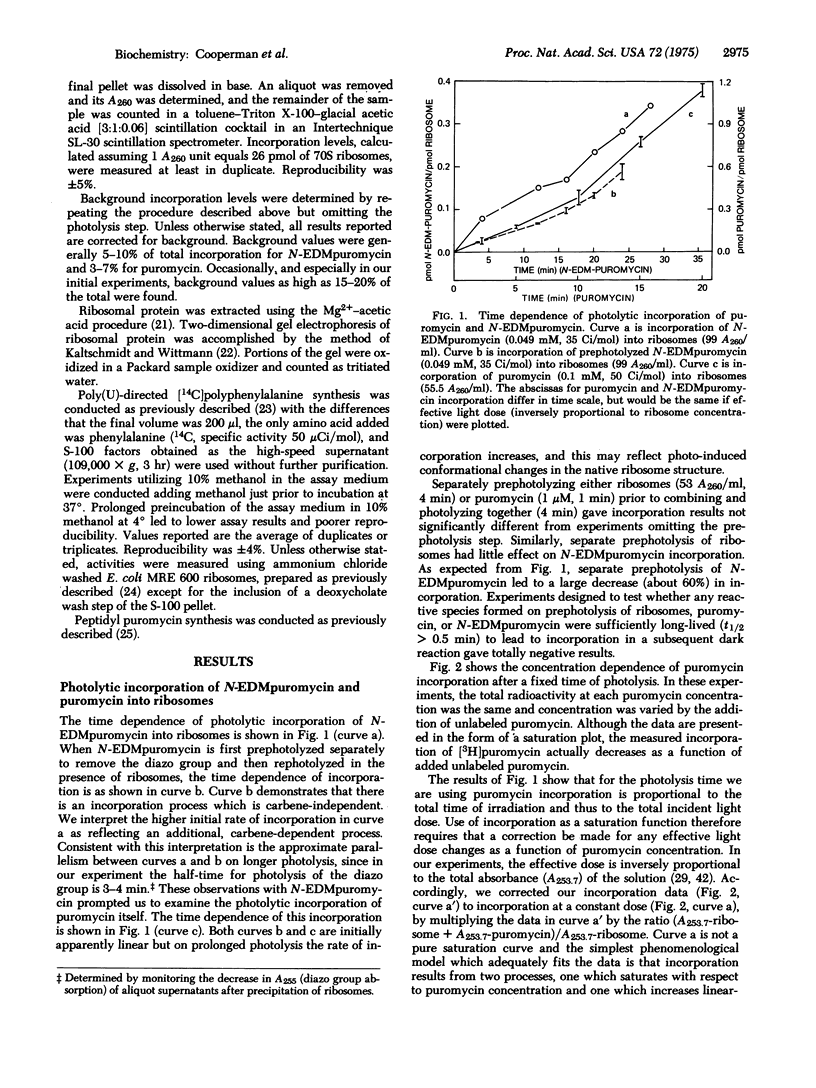
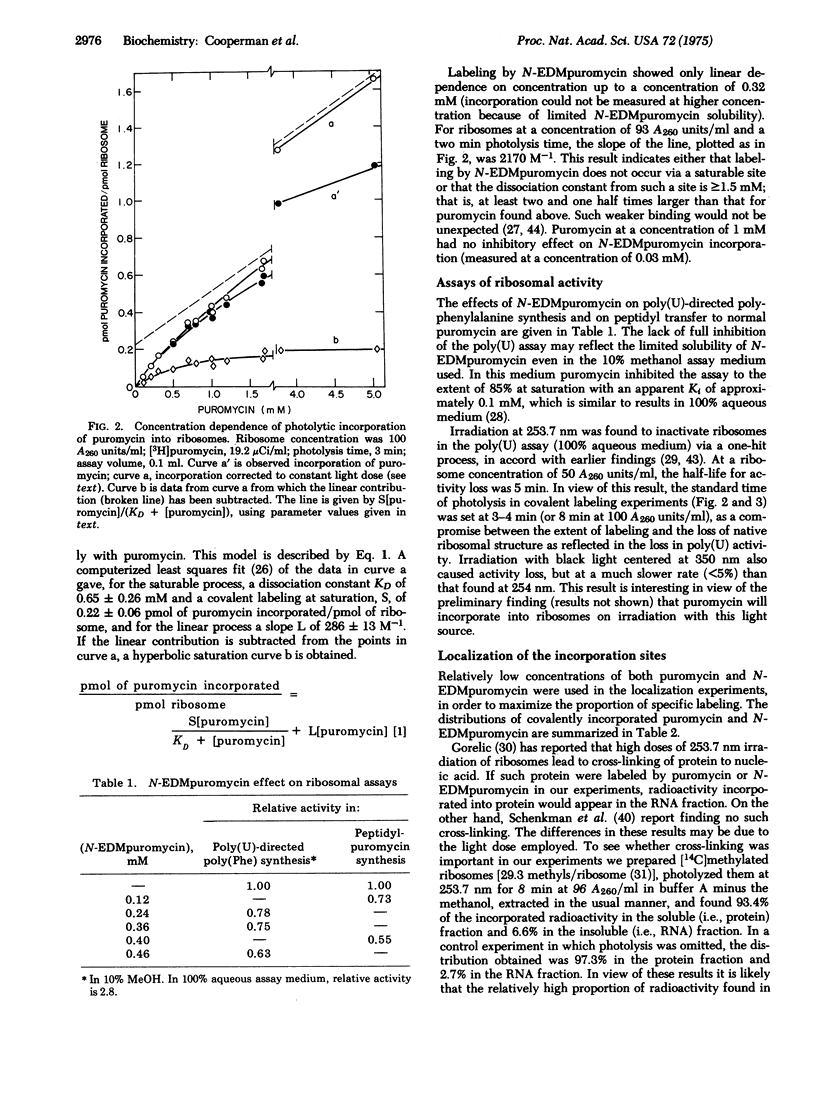
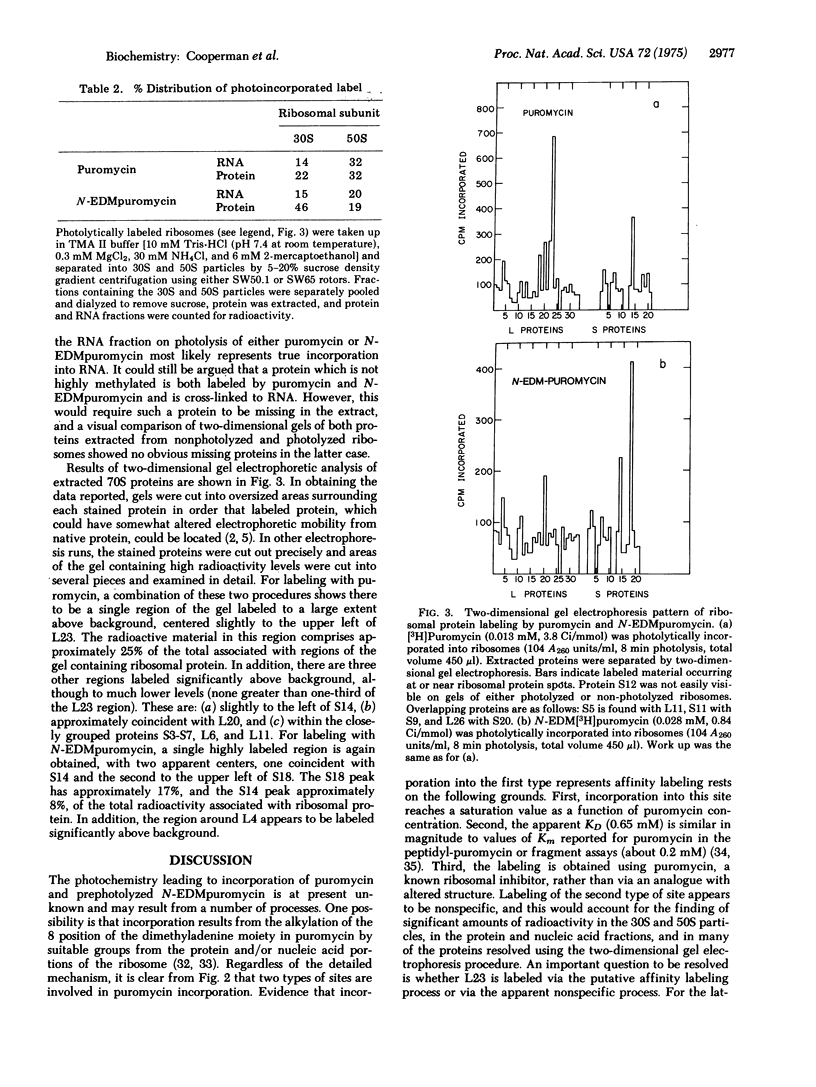
[3H]Puromycin and N-(ethyl-2-diazomalonyl)[3H]puromycin are incorporated into E. coli ribosomes on irradiation at 253.7 nm. Both compounds incorporate into both protein and nucleic acid. Two-dimensional gel electrophoresis of ribosomal protein shows that L23 is the major protein labeled by puromycin. Although incorporation is clearly a complex process, evidence is presented that L23 is labeled via an affinity labeling process, thus placing L23 at the aminoacyl-tRNA receptor (A) site. N-(ethyl-2-diazomalonyl)puromycin is a ribosomal ligand, as shown by its inhibition of two ribosomal assays, but it is not a good puromycin analog, and it is unclear whether its incorporation, which proceeds via both carbene-dependent and carbene-independent processes, results from affinity labeling.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya A. S., Moore P. B. Reaction of ribosomal sulfhydryl groups with 5,5'-dithiobis(2-nitrobenzoic acid). J Mol Biol. 1973 May 15;76(2):207–221. doi: 10.1016/0022-2836(73)90385-9. [DOI] [PubMed] [Google Scholar]
- Almquist R. G., Vince R. Puromycin analogs. Synthesis and biological activity of 5'-deoxypuromycin and its aminonucleoside, 6-dimethylamino-9(3'-amin-3',5-dideoxy-beta-D-ribofuranosyl)purine. J Med Chem. 1973 Dec;16(12):1396–1398. doi: 10.1021/jm00270a018. [DOI] [PubMed] [Google Scholar]
- Bispink L., Matthaei H. Photoaffinity labeling of 23 S rRNA in Escherichia coli ribosomes with poly(U)-coded ethyl 2-diazomalonyl-Phe-tRNA. FEBS Lett. 1973 Dec 1;37(2):291–294. doi: 10.1016/0014-5793(73)80480-6. [DOI] [PubMed] [Google Scholar]
- Brunswick D. J., Cooperman B. S. Synthesis and characterization of photoaffinity labels for adenosine 3':5'-cyclic monophosphate and adenosine 5'-monophosphate. Biochemistry. 1973 Oct 9;12(21):4074–4078. doi: 10.1021/bi00745a008. [DOI] [PubMed] [Google Scholar]
- Czernilofsky A. P., Collatz E. E., Stöffler G., Kuechler E. Proteins at the tRNA binding sites of Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1974 Jan;71(1):230–234. doi: 10.1073/pnas.71.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekert B., Muel B., Latarjet R. Irradiation de ribosomes par des rayons ultraviolets: spectre d'action de l'inactivation de la fonction de synthèse. Biochim Biophys Acta. 1970 Mar 19;204(1):275–277. [PubMed] [Google Scholar]
- Fahnestock S., Neumann H., Shashoua V., Rich A. Ribosome-catalyzed ester formation. Biochemistry. 1970 Jun 9;9(12):2477–2483. doi: 10.1021/bi00814a013. [DOI] [PubMed] [Google Scholar]
- Gorelic L. Photoinduced convalent crosslinkage, in situ, of Escherichia coli 50 S ribosomal proteins to rRNA. Biochim Biophys Acta. 1975 May 1;390(2):209–225. doi: 10.1016/0005-2787(75)90342-1. [DOI] [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Harris R. J., Greenwell P., Symons R. H. Affinity labelling of ribosomal peptidyl transferase by a puromycin analogue. Biochem Biophys Res Commun. 1973 Nov 1;55(1):117–124. doi: 10.1016/s0006-291x(73)80067-1. [DOI] [PubMed] [Google Scholar]
- Hishizawa T., Lessard J. L., Pestka S. Studies on the formation of transfer ribonucleic acid-ribosomes complexes. XII. Phenylalanyl-oligonucleotide binding to E. coli ribosomes: necessity for a free amino group. Proc Natl Acad Sci U S A. 1970 Jun;66(2):523–530. doi: 10.1073/pnas.66.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung N., Cantor C. R. A new simpler photoaffinity analogue of peptidyl tRNA. Nucleic Acids Res. 1974 Dec;1(12):1753–1762. doi: 10.1093/nar/1.12.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung N., Reines S. A., Cantor C. R. Investigation of the ribosomal peptidyl transferase center using a photoaffinity label. J Mol Biol. 1974 Oct 5;88(4):841–855. doi: 10.1016/0022-2836(74)90403-3. [DOI] [PubMed] [Google Scholar]
- Kagawa H., Fukutome H., Kawade Y. Inactivation of Escherichia coli ribosomes by ultraviolet irradiation. I. Activity of poly U-directed polyphenylalanine synthesis. J Mol Biol. 1967 Jun 14;26(2):249–265. doi: 10.1016/0022-2836(67)90295-1. [DOI] [PubMed] [Google Scholar]
- Kahan L., Kaltschmidt E. Glutaraldehyde reactivity of the proteins of Escherichia coli ribosomes. Biochemistry. 1972 Jul 4;11(14):2691–2698. doi: 10.1021/bi00764a022. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. XII. Number of proteins in small and large ribosomal subunits of Escherichia coli as determined by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1276–1282. doi: 10.1073/pnas.67.3.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland C. G. The requirements for specific sRNA binding by ribosomes. J Mol Biol. 1966 Jun;18(1):90–108. doi: 10.1016/s0022-2836(66)80079-7. [DOI] [PubMed] [Google Scholar]
- Lessard J. L., Pestka S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. XXII. Binding of aminoacyl-oligonucleotides to ribosomes. J Biol Chem. 1972 Nov 10;247(21):6901–6908. [PubMed] [Google Scholar]
- Maassen J. A., Möller W. Identification by photo-affinity labeling of the proteins in Escherichia coli ribosomes involved in elongation factor G-dependent GDP binding. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1277–1280. doi: 10.1073/pnas.71.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G., Crichton R. R. Reductive methylation: a method for preparing functionally active radioactive ribosomes. FEBS Lett. 1973 Nov 15;37(1):74–78. doi: 10.1016/0014-5793(73)80429-6. [DOI] [PubMed] [Google Scholar]
- Oen H., Pellegrini M., Eilat D., Cantor C. R. Identification of 50S proteins at the peptidyl-tRNA binding site of Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2799–2803. doi: 10.1073/pnas.70.10.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M., Oen H., Eilat D., Cantor C. R. The mechanism of covalent reaction of bromoacetyl-phenylalanyl-transfer RNA with the peptidyl-transfer RNA binding site of the Escherichia coli ribosome. J Mol Biol. 1974 Oct 5;88(4):809–829. doi: 10.1016/0022-2836(74)90401-x. [DOI] [PubMed] [Google Scholar]
- Pestka S. Peptidyl-puromycin synthesis on polyribosomes from Escherichia coli. Proc Natl Acad Sci U S A. 1972 Mar;69(3):624–628. doi: 10.1073/pnas.69.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. 8. Survey of the effect of antibiotics of N-acetyl-phenylalanyl-puromycin formation: possible mechanism of chloramphenicol action. Arch Biochem Biophys. 1970 Jan;136(1):80–88. doi: 10.1016/0003-9861(70)90329-2. [DOI] [PubMed] [Google Scholar]
- Pongs O., Bald R., Erdmann V. A. Identification of chloramphenicol-binding protein in Escherichia coli ribosomes by affinity labeling. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2229–2233. doi: 10.1073/pnas.70.8.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O., Bald R., Wagner T., Erdmann V. A. Irreversible binding of N-iodoacetylpuromycin to E. coli ribosomes. FEBS Lett. 1973 Sep 1;35(1):137–140. doi: 10.1016/0014-5793(73)80595-2. [DOI] [PubMed] [Google Scholar]
- Pongs O., Erdmann V. A. Affinity labeling of E. coli ribosomes with a streptomycin-analogue. FEBS Lett. 1973 Nov 15;37(1):47–50. doi: 10.1016/0014-5793(73)80423-5. [DOI] [PubMed] [Google Scholar]
- Schenkman M. L., Ward D. C., Moore P. B. Covalent attachment of a messenger RNA to the Escherichia coli ribosome. Biochim Biophys Acta. 1974 Jul 24;353(4):503–508. doi: 10.1016/0005-2787(74)90056-2. [DOI] [PubMed] [Google Scholar]
- Schwartz I., Ofengand J. Photo-affinity labeling of tRNA binding sites in macromolecules. I. Linking of the phenacyl-p-azide of 4-thiouridine in (Escherichia coli) valyl-tRNA to 16S RNA at the ribosomal P site. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3951–3955. doi: 10.1073/pnas.71.10.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J. Covalent labeling of active sites. Adv Protein Chem. 1967;22:1–54. doi: 10.1016/s0065-3233(08)60040-6. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Wilchek M., Zamir A. Mapping of Escherichia coli ribosomal components involved in peptidyl transferase activity. Proc Natl Acad Sci U S A. 1973 May;70(5):1423–1426. doi: 10.1073/pnas.70.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Zamir A., Wilchek M. A photo-induced reaction of chloramphenicol with E. coli ribosomes: covalent binding of the antibiotic and inactivation of peptidyl transferase. Biochem Biophys Res Commun. 1974 Jul 24;59(2):693–696. doi: 10.1016/s0006-291x(74)80035-5. [DOI] [PubMed] [Google Scholar]
- Sopori M., Pellegrini M., Lengyel P., Cantor C. R. Affinity labeling of Escherichia coli ribosomal proteins with an analog of the natural initiator tRNA. Biochemistry. 1974 Dec 17;13(26):5432–5439. doi: 10.1021/bi00723a030. [DOI] [PubMed] [Google Scholar]
- Steinmaus H., Rosenthal I., Elad D. Light- and -ray-induced reactions of purines and purine nucleosides with alcohols. J Org Chem. 1971 Nov 19;36(23):3594–3598. doi: 10.1021/jo00822a029. [DOI] [PubMed] [Google Scholar]



