Abstract
We evaluated the clinical benefits of novel predictive markers for distant recurrence with colorectal cancer using lectin microarray analysis of cell surface glycan modifications. Glycoproteins were extracted from formalin-fixed, paraffin-embedded tumor specimens and normal epithelium from 53 consecutive curatively resected stage I–III colorectal cancer cases and then subjected to lectin microarray to obtain lectin–glycan interaction (LGI) values. In addition, clinicopathological factors associated with distant recurrence were identified. LGI values that were associated with distant recurrence were validated with an additional 55 curatively resected stage II colorectal cancer cases. LGI values for Agaricus bisporus (ABA) lectin, prominent in cancer tissues, were statistically associated with distant recurrence. ABA lectin staining exhibited strikingly intense signals in the cytoplasm and apical surfaces of cancer cells, while weak staining was observed in the supranuclear regions of normal epithelium. This ABA tumor/normal LGI ratio may be a new predictive biomarker for distant recurrence of curatively resected colorectal cancer.
Keywords: Colorectal cancer, glycoprotein, lectin, prediction, recurrence
Introduction
Colorectal cancer is a major cause of morbidity and mortality, with a worldwide annual incidence that ranks third among men (746,000 cases; 10.0% of total cancers per year) and second among women (614,000 cases; 9.2%) 1. Surgery remains the main curative therapy for colorectal cancer. Adjuvant chemotherapy after complete tumor resection is effective for reducing the recurrence risk 2–4. Although adjuvant chemotherapy is standard care for patients with stage III colon cancer, its role for stage II colon cancer remains controversial 3,5. Thus, determining the optimal indications for adjuvant chemotherapy, minimizing the side effects, and decreasing the risk of recurrence are important goals.
Investigators have attempted to identify predictive factors other than the pathological stage for colorectal cancer recurrence. Both oncogenes, such as c-myc, TGF-β, BRAF, and p53, and clinicopathological factors, including lymphatic or venous invasion and budding 6, reportedly predicted recurrence; however, to date, none of these proved to be statistically reliable as predictive factors for use in clinical practice 7. Currently, the glycoproteins CEA and CA19-9 are frequently used to diagnose recurrence or metastasis with colorectal cancer 8–10.
Changes in the glycosylation patterns on cells and specific glycotransferases are reportedly related to cell proliferation, differentiation, tissue adhesion, and carcinogenesis 11. Aberrant glycosylation patterns are associated with human breast 12, prostate 13, thyroid 14, and ovarian 15 cancers as well as human colon cancer 16,17. However, analyzing the structural changes of glycoproteins on cell surfaces has been very difficult because the glycosylation structure is highly susceptible to changes in the extracellular environment. Recently, a novel lectin microarray system was developed to comprehensively analyze glycan profiles using lectins that specifically recognized various sugar moieties 18–21. A recent review also proposed its potential application for the diagnosis of adult T-cell leukemia 22.
Thus, in the present study, we used a lectin microarray system to identify predictive distant recurrence markers from resected specimens of colorectal cancer and assessed their statistical significance.
Materials and Methods
Patients and clinical samples
We selected surgical specimens and the medical records of colorectal cancer patients who underwent resection with curative intent from 1997 to 2010 at the Department of Surgery I, Oita University. We excluded patients who had received preoperative chemotherapy/chemoradiotherapy. As a learning set, consecutive stage I–III colorectal cancer patients (n = 53) were included in this study. All patients were classified using the UICC-TNM staging system 7. All patients were followed up at our hospital's outpatient clinic for an average of 68 months after surgery. Among these 53 patients, 11 developed recurrences that involved at least one distant organ: three in the liver; two in the lung; two in distant LNs and local; and one each in the liver and peritoneum, lung and adrenal gland, lung and local, and peritoneum.
Independent of our primary set, consecutive stage II colorectal cancer patients (n = 55) with a median follow-up of 56 months were included for a validation study. Distant recurrence developed in 12 patients: four in the liver and lung; two each in the liver and lung; two in lungs and peritoneum; one each in the peritoneum and a distant lymph node. Written informed consent was obtained from all the patients.
Sample preparation
Formalin-fixed, paraffin-embedded sections (10 μm for lectin microarray, 3 μm for lectin staining) of colorectal cancer tissue and normal epithelium in the same clinical specimen were placed on glass slides and deparaffinized. Areas of malignant cells were identified for macrodissection under a magnifying glass after staining with hematoxylin and eosin. In addition, regions that contained normal epithelial tissues were macrodissected.
Protein extraction and lectin microarray
The lectin microarray procedures were performed according to the manufacturer's protocol. After washing three times with phosphate-buffered saline (PBS), tissue pellets were collected by centrifugation, resuspended with 20 μL of PBS containing 0.5% NP40 (Nonidet P40), and then sonicated with a Bioruptor UCW-310 (Cosmo Bio, Tokyo, Japan). Protein concentrations were determined with a Micro BCA Protein Assay Reagent Kit (Cat No. 23235; Thermo Scientific, Rockford, IL) and diluted to 15 μg/mL with PBS. Glycoprotein fractions (25 ng each) were labeled with Cy3 using a Probing Solution (GP Biosciences Ltd., Tokyo, Japan) in a 100 μL volume, applied to each well of a LecChip™ (GP Biosciences Ltd.), and then incubated in the dark in a chamber (>80% humidity at 20°C) for 15 h. Wells were washed three times with probing buffer before each application to reduce nonspecific background staining. After the binding reaction, fluorescent images of the lectin microarrays were acquired using a GlycoStation™ Reader 1200 evanescent-field fluorescence scanner (GP Biosciences Ltd.). Data were analyzed using GlycoStation™ Tools Pro Suite 1.5. Lectin–glycan interaction (LGI) values were normalized over 45 different lectins by setting the average intensity of the 45 lectins to 100%.
β-actin staining
We performed β-actin staining for randomly selected samples (four cancerous and four normal epithelia) for normalization. Tissue sections were deparaffinized and soaked in 0.01 mol/L sodium citrate buffer. A primary antibody against β-actin (cat. no. ab8226; Abcam, Cambridge, MA) was used at a 1:500 dilution and staining was developed using a biotin–avidin–peroxidase method. Rabbit serum was used to reduce nonspecific background staining. All sections were counterstained with hematoxylin.
Lectin staining of colorectal tissue
After deparaffinization using xylene and a graded alcohol series, slides were immersed in diluted Antigen Unmasking Solution (Vector Laboratories, Burlingame, CA) to enhance lectin activity. The slides were transferred to 10 mmol/L citrate buffer (pH 6.0) and heated at 95°C for 40 min. After cooling to room temperature, the sections were washed in PBS (pH 7.5) for 5 min. The glass slides were incubated in methanol containing 0.3% hydrogen peroxide for 10 min to reduce endogenous peroxidase activity. To block endogenous biotin and streptavidin, the glass slides were pretreated by incubating in a solution prepared from four drops of streptavidin solution with 1 mL of Carbo-Free Blocking Solution (1 × concentration) at room temperature for 15 min. After briefly rinsing with PBS, the slides were soaked with four drops of Biotin Blocking Solution at room temperature for 15 min. The sections were then incubated with biotinylated ABA lectin purified from Agaricus bisporus lectin (J-OIL MILLS, Tokyo, Japan) used at a concentration of 2 μg/mL for 30 min, incubated with VECTASTAIN® Elite ABC Reagent (Vector Laboratories, Burlingame, CA) for an additional 30 min, and washed in PBS for 5 min. Finally, the reaction products were visualized by incubating the glass slides with diaminobenzidine (DAB) reagent (Vector Laboratories). The sections were counterstained with hematoxylin. Because ABA has dual sugar-binding affinities, Galβ1–3GalNAcα or GalcNAc), the specificity of ABA binding was tested by adding 10 mmol/L Galβ1–3GalNAcα-Thr (Tokyo Chemical Industry Co. Ltd, Tokyo, Japan) or N-acetyl-d-glucosamine (Sigma, St. Louis, MO), respectively.
Statistical analysis
The net intensity value for each microarray spot was calculated by subtracting the background value from the raw signal intensity value. Triplicate readings for each lectin were averaged. Signal intensity results were compared between sample groups by a Mann–Whitney test or Welch's t-test. Strong correlations (R2 = 0.999) were found between normalized and non-normalized data sets. Prognostic factors in patient subgroups were compared by a chi-square test. The Kaplan–Meier method was used to generate survival curves, and statistical comparisons of these curves were made using a log-rank test. Multivariate survival analyses were performed using a Cox proportional hazards model. The cutoff point for a particular lectin was determined using receiver operating characteristic (ROC) curve analysis. A P value of <0.05 was considered statistically significant, and for the log-rank test a P value of <0.01 was considered statistically significant. All analyses were performed using SPSS release 20 (SPSS Inc., Chicago, IL).
Results
Lectin microarray profiles of colorectal cancer tissue
Table1 shows the differential glycan analysis results for 45 lectins (LGI values) expressed by cancerous and normal epithelia in colorectal tissues. The following 12 lectins exhibited significantly increased LGI values for cancer tissues compared with those for normal epithelia: PSA, SNA, SSA, TJA-I, NPA, ConA, GNA, HHL, ABA, PWN, MPA, and Calsepa. In contrast, the following 11 lectins exhibited significantly decreased LGI values for binding to cancer tissues: PHA(L), ECA, RCA120, PHA(E), ACG, BPL, TJA-II, WFA, VVA, DBA, and SBA.
Table 1.
Differential glycan analysis between colorectal cancer tissue (n = 53) and normal control (n = 53) using data from 45 lectins
| Lectin | Normal average | Tumor average | T/N ratio | P-value |
|---|---|---|---|---|
| LTL | 17.4 | 21.6 | 1.24 | N.S. |
| PSA | 53.1 | 62.0 | 1.17 | <0.005 |
| LCA | 68.3 | 74.1 | 1.08 | N.S. |
| UEA_I | 47.7 | 54.2 | 1.14 | N.S. |
| AOL | 173.8 | 170.8 | 0.98 | N.S. |
| AAL | 221.4 | 211.9 | 0.96 | N.S. |
| MAL_I | 38.2 | 38.0 | 1.00 | N.S. |
| SNA | 64.9 | 113.9 | 1.75 | <0.005 |
| SSA | 69.7 | 102.1 | 1.46 | <0.005 |
| TJA-I | 114.6 | 131.2 | 1.15 | 0.024 |
| PHA(L) | 61.4 | 44.1 | 0.72 | <0.005 |
| ECA | 40.5 | 32.2 | 0.79 | <0.005 |
| RCA120 | 178.6 | 145.3 | 0.81 | <0.005 |
| PHA(E) | 158.5 | 136.9 | 0.86 | <0.005 |
| DSA | 256.8 | 248.3 | 0.97 | N.S. |
| GSL-II | 25.2 | 29.7 | 1.18 | N.S. |
| NPA | 69.0 | 106.3 | 1.54 | <0.005 |
| ConA | 38.3 | 49.2 | 1.28 | 0.005 |
| GNA | 52.6 | 88.2 | 1.68 | <0.005 |
| HHL | 32.8 | 52.3 | 1.60 | <0.005 |
| ACG | 257.3 | 207.3 | 0.81 | <0.005 |
| TxLC_I | 83.4 | 87.9 | 1.05 | N.S. |
| BPL | 122.5 | 106.3 | 0.87 | <0.005 |
| TJA-II | 176.2 | 145.6 | 0.83 | <0.005 |
| EEL | 32.9 | 32.0 | 0.97 | N.S. |
| ABA | 67.2 | 90.2 | 1.34 | <0.005 |
| LEL | 313.8 | 323.9 | 1.03 | N.S. |
| STL | 265.5 | 271.9 | 1.02 | N.S. |
| UDA | 235.8 | 242.8 | 1.03 | N.S. |
| PWM | 44.3 | 59.3 | 1.34 | <0.005 |
| Jacalin | 138.9 | 144.4 | 1.04 | N.S. |
| PNA | 33.4 | 35.9 | 1.07 | N.S. |
| WFA | 121.3 | 80.4 | 0.66 | <0.005 |
| ACA | 144.6 | 137.8 | 0.95 | N.S. |
| MPA | 41.5 | 48.3 | 1.16 | 0.016 |
| HPA | 67.1 | 54.7 | 0.81 | N.S. |
| VVA | 32.2 | 23.2 | 0.72 | <0.005 |
| DBA | 75.9 | 43.2 | 0.57 | <0.005 |
| SBA | 76.9 | 50.6 | 0.66 | <0.005 |
| Calsepa | 56.8 | 72.3 | 1.27 | <0.005 |
| PTL_I | 34.2 | 32.3 | 0.94 | N.S. |
| MAH | 50.8 | 47.5 | 0.94 | N.S. |
| WGA | 190.0 | 194.8 | 1.03 | N.S. |
| GSL_I_A4 | 37.2 | 36.6 | 0.98 | N.S. |
| GSL_I_B4 | 17.1 | 17.8 | 1.04 | N.S. |
The ratios of tumor versus normal (T/N) were calculated. N.S., not significant; Mann–Whitney U test.
β-actin staining
We used β-actin staining to establish that the specimens analyzed by lectin microarray had maintained protein molecules both in the membrane and cytosolic fractions. Moderate β-actin staining was observed either in the apical surface or the cytoplasm of all cancerous tissues (Fig1A) and lymphocytes or the cytoplasm of surface epithelia of normal mucosa (Fig1C).
Figure 1.
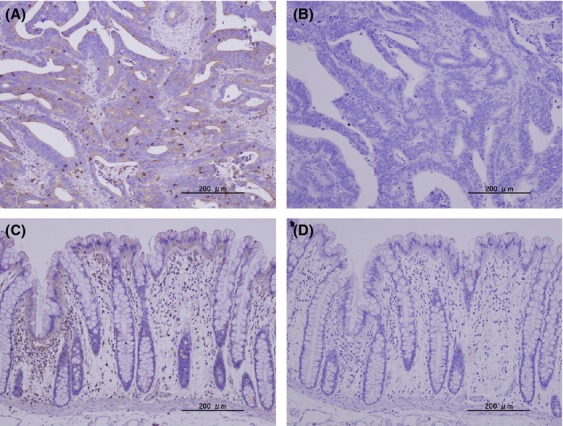
β-actin staining. β-actin in the colorectal cancer tissues (A) or normal colorectal tissues (C) were visualized with anti-human β-actin mAb and biotin–avidin–peroxidase method. Hematoxylin staining of the colorectal cancer tissues (B) or normal colorectal tissues (D) as a control were also performed (×100).
Associations between distant recurrence and clinicopathological factors including lectin signals of postoperative colorectal cancer patients
We examined the clinicopathological factors and lectin expression related to distant recurrence. By univariate analysis, distant recurrence was associated with tumor invasion (P = 0.050), the cancer site (P = 0.002), and the lymph node status (P = 0.023). Of the 45 lectins tested, only HHL- and ABA-binding signals (T/N ratios) were significantly associated with distant recurrence (Table2).
Table 2.
Correlation between distant recurrence and clinicopathological factors, including lectin signals, in patients who underwent curative resection for stage I–III colorectal cancer
| Factors | Category | Recurrence (−) | Recurrence (+) | Univariate analysis | Multivariate P-value, relative risk (95% CI) |
|---|---|---|---|---|---|
| (n = 42) | (n = 11) | P-value | |||
| Age, mean ± SD (range) | 68 ± 13.0 (40–93) | 67 ± 16.5 (26–78) | 0.249 | ||
| Sex | Male | 20 | 8 | 0.138 | |
| Female | 22 | 3 | |||
| CEA (mean, ng/mL) | <5 | 29 | 6 | 0.140 | |
| ≥5 | 8 | 5 | |||
| CA19-9 (mean, U/mL) | <37 | 32 | 8 | 0.598 | |
| ≥37 | 4 | 2 | |||
| Ileus | No | 37 | 11 | 0.296 | |
| Yes | 5 | 0 | |||
| Adjuvant chemotherapy | No | 31 | 5 | 0.143 | |
| Yes | 11 | 6 | |||
| Site | Colon | 33 | 3 | 0.002 | N.S. |
| Rectum | 9 | 8 | |||
| Tumor invasion | pT1/T2 | 17 | 1 | 0.050 | |
| pT3/T4 | 25 | 10 | |||
| Lymph node status | pN0-1 | 37 | 6 | 0.023 | N.S. |
| pN2- | 5 | 5 | |||
| Lymphatic invasion | ly− | 20 | 5 | 0.898 | |
| ly+ | 22 | 6 | |||
| Venous invasion | v− | 31 | 8 | 0.942 | |
| v+ | 11 | 3 | |||
| Histology | tub1/tub2 | 39 | 11 | 1.000 | |
| por/muc | 3 | 0 | |||
| pStage | I | 17 | 1 | 0.122 | |
| II(A+B) | 10 | 3 | |||
| III(A+B+C) | 15 | 7 | |||
| PSA2.367 | <2.367 | 41 | 10 | 0.375 | |
| ≥2.367 | 1 | 1 | |||
| SNA | <1.956 | 30 | 5 | 0.105 | |
| ≥1.956 | 12 | 6 | |||
| SSA | <1.650 | 29 | 5 | 0.136 | |
| ≥1.650 | 13 | 6 | |||
| TJA-I | <0.866 | 10 | 0 | 0.075 | |
| ≥0.866 | 32 | 11 | |||
| PHA(L) | <0.839 | 29 | 4 | 0.052 | |
| ≥0.839 | 13 | 7 | |||
| ECA | <0.781 | 21 | 2 | 0.057 | |
| ≥0.781 | 21 | 9 | |||
| RCA120 | <1.068 | 35 | 8 | 0.340 | |
| ≥1.068 | 7 | 3 | |||
| PHA(E) | <0.664 | 5 | 3 | 0.206 | |
| ≥0.644 | 37 | 8 | |||
| NPA | <2.434 | 39 | 8 | 0.096 | |
| ≥2.434 | 3 | 3 | |||
| ConA | <1.929 | 33 | 7 | 0.257 | |
| ≥1.929 | 9 | 4 | |||
| GNA | <2.053 | 29 | 5 | 0.136 | |
| ≥2.053 | 13 | 6 | |||
| HHL | <4.00 | 41 | 8 | 0.025 | 0.001, 1.69 (1.25–2.28) |
| ≥4.00 | 1 | 3 | |||
| ACG | <0.951 | 35 | 7 | 0.154 | |
| ≥0.951 | 7 | 4 | |||
| BPL | <1.522 | 41 | 9 | 0.106 | |
| ≥1.522 | 1 | 2 | |||
| TJA-II | <0.974 | 36 | 7 | 0.112 | |
| ≥0.974 | 6 | 4 | |||
| ABA | <2.180 | 36 | 4 | 0.002 | 0.011, 3.25 (1.31–8.05) |
| ≥2.180 | 6 | 7 | |||
| PWM | <1.846 | 31 | 5 | 0.079 | |
| ≥1.846 | 11 | 6 | |||
| WFA | <0.802 | 32 | 8 | 0.545 | |
| ≥0.802 | 10 | 3 | |||
| MPA | <2.456 | 41 | 9 | 0.106 | |
| ≥2.456 | 1 | 2 | |||
| VVA | <2.592 | 41 | 10 | 0.375 | |
| ≥2.592 | 1 | 1 | |||
| DBA | <0.537 | 22 | 4 | 0.344 | |
| ≥0.537 | 20 | 7 | |||
| SBA | <0.997 | 22 | 4 | 0.344 | |
| ≥0.997 | 20 | 7 | |||
| Calsepa | <1.541 | 31 | 5 | 0.079 | |
| ≥1.541 | 11 | 6 |
Multivariate analyses showed that increased HHL- and ABA-binding signals (T/N ratios) were independent predictive factors for worse recurrence-free survival after primary colorectal cancer resection. For HHL: relative risk (RR) = 1.69; 95% confidence interval (CI): 1.25–2.28; P = 0.001. For ABA: RR = 3.25; 95% CI: 1.31–8.05; P = 0.011 (Table2). Figure2 shows the Kaplan–Meier recurrence-free survival curves with log-rank comparisons based on (A) ABA- and (B) HHL-binding signals. The 5-year recurrence-free survival were 82.0% and 24.7% for patients with ABA of <2.18 and those with ABA of ≥2.18, respectively (P = 0.001; Fig2A). The 5-year recurrence-free survival were 88.8% and 40.9% for patients with HHL of <4.00 and those with HHL of ≥4.00, respectively (P = 0.001; Fig2B).
Figure 2.
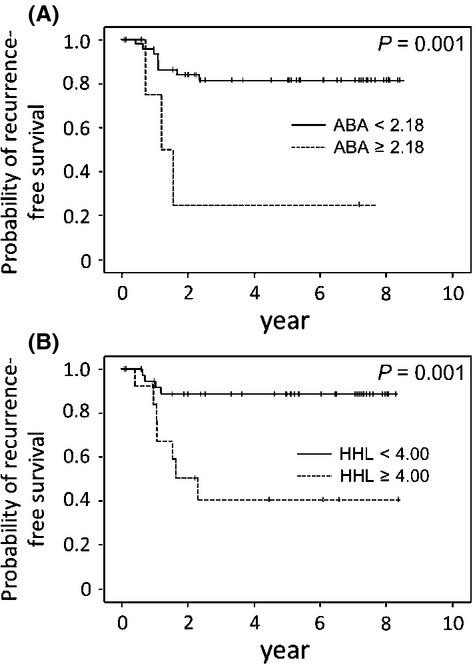
Recurrence-free survival analysis of stage I–III colorectal cancer patients (n = 53) with Agaricus bisporus (ABA) and Hippeastrum hybrid (HHL) status. (A) Five-year recurrence-free survival were 82.0% and 24.7% for the patients with ABA <2.18, and ABA ≥2.18 (P = 0.001). (B) Five-year recurrence-free survival were 88.8% and 40.9% for the patients with HHL <4.00, and HHL ≥4.00 (P = 0.001).
As shown in Table1, PHA(L) showed significantly increased LGI values for cancer tissue compared with those in normal epithelia. However, Kaplan–Meier recurrence-free survival curves (Fig. S1) indicated only a marginally significant difference, 84.3% with PHA(L) of <0.839 and 52.9% with PHA(L) of ≥0.893 (P = 0.021). Thus, we selected ABA and HHL LGI values for further investigation.
In our validation set, univariate analysis showed that distant recurrence was significantly associated only with ABA-binding signals (T/N ratios) (P = 0.029; Table3). Figure3 shows the Kaplan–Meier recurrence-free survival curves with log-rank comparisons based on ABA-binding signals. ABA staining was identified as an independent predictive factor for worse recurrence-free survival after primary colorectal resection.
Table 3.
Correlation between distant recurrence and clinicopathological factors, including lectin signals, in patients who underwent curative resection for stage II colorectal cancer in the validation setting
| Factors | Category | Recurrence (−) (n = 43) | Recurrence(+) (n = 12) | Univariate analysis P-value |
|---|---|---|---|---|
| Age, mean ± SD (range) | 70.3 ± 10.0 (49–93) | 74.0 ± 10.4 (53–89) | 0.266 | |
| Sex | Male | 23 | 9 | 0.182 |
| Female | 20 | 3 | ||
| CEA (ng/mL) | <5 | 35 | 7 | 0.129 |
| ≥5 | 8 | 5 | ||
| CA19-9 (U/mL) | <37 | 40 | 12 | 1.000 |
| ≥37 | 3 | 0 | ||
| Ileus | No | 39 | 10 | 0.602 |
| Yes | 4 | 2 | ||
| Adjuvant chemotherapy | No | 43 | 11 | 0.218 |
| Yes | 0 | 1 | ||
| Site | Colon | 42 | 12 | 1.000 |
| Rectum | 1 | 0 | ||
| Tumor invasion | pT3 | 42 | 12 | 1.000 |
| pT4 | 1 | 0 | ||
| Lymphatic invasion | ly− | 25 | 5 | 0.311 |
| ly+ | 18 | 7 | ||
| Venous invasion | v− | 36 | 8 | 0.230 |
| v+ | 7 | 4 | ||
| Histology | tub1/tub2 | 40 | 11 | 1.000 |
| por/muc | 3 | 1 | ||
| HHL | <4.00 | 28 | 6 | 0.503 |
| ≥4.00 | 15 | 6 | ||
| ABA | <2.180 | 42 | 9 | 0.029 |
| ≥2.180 | 1 | 3 |
Figure 3.
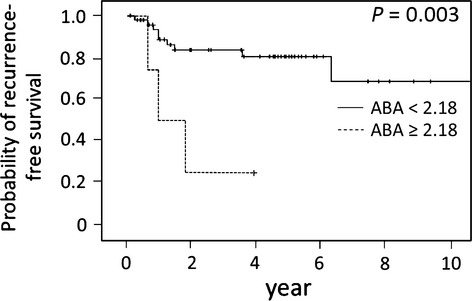
Recurrence-free survival analysis of stage II colorectal cancer patients (n = 55) with Agaricus bisporus (ABA) status in the validation setting. Recurrence-free survival according to ABA status (P = 0.003).
Lectin staining
We attempted to confirm ABA histological binding using histochemical analysis using biotin-labeled ABA for randomly selected samples (15 cancerous and 15 normal epithelia). There was a high frequency of strong ABA staining in the cytoplasm and moderate staining in apical surfaces of cancerous tissues (Fig.4A), whereas ABA staining was minimally detected in the supranuclear regions of glandular epithelium in normal mucosa (Fig.4B). For all of these cases, staining was inhibited or greatly diminished after absorption with Galβ1–3GalNAcα-Thr or N-acetyl-d-glucosamine.
Figure 4.
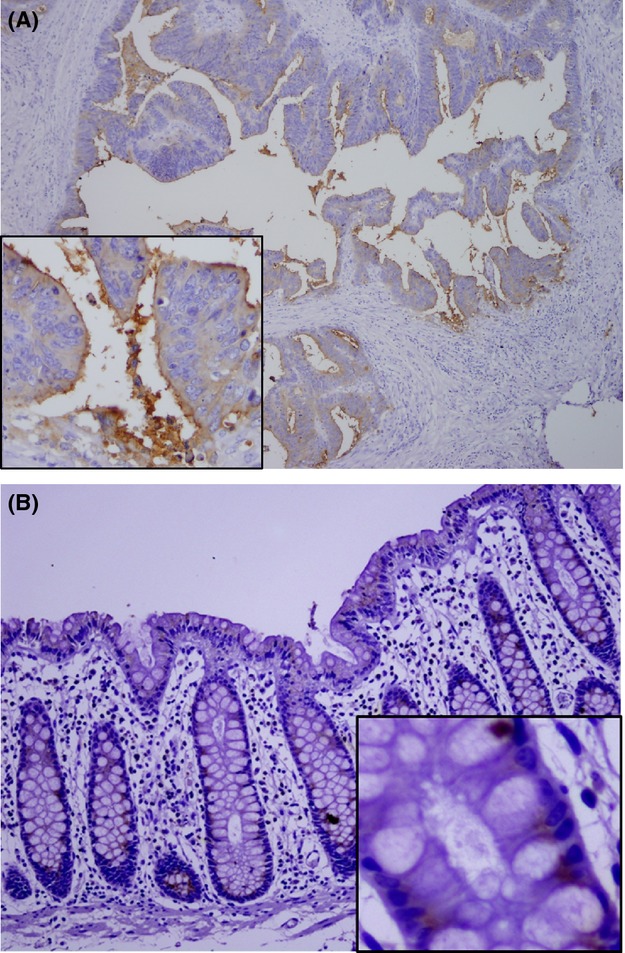
Agaricus bisporus (ABA) staining for colorectal cancer tissues. (A) Strong ABA staining was detected within the cytoplasm of cancer cells and the apical surface (×100, ×400). (B) ABA staining was detected in the supranuclear region of the glandular epithelium of normal colorectal tissues (×100, ×400).
Discussion
In this study, we evaluated multiple glycan expression profiles using a lectin microarray system for the tissues of curatively treated subjects, to identify predictive markers for distant colorectal cancer recurrence. The clinicopathological factors and 45 lectins in two independent cohorts showed that an increased ABA-binding signal could be a predictive factor for distant colorectal cancer recurrence (Figs.2A and 3). This is the first report to show that LGI values obtained from a lectin microarray could be used to identify predictive biomarkers for distant colorectal cancer recurrence, and that increased ABA-binding signals were associated with distant recurrence in these patients.
Although multiple methods, such as enzyme-linked immunosorbent assay (ELISA), liquid chromatography, and mass spectrometry, have been used to analyze glycans on the surfaces of cancerous cells, the disadvantages of these methods, including their relatively low sensitivity, low throughput, labor-intensive sample preparation, and their requirements of specialized knowledge and skills, has hindered their widespread use for diagnosis 23. In comparison, the lectin microarray system used in the present study provides comprehensive, highly reproducible glycan expression profiles and requires only basic biochemical techniques. The 45 independent LGI values could be obtained from formalin-fixed clinical specimens 15. Although this method requires meticulous sample preparation, simultaneous, quantitative profiling of N- and O-linked glycans (LGI values) can easily be achieved with this system.
Histochemical analysis has shown that there is regionally variable glycan expression in the colon 24. However, it is not known whether the comorbidity that occurs in obstructive colitis with ileus or diabetes mellitus patients can affect the glycan structures of the colorectal epithelium 25. To confirm the results of lectin microarray analysis, which indicated increased ABA LGI values in the cancerous tissues of colorectal cancer subjects, we used ABA lectin staining and detected strong, concomitant signals both on the surfaces and in the cytoplasm of cancerous tissues (Fig.4A). In contrast, ABA staining was weak and limited to lymphocytes and on the surfaces or glandular parts in normal epithelium (Fig.4B). Because we noted that each LGI value varied among normal specimens, we decided to employ a tumor/normal ratio for each clinical specimen to evaluate these values.
Because the available amount of a particular glycoprotein fraction was limited, we adjusted each fraction's concentration and each LGI value was normalized by setting the average of the 45 independent LGI values to 100%. In addition, we used β-actin staining to establish that each sample contained proteins (Fig.1).
In this study, ABA was identified as a predictive factor for distant recurrence and was shown to be a prognostic factor. ABA reportedly has two distinct binding sites: Galβ1–3GalNAcα (TF antigen) and N-acetylgalactosamine 26. The TF antigen appears on tumors, such as those in lung cancer 27, bladder carcinoma 28, and colon cancer 29. Langkilde et al. reported that high-level TF antigen expression was associated with poor prognoses for patients with bladder carcinoma 28. Hanish et al. reported that TF antigen expression was associated with tumor progression and metastasis in cells or tissues 30. Cao et al. found that TF antigen expression on primary colon carcinoma was associated with an enhanced risk of liver metastases 31. The results in these previous reports are consistent with our results.
Shigeoka et al. found that treating colon 26 cells with neuraminidase resulted in TF antigen exposure and consequently a higher frequency of liver metastases in an animal model. In addition, they showed that metastasis to the liver could be prevented by an antibody directed against the TF antigen 32. An antibody against the TF antigen may have a possible therapeutic effect for prophylaxis against metastasis to the liver.
The American Society of Clinical Oncology stated that patients with stage II disease could be considered for adjuvant therapy, including those with inadequately sampled nodes, T4 lesions, perforation, or poorly differentiated tumors 3. The European Society for Medical Oncology guidelines recommended that patients with stage II disease should be considered for adjuvant therapy in cases that included <12 lymph nodes, vascular invasion, lymphatic invasion, perineural invasion, poorly differentiated tumors, obstruction, perforation, or a pT4 tumor 33. In addition to these clinicopathological factors, previous studies have investigated the potential of various molecular and genetic biomarkers, including MSI 34, LOH18q 35, p53 36, KRAS 37, and BRAF 38, for better patient selection and classification for adjuvant therapy. However, no appropriate biomarker has yet been identified to decide the adjuvant therapy for stage II colorectal cancer patients.
In this study, using a lectin microarray, we showed that ABA intensity measurements could be an effective predictive biomarker for distant colorectal cancer recurrence. This system has significant advantages, including high-throughput performance with simple pretreatments, ultrasensitivity, and high reproducibility. However, various factors that could affect the glycan structure of the normal epithelium, such as tumor location, presence or absence of obstructive colitis, and diabetes mellitus, need to be clarified. In addition, lectin profiling analysis of different types of lesions is required because cancer tissue heterogeneity may affect its lectin-binding profile.
The major limitation of the current study was the relatively small number of events available for analysis, and other candidates may have been overlooked because of a lack of statistical significance. Nevertheless, those factors that were shown to be significant in a validation set are worth considering in further research. Experiments using cell lines or animal models should be developed to verify whether or not this altered ABA binding could actually induce metastases.
In conclusion, our results indicated that the tumor/normal ratio for ABA binding could be a novel predictive biomarker for distant recurrence of curatively resected colorectal cancer.
Acknowledgments
We thank Mayumi Takeda, Yuiko Aso, Hiroko Taguchi, and Emi Ikebe for technical assistance. We are very grateful to Jun Hirabayashi for helping us with the experiments.
Conflict of Interest
None declared.
Supporting Information
Figure S1. Recurrence- free survival curve of stage I–III colorectal cancer patients (n=53) with PHA(L) status.
References
- The International Agency for Research on Cancer (IARC), the World Health Organization. 2012. Available at http://globocan.iarc.fr/factsheet.asp (accessed 9 August 2014)
- Schippinger W, Samonigg H, Schaberl-Moser R, Greil R, Thodtmann R, Tschmelitsch J, et al. A prospective randomised phase III trial of adjuvant chemotherapy with 5-fluorouracil and leucovorin in patients with stage II colon cancer. Br. J. Cancer. 2007;97:1021–1027. doi: 10.1038/sj.bjc.6604011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson AB, III, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J. Clin. Oncol. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- Gray R, Barnwell J, McConkey C, Hills RK, Williams NS. Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- Andre T, Sargent D, Tabernero J, O'Connell M, Buyse M, Sobrero A, et al. Current issues in adjuvant treatment of stage II colon cancer. Ann. Surg. Oncol. 2006;13:887–898. doi: 10.1245/ASO.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Inomata M, Ochiai A, Sugihara K, Moriya Y, Yamaguchi N, Adachi Y, et al. Macroscopic features at the deepest site of tumor penetration predicting liver metastases of colorectal cancer. Jpn. J. Clin. Oncol. 1998;28:123–128. doi: 10.1093/jjco/28.2.123. [DOI] [PubMed] [Google Scholar]
- Deschoolmeester V, Baay M, Specenier P, Lardon F. Vermorken JB. A review of the most promising biomarkers in colorectal cancer: one step closer to targeted therapy. Oncologist. 2010;15:699–731. doi: 10.1634/theoncologist.2010-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z, Cohen AM. Urmacher C. Usefulness of carcinoembryonic antigen monitoring despite normal preoperative values in node-positive colon cancer patients. Dis. Colon Rectum. 1993;36:1063–1068. doi: 10.1007/BF02047301. [DOI] [PubMed] [Google Scholar]
- Park IJ, Choi GS, Lim KH, Kang BM. Jun SH. Serum carcinoembryonic antigen monitoring after curative resection for colorectal cancer: clinical significance of the preoperative level. Ann. Surg. Oncol. 2009;16:3087–3093. doi: 10.1245/s10434-009-0625-z. [DOI] [PubMed] [Google Scholar]
- Bhattacharjya S, Aggarwal R. Davidson BR. Intensive follow-up after liver resection for colorectal liver metastases: results of combined serial tumour marker estimations and computed tomography of the chest and abdomen – a prospective study. Br. J. Cancer. 2006;95:21–26. doi: 10.1038/sj.bjc.6603219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Nishidate T, Kijima K, Ohashi T, Takegawa K, Fujikane T, et al. Critical roles of mucin 1 glycosylation by transactivated polypeptide N-acetylgalactosaminyltransferase 6 in mammary carcinogenesis. Cancer Res. 2010;70:2759–2769. doi: 10.1158/0008-5472.CAN-09-3911. [DOI] [PubMed] [Google Scholar]
- Lange T, Ullrich S, Muller I, Nentwich MF, Stubke K, Feldhaus S, et al. Human prostate cancer in a clinically relevant xenograft mouse model: identification of beta(1,6)-branched oligosaccharides as a marker of tumor progression. Clin. Cancer Res. 2012;18:1364–1373. doi: 10.1158/1078-0432.CCR-11-2900. [DOI] [PubMed] [Google Scholar]
- Chen G, Wang Y, Qiu L, Qin X, Liu H, Wang X, et al. Human IgG Fc-glycosylation profiling reveals associations with age, sex, female sex hormones and thyroid cancer. J. Proteomics. 2012;75:2824–2834. doi: 10.1016/j.jprot.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Saldova R, Dempsey E, Perez-Garay M, Marino K, Watson JA, Blanco-Fernandez A, et al. 5-AZA-2′-deoxycytidine induced demethylation influences N-glycosylation of secreted glycoproteins in ovarian cancer. Epigenetics. 2011;6:1362–1372. doi: 10.4161/epi.6.11.17977. [DOI] [PubMed] [Google Scholar]
- Dennis JW, Laferte S, Waghorne C, Breitman ML. Kerbel RS. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987;236:582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Kim YS, Tsao D. Whitehead J. Biology of colon cancer: an overview. Cancer. 1977;40:2473–2478. doi: 10.1002/1097-0142(197711)40:5+<2473::aid-cncr2820400913>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Fry SA, Afrough B, Lomax-Browne HJ, Timms JF, Velentzis LS. Leathem AJ. Lectin microarray profiling of metastatic breast cancers. Glycobiology. 2011;21:1060–1070. doi: 10.1093/glycob/cwr045. [DOI] [PubMed] [Google Scholar]
- Matsuda A, Kuno A, Ishida H, Kawamoto T, Shoda J. Hirabayashi J. Development of an all-in-one technology for glycan profiling targeting formalin-embedded tissue sections. Biochem. Biophys. Res. Commun. 2008;370:259–263. doi: 10.1016/j.bbrc.2008.03.090. [DOI] [PubMed] [Google Scholar]
- Matsuda A, Kuno A, Kawamoto T, Matsuzaki H, Irimura T, Ikehara Y, et al. Wisteria floribunda agglutinin-positive mucin 1 is a sensitive biliary marker for human cholangiocarcinoma. Hepatology. 2010;52:174–182. doi: 10.1002/hep.23654. [DOI] [PubMed] [Google Scholar]
- Nishijima Y, Toyoda M, Yamazaki-Inoue M, Sugiyama T, Miyazawa M, Muramatsu T, et al. Glycan profiling of endometrial cancers using lectin microarray. Genes Cells. 2012;17:826–836. doi: 10.1111/gtc.12003. [DOI] [PubMed] [Google Scholar]
- Iha H. Yamada M. Chapter 5 in ‘T-cell leukemia - characteristics, treatment and prevention’. 2013. Glycan profiling of adult T-cell leukemia (ATL) cells with the high resolution lectin microarrays; pp. 89–103. , and. In Tech, doi: 10.5772/45997. [Google Scholar]
- Vanderschaeghe D, Festjens N, Delanghe J. Callewaert N. Glycome profiling using modern glycomics technology: technical aspects and applications. Biol. Chem. 2010;391:149–161. doi: 10.1515/bc.2010.031. [DOI] [PubMed] [Google Scholar]
- McMahon RF, Panesar MJ. Stoddart RW. Glycoconjugates of the normal human colorectum: a lectin histochemical study. Histochem. J. 1994;26:504–518. doi: 10.1007/BF00157896. [DOI] [PubMed] [Google Scholar]
- Ohtsubo K, Chen MZ, Olefsky JM. Marth JD. Pathway to diabetes through attenuation of pancreatic beta cell glycosylation and glucose transport. Nat. Med. 2011;17:1067–1075. doi: 10.1038/nm.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrizo ME, Capaldi S, Perduca M, Irazoqui FJ, Nores GA. Monaco HL. The antineoplastic lectin of the common edible mushroom (Agaricus bisporus) has two binding sites, each specific for a different configuration at a single epimeric hydroxyl. J. Biol. Chem. 2005;280:10614–10623. doi: 10.1074/jbc.M411989200. [DOI] [PubMed] [Google Scholar]
- Stein R, Chen S, Grossman W. Goldenberg DM. Human lung carcinoma monoclonal antibody specific for the Thomsen-Friedenreich antigen. Cancer Res. 1989;49:32–37. [PubMed] [Google Scholar]
- Langkilde NC, Wolf H, Clausen H, Kjeldsen T. Orntoft TF. Nuclear volume and expression of T-antigen, sialosyl-Tn-antigen, and Tn-antigen in carcinoma of the human bladder. Relation to tumor recurrence and progression. Cancer. 1992;69:219–227. doi: 10.1002/1097-0142(19920101)69:1<219::aid-cncr2820690136>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Itzkowitz SH, Yuan M, Montgomery CK, Kjeldsen T, Takahashi HK, Bigbee WL, et al. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 1989;49:197–204. [PubMed] [Google Scholar]
- Hanisch FG. Baldus SE. The Thomsen-Friedenreich (TF) antigen: a critical review on the structural, biosynthetic and histochemical aspects of a pancarcinoma-associated antigen. Histol. Histopathol. 1997;12:263–281. [PubMed] [Google Scholar]
- Cao Y, Karsten UR, Liebrich W, Haensch W, Springer GF. Schlag PM. Expression of Thomsen-Friedenreich-related antigens in primary and metastatic colorectal carcinomas. A reevaluation. Cancer. 1995;76:1700–1708. doi: 10.1002/1097-0142(19951115)76:10<1700::aid-cncr2820761005>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Shigeoka H, Karsten U, Okuno K. Yasutomi M. Inhibition of liver metastases from neuraminidase-treated colon 26 cells by an anti-Thomsen-Friedenreich-specific monoclonal antibody. Tumour Biol. 1999;20:139–146. doi: 10.1159/000030056. [DOI] [PubMed] [Google Scholar]
- Labianca R, Nordlinger B, Beretta GD, Brouquet A. Cervantes A. Primary colon cancer: ESMO Clinical Practice Guidelines for diagnosis, adjuvant treatment and follow-up. Ann. Oncol. 2010;21(Suppl. 5):v70–v77. doi: 10.1093/annonc/mdq168. [DOI] [PubMed] [Google Scholar]
- Popat S, Hubner R. Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J. Clin. Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Wu TT, Catalano PJ, Ueki T, Satriano R, Haller DG, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N. Engl. J. Med. 2001;344:1196–1206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro AJ, Lain S. Lane DP. P53 abnormalities and outcomes in colorectal cancer: a systematic review. Br. J. Cancer. 2005;92:434–444. doi: 10.1038/sj.bjc.6602358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino S, Meyerhardt JA, Irahara N, Niedzwiecki D, Hollis D, Saltz LB, et al. KRAS mutation in stage III colon cancer and clinical outcome following intergroup trial CALGB 89803. Clin. Cancer Res. 2009;15:7322–7329. doi: 10.1158/1078-0432.CCR-09-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tol J, Nagtegaal ID. Punt CJ. BRAF mutation in metastatic colorectal cancer. N. Engl. J. Med. 2009;361:98–99. doi: 10.1056/NEJMc0904160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Recurrence- free survival curve of stage I–III colorectal cancer patients (n=53) with PHA(L) status.


