Abstract
Interacting parenting thoughts and behaviors, supported by key brain circuits, critically shape human infants’ current and future behavior. Indeed, the parent–infant relationship provides infants with their first social environment, forming templates for what they can expect from others, how to interact with them and ultimately how they go on to themselves to be parents. This review concentrates on magnetic resonance imaging experiments of the human parent brain, which link brain physiology with parental thoughts and behaviors. After reviewing brain imaging techniques, certain social cognitive and affective concepts are reviewed, including empathy and trust—likely critical to parenting. Following that is a thorough study-by-study review of the state-of-the-art with respect to human neuroimaging studies of the parental brain—from parent brain responses to salient infant stimuli, including emotionally charged baby cries and brief visual stimuli to the latest structural brain studies. Taken together, this research suggests that networks of highly conserved hypothalamic–midbrain–limbic–paralimbic–cortical circuits act in concert to support parental brain responses to infants, including circuits for limbic emotion response and regulation. Thus, a model is presented in which infant stimuli activate sensory analysis brain regions, affect corticolimbic limbic circuits that regulate emotional response, motivation and reward related to their infant, ultimately organizing parenting impulses, thoughts and emotions into coordinated behaviors as a map for future studies. Finally, future directions towards integrated understanding of the brain basis of human parenting are outlined with profound implications for understanding and contributing to long term parent and infant mental health.
Keywords: Attachment, Brain imaging, fMRI, Parent–child relationships, Parenting and caregiving
1. Brain imaging of human parent–infant relationships
Functional magnetic resonance imaging (fMRI) is a non-invasive technique that may be used to acquire data on the brain basis of human parental behavior and thoughts. Structural as well as functional data may be gathered by measuring the physical and blood–oxygen-dependant signals in response to infant auditory and visual stimuli. Thus, brain activity may be indirectly measured as changes in regional blood oxygenation. The differences between a region’s oxygenated and deoxygenated hemoglobin, between states of action vs. inaction for instance, provide characteristic magnetic signals localized to millimeters that are detected by scanners positioned around each subject’s head. An important caveat throughout the interpretation of parenting fMRI studies, however, is that that brain activity measurements represent an integration of electrical brain activity that may be nearly instantaneous yet the associated blood flow change lags over seconds. Furthermore, experimental design captures brain activity over periods of a few seconds or 10s of seconds. So, on the one hand, short blocks or events may capture briefly held mental states, but miss highly transient or more sustained emotion; while on the other hand longer blocks may capture more complex and delayed brain responses, but also average them out making subtle aspects more difficult to detect. Brain activity during these periods may then be measured and compared between periods of attending to stimuli of interest and control stimuli to generate maps of the brain indicating differences in brain activity that are important for one set of perceptions and thoughts versus another. Building from these techniques, the focus question of this review, which at this point of limited studies remains broad, is whether the complex thoughts and behaviors of parents may be mapped with brain imaging techniques in such as way to confirm and extend findings from related fields and help form and test hypotheses about the normal parenting as well as parenting at risk and affected by adversity such as psychopathology.
A gradually increasing, but still modest number of studies have been published in which parents have been the subjects and infant cries and pictures have been stimuli. In such studies, for example, comparisons of brain activity measured during baby cry vs. control sound, or own vs. other baby stimulus yield significant differences in the functional signal emanating from certain brain regions that may then be said to relate to the parental experience of a the baby signal. Such findings are particularly compelling when the magnitude of the difference is correlated with a measure of associated parenting thoughts and behaviors. Some of the earliest studies had limited subject numbers and fixed effects analyses that do not take inter-subject variability into account and require replication for generalization, while most studies now employ random effects analyses and even correlate brain responses in specific regions with parental thoughts and behaviors. The choice of stimuli used for brain activity comparisons in these experiments are of critical importance in their interpretation, so I have grouped them according to the two modalities currently used: audio only baby cry, and visuals with no audio. However, before describing parental brain studies, in the later part is a brief introductory selective review of recent literature on social thoughts and affect that make the interpretation of parental brain responses seem possible in humans.
2. Connecting the psychology and functional neuroanatomy of parenting
Brain imaging combined with behavioral paradigms over the last 10 years have made possible an increasingly sophisticated neurobiological understanding of affect and its regulation as well as empathy, and sociocultural cognitions such as inclusion/exclusion, trust and even attachment. In this section, I selectively review key studies in these fields.
Empathy, defined as appropriate perception, experience and response to another’s emotion, is especially relevant to parenting in which infants’ needs are great, yet most communication is exclusively nonverbal. The growing field of cognitive neuroscience, propelled by modern brain imaging techniques and interest in deficits of empathy in such disorders as autism, has revealed networks of brain activity relating to empathy and emotional mirroring (Gallese et al., 2004; Iacoboni, 2009; Uddin et al., 2007). These empathy systems seem to overlap significantly with brain responses of parents to infant stimuli reviewed in this paper. Two of these overlapping regions are the cingulate and insular cortices.
In one fascinating study, focusing on the neuroanatomy of empathy using fMRI techniques, Singer and colleagues measured brain activity while volunteers experienced a painful stimulus or observed a signal indicating that their loved one (“other”), present in the same room, had received a similar pain stimulus (Singer et al., 2004). They found a separation of circuits responding to the sensory-discriminative components of pain from the autonomic–affective aspects. Specifically, both experiencing pain and experiencing the pain of a loved one activated the insula and anterior cingulate with the latter activating relatively more anterior regions of these structures. The experience of one’s own pain oneself also activated the brainstem, cerebellum, and sensorimotor cortex. Such decoupled yet parallel representations of empathy in cortical structures such as the insula and anterior cingulate have been postulated to be necessary for our empathic abilities to mentalize, that is, to understand the thoughts, beliefs, and intentions of others in relation to ourselves (Frith and Frith, 2003; Hein and Singer, 2008). It may well be that humans use separate circuits to decouple representations of the external vs. internal information to understand physical properties and assess personal emotional values. This framework may be of great importance to those studying the brain substrates of relationships, including the interface of external experiences with the internal representations that pervade our mood and support behavioral planning.
In another wonderful study of the cingulate in mediating the brain basis of social behavior, Eisenberger and colleagues utilized virtual reality to simulate feelings of social isolation. In this study (Eisenberger et al., 2003), the subject is involved in a virtual game of “Cyberball”, which includes three players. Suddenly, the subject player is excluded from the virtual game and there is a rapid change in the anterior cingulate cortex activity. Perhaps the cingulate mediates a social loss/separation/attachment/grief system, which may be so important to parenting as well as critical developments in each individual. More recent studies imaging are showing that the distress of peer rejection involves linked activity in the anterior cingulate with insula, frontal cortical regions and striatum that varies over our childhood and adolescence (Crowley et al., 2010; Masten et al., 2009). This suggests developmental windows for considering the importance of mental health risks as well as resilience factors, such as parenting and interventions that may also be studied with neuroimaging. Thus, in addition to registering physical pain, anterior cingulate may also be an important circuit in thinking about a range of emotional signals (including social pain such as in witnessing the pain of a loved one, social rejection, or stimuli of one’s child or romantic love), involving coordinated shifts in attention, decision-making, memory, mood regulation, and directed behavior.
The insula has also been raised as an important center for integrating across emotional and cognitive information (Carr et al., 2003; Critchley, 2009; Perlman and Pelphrey, 2010) with connections to mirror areas in the posterior parietal, inferior frontal, and superior temporal cortices also of interest in addition to limbic regions so well studied in animal models, but not nearly the whole story for humans. In one study subjects were shown pictures of standard emotional faces (happy, sad, angry, surprised, disgusted, and afraid) and fMRI was used to measure responses to two behavioral tasks: (i) mere observation and (ii) observation as well as internal simulation of the emotion observed.
As expected, imitation produced greater activity in frontotemporal areas in the mirror network, including the premotor face area, the dorsal pars opercularis of the inferior frontal cortex, and the superior temporal sulcus. Imitation also produced greater activity in the right anterior insula and right amygdala. This is particularly intriguing in light of evidence that the anterior insula responds to pleasant “caress-like” touch (Olausson et al., 2002) and that the insula plays a crucial role in emotional and interpersonal interaction in health and mental illness such as autism (Dapretto et al., 2006). A further confirmation of the insula’s role in emotion recognition comes from the study of patients with strokes. Stroke patients with insular lesions showed a significantly greater deficit in emotion recognition than other stroke patients (Bodini et al., 2004). The cingulate and insula will likely continue to emerge as key areas of importance during the social recognition of others important to the personal transformations that are typical in the initial formation of a new family as well as other relationships. Further research on the brain basis of thinking about other minds (mentalization) and empathy is also beginning to dissect the brain basis of complex social emotional thinking (Pelphrey et al., 2005; Saxe, 2006b). This research suggests that specific regions in the medial prefrontal cortex and temporal cortex mediate empathic thoughts and collaborative behaviors. Perhaps studies of high risk families will show aberrations in these patterns of activation. Since early intervention programs such as nurse home visitation and circle-of-security interventions have been shown to have beneficial effects on parenting and child development (Cassidy et al., 2010; Olds et al., 2004; Olds et al., 2007), perhaps evidence of brain “corrections” in the activation patterns seen in these limbic cortical regions in response to infant stimuli may be found in future research.
In another innovative approach to explicitly study the biological bases of adult attachment, line drawings were presented to subjects during brain imaging which were meant to activate the attachment system with depictions of illness, solitude, separation and abuse (Buchheim et al., 2006). Subjects with organized compared to disorganized attachment patterns showed increased activity in the right amygdala, left hippocampus and right inferior frontal gyrus—areas considered to be important to social bonding and attachment. In a follow-up study (Buchheim et al., 2008), a similar paradigm showed that patients with borderline personality, a disorder of emotional instability connected with early-life trauma, showed significantly more anterior midcingulate cortex activation compared with controls in response to separation stimuli. In response to dyadic (pro-attachment) stimuli, patients showed more activation of the right superior temporal sulcus and less activation of the right parahippocampal gyrus compared to controls. These results suggest neural substrates of attachment trauma underlying interpersonal reactions.
With the previously discussed selective review, the following sections review experiments specifically designed to understand the brain basis of parental attachment by presenting emotionally charged infant stimuli during brain imaging. Such studies are beginning to incorporate psychological, behavioral and even hormone level measures pertaining to parenting. We may expect that “parental” brain circuits would share much in common with those that regulate other social cognitions, emotions and behaviors, including emotion mediation and drive regions of the basal ganglia (insula, striatum, hypothalamus and amygdala) as well as executive and regulatory regions (anterior cingulate, medial frontal and orbitofrontal cortices). Understanding how these regions respond to infants in parents promises earlier problem detection and refinement of treatments tailored to individual parent–infant dyads.
The experiments to date on human parents using baby sound and visual stimuli with brain fMRI are summarized in Tables 1 and 2, respectively. These inclusive reference tables are intended to suggest patterns of response across all studies and stimuli at a glance and to stimulate future studies. Parent brain areas of increased activity with baby stimuli are indicated in these tables with “ACT”, while areas of decreased activity are indicated by “DEACT”. It is important to keep in mind that human brains are awash with blood at all times and it is just possible to measure differences between different periods—not yet absolute or nuanced high temporal resolution mental states. Also indicated are the number of subjects, age of infants at time of scan, type of study (magnet strength and block or event design), and stimuli used in each study. Statistical methods vary across studies, but all findings reported here satisfy the criteria of fixed effects at p<0.001, or random effects at p<0.05. Each of these studies along with closely related research is detailed in the following sections. Despite the advances, these experiments also reflect limitations that are being addressed in newer generations of studies now underway. These limitations include the only partial realism of baby stimuli, limited response possibilities of subjects as they are being imaged as well as the inherent methodological problems in which brain activity can only be compared across time points if the same stimuli are used—while the actual baby and nature of the relationship is a complex set of “moving targets”, developing in the parent–infant dyad.
Table 1.
Human parent brain responses to own and other infant cries. Anatomical brain regions with increased activity (ACT), and areas of decreased activity (DEACT) are indicated. Empty boxes indicate no significant changes in brain activity with exposure to baby sounds.
| Authors Year: |
Lorberbaum et al. (1999) |
Lorberbaum et al. (2002) |
Seifritz et al. (2003) |
Swain et al. (2003, 2004a, 2006) |
Swain et al. (2003, 2004a) |
Swain et al. (2008, submitted for publication) |
Kim et al. (2010a) | Kim et al. (2010c) | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
||
| Number of subjects: | n=4 | n=10 | n=20 | n=7–14 | n=7–8 | n=12 | n=26 | n=20 | ||
|
|
|
|
|
|
|
|
|
|
||
| Age of infants at time of scan: | 3 weeks– 3.5 years |
1–2 months | <3 years | Time 1: 2–4 weeks
|
Time 1: 2– 4 weeks
|
2–4 weeks | 2–4 weeks | 2–4 weeks | ||
| Time 2: 3–4 months | Time 2: 3– 4 months | |||||||||
|
|
|
|
|
|
|
|
|
|
||
| Parental groups: | Mothers only | Mothers only | Mothers+fathers+ non-parents |
Novice+multiparous mothers |
Novice+multiparous fathers |
Novice mothers (vaginal>c/s) |
Novice+multiparous mothers (PBI: high>low) |
Novice+multiparous mothers (breast>formula feeding) |
||
|
|
|
|
|
|
|
|
|
|
||
| Type of study: | 1.5 T, 30 s blocks, fixed effects |
1.5 T, 30 s blocks, random effects |
1.5 T, 6 s events, random effects |
3 T, 30 s blocks, fixed effects |
3 T, 30 s blocks, fixed effects |
3 T, 30 s blocks, random effects |
3 T, 30 s blocks, random effects |
3 T, 30 s blocks, random effects | ||
|
|
|
|
|
|
|
|
|
|
||
| Baby cry used: | Other cry> white noise |
Other cry> control noise |
Other cry+laugh | Own cry> control |
Other cry> control |
Own cry> control |
Other cry> control |
Own baby cry> other baby cry |
Cry>control sound | Own>Other Cry; Breast> Formula |
| Septal regions (MPOA/VBNST/caudate head) | ACT | ACT | ACT | ACT (vaginal>c/s) | ||||||
| Hypothalamus | ACT | ACT | ACT | |||||||
| Thalamus | ACT | ACT | ACT | ACT (vaginal>c/s) | ACT (Breast>formula) | |||||
| Striatum/Putamen/Nucleus accumbens | ACT | ACT | ACT | ACT | ACT (vaginal>c/s) | ACT (Breast>formula) | ||||
| Anterior cingulate | ACT | ACT | DEACT | ACT | ACT | ACT | ACT | |||
| Middle cingulate | ACT | ACT | ACT (Breast>formula) | |||||||
| Posterior cingulate | ACT | ACT | ||||||||
| Amygdala | ACT (cry–rest) | ACT | ACT | ACT (vaginal>c/s) | ||||||
| Lentiform nucleus globus pallidus | ACT | ACT | ACT | |||||||
| Hippocampus | ACT | ACT | ||||||||
| Midbrain | ACT | ACT | ACT | ACT | ||||||
| Insula | ACT | ACT | ACT | ACT | ACT | ACT (Breast>formula) | ||||
| Orbitofrontal/inferior frontal gyri | ACT | ACT | ACT | ACT | ACT | ACT | ||||
| Medial frontal gyrus | ACT | ACT | DEACT | DEACT | ACT | ACT (vaginal>c/s) | ACT+high>low PMC | |||
| Ventral prefrontal cortex | ACT | ACT | ACT | ACT+DLPFChigh> low PMC | ||||||
| Temporoparietal cortex | ACT | ACT | ACT | ACT | ACT | ACT | ACT (vaginal>c/s) | |||
| Parahippocamal/limbic lobe | ACT | ACT | ||||||||
| Occipital cortex | Not examined | Not examined | ACT | ACT | ACT | |||||
| Fusiform gyrus | ACT | ACT | ACT | ACT | ACT+high>low PMC | ACT (Breast>formula) | ||||
| Superior temporal/auditory cortex | ACT | ACT | ACT | ACT (vaginal>c/s) | ACT+high>low PMC | ACT (Breast>formula) | ||||
| Cerebellum | Not examined | Not examined | ACT | ACT | ACT | |||||
Table 2.
Human parent brain responses to infant pictures. Anatomical brain regions with increased activity (ACT) during infant cry, and areas of decreased activity (DEACT) are indicated. Empty boxes indicate no significant changes in brain activity with exposure to baby pictures.
| Authors
|
Bartels and Zeki (2004a, 2004b) |
Nitschke et al. (2004) |
Ranote et al. (2004) |
Strathearn et al. (2005, 2008, 2009) |
Swain et al. (2003, 2004a, b, 2005) |
Swain et al. (2003, 2004a, b, 2005) |
Noriuchi et al. (2008) |
Lenzi et al. (2008) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | ||||||||||
|
|
|
|
|
|
|
|
|
|
||
| Number of subjects | n=19 | n=6 | n=10 | n=8–28** | n=9–14 | n=4–9 | n=13 | n=16 | ||
|
|
|
|
|
|
|
|
|
|
||
| Age of infants at time of scan |
9 month– 3.5 years |
2–4 months | 4–8 months | 3–18 months | Time 1: 2–4 weeks |
Time 1: 2–4 weeks |
16.5 months ± 3.8 |
6–12 months | ||
| Time 2: 3–4 months |
Time 2: 3–4 months |
|||||||||
|
|
|
|
|
|
|
|
|
|
||
| Parental groups | Mothers only | Mothers only | Mothers only | Mothers only | Novice +multiparous mothers |
Novice+ multiparous fathers |
Mothers only | Novice | ||
|
|
|
|
|
|
|
|
|
|
||
| Type of study: (all silent visuals) |
2 T,15 s blocks | 1.5 T, 30 s blocks |
1.5 T,20–40 s videos |
3 T, 6 s events | 3 T, 30 s blocks | 3 T, 30 s blocks | 1.5 T, 32 s video blocks |
3 T,2 s mini-blocks, | ||
|
|
|
|
|
|
|
|
|
|||
| Random effects | Fixed effects | Random effects |
Fixed effects | Fixed effects | Fixed effects | Random effects |
Random effects | |||
|
|
|
|
|
|
|
|
|
|
||
| Baby visual | Own>other | Own>other | Own>other | Own>other | Own>other/ baby>house |
Own>other/ baby>house |
Own>other | Own, other—joy, distress, ambiguous, neutral |
||
| Septal regions (MPOA/VBNST/ caudate head) | ACT | ACT (joy-other) | ||||||||
| Hypothalamus | ACT | ACT | ||||||||
| Thalamus | ACT | ACT | ACT | ACT | ACT | ACT | ACT | ACT | ACT (joy-other) | |
| Striatum/putamen/ nucleus accumbens | ACT | ACT | ACT | ACT | ACT | |||||
| Anterior cingulate | ACT | ACT | ACT | ACT | ACT | |||||
| Middle cingulate | ACT | ACT | ACT | ACT | ||||||
| Posterior cingulate | DEACT | ACT | ||||||||
| Amygdala | DEACT | ACT | ACT | |||||||
| Lentiform nucleus/ globus pallidus | ACT | ACT | ACT | ACT (joy-other) | ||||||
| Hippocampus | ACT | ACT | ||||||||
| Midbrain | ACT | ACT | ACT | ACT | ACT | ACT | ||||
| Insula | ACT | ACT | ACT (emo-neutral, own-other*) | |||||||
| Orbitofrontal/inferior frontal | ACT | ACT | ACT | ACT | ACT | ACT | ACT | ACT (own-other*) | ||
| Medial frontal gyrus | DEACT | DEACT | ACT | ACT | ACT | |||||
| Ventral prefrontal | ACT | ACT (emo-neutral, own-other*) | ||||||||
| Temporoparietal | DEACT | ACT | ACT | ACT | ACT | ACT | ACT (emo-neutral, own-other*) | |||
| Parahippocamal/ limbic lobe | ACT | ACT (joy-other) | ||||||||
| Occipital cortex | ACT | ACT | ACT | ACT | ACT | ACT | ACT | |||
| Fusiform gyrus | ACT | ACT | ACT | ACT | ACT | ACT | ACT | ACT | ACT (joy-other) | |
| Superior temporal/ auditory cortex | ACT | ACT | ACT (emo-neutral, own-other*) | |||||||
| Cerebellum | ACT | ACT | ACT | ACT | ACT | |||||
3. Parental brains and baby cry stimuli
The first experiments using the pioneering approach of studying brain activity in mothers while they listen to infant cries were conducted by Lorberbaum and colleagues. Building on the thalamocingulate theory of maternal behavior in animals (MacLean, 1990), they initially predicted that baby cries would selectively activate cingulate and thalamus in mothers (ranging from 3 weeks to 3.5 years postpartum) exposed to an audio taped 30 s standard baby cry, not from their own infant (Lorberbaum et al., 1999). They later expanded their hypotheses to include the basal forebrain’s medial pre-optic area and ventral bed nucleus of the stria terminalis and its rich reciprocal connections as being critical to parental behaviors (Lorberbaum et al., 2002). These include the descending connections to modulate more basic reflexive caring behaviors such as nursing, licking, grooming and carrying reflexes in rodent studies, and ascending connections such as the mesolimbic and mesocortical dopamine systems for more general motivation and flexible responses to tend a crying infant or prepare for a threat. In their first study (Lorberbaum et al., 1999), a group of 4 mothers were studied for their response to 30 s of a standard cry compared with 30 s of a control sound consisting of white noise that was shaped to the temporal pattern and amplitude of the cry. With cry versus control sound, the 4 mothers showed increased activity in the subgenual anterior cingulate and right mesial prefrontal/orbitofrontal cortex to infant cries using a fixed effects data analysis. In a methodologically more stringent follow-up study that used a larger sample size and a random effects analysis, brain activity was measured in10 healthy, breastfeeding, first-time mothers with infants 1–2 months old. Subjects listened to standard infant cry recordings (not from their own baby) compared to control sounds varying in the same pattern as the cry (Lorberbaum et al., 2002) Brain regions posterior to the brainstem were not imaged. Activated regions included the anterior and posterior cingulate, thalamus, midbrain, hypothalamus, septal regions, dorsal and ventral striatum, medial prefrontal cortex, right orbitofrontal/insula/temporal polar cortex region, and right lateral temporal cortex and fusiform gyrus. Additionally, when cry response was compared with the inter-stimulus rest periods, instead of the control sound (which some mothers judged to be aversive), the amygdala was active. Activation of the fusiform gyrus is interesting because this structure has been implicated in human face and voice recognition along with related social cognitions that might be impaired in autism (Schultz, 2005).
These initial studies also fit human parent activations to baby cry with the rich literature on the regulation of animal parenting behavior (Numan and Insel, 2003), even though the cry stimuli did not originate from the parent’s own infant and the control sounds were emotionally negative (sounded like harsh static on the television). In addition, human activate to cortical regulatory regions that may be impossible to study in non-human brains. In human studies it will be important to tease apart which responses relate to paying attention to infants as simply another instance of attention in general, and that peculiar to parenting attention per se. For example, it has been shown by event-related brain potential studies that auditory attention requires some of the parent response regions such as anterior cingulate and temporal cortices (Tzourio et al., 1997). However, in another study using similar techniques, women responded significantly more to a baby cry than to an emotionally neutral vocalization in these regions (Purhonen et al., 2001b); in a third study, mothers responded more than control women to infant cries (Purhonen et al., 2001a). These results suggest that increases in the mother’s alertness and arousal toward baby signals may function to assist the mother her ability to be continuously alert or attuned to the infant’s needs. Support for this view might be found in studying parents who are having difficulty sustaining or appropriately modulating their attention and arousal in response to infant cries i.e. those suffering postpartum depression or who mistreat or neglect their children. In one such physiological study of parents who maltreat their children (Frodi and Lamb, 1980), audiovisual infant stimuli elicited exaggerated physiological responses. Indeed, infant crying is a proximate risk factor for infanticide (Soltis, 2004), perhaps due to parents’ failure to regulate their arousal and maintain a caring stance. Future work may shed light on this critical question: What is going on in a healthy parent’s brain and parent–infant relationship (Swain et al., 2004c) compared to a parent at risk for neglect and abuse? One might think that healthy parents would attend to infant cues and respond appropriately with adequate cortical regulation, but not be so aroused as to make impulsive or disinhibited decisions with excess drive centers, or under-aroused to result in neglect.
Hypothesizing that gender and experience would also influence neural responses to baby sounds such as baby cry and laughter, Seifritz et al. (2003) studied four groups: mothers and fathers of children under age 3, and non-parent males and females, with10-subjects in each group. They used a more event-related fMRI design, measuring brain responses to brief 6-s sounds. Over the entire sample, intensity-matched baby sounds of crying and laughing compared to “neutral” sounds (white noise pulsed at 5-Hz with an averaged frequency spectrum similar to the infant vocalizations) produced more brain activity in bilateral temporal regions. These regions might be important for hearing processes (Heshyl’s gyrus and temporal poles), processing human vocalizations, and empathic emotion processing. They also reported that women as a group including, parents and non-parents (but not males), had a decrease in activity in response to both baby cry and laughter in the subgenual anterior cingulate cortex. This finding is, however, contrary to the other studies (Lorberbaum et al., 1999; Lorberbaum et al., 2002; Swain et al., 2003; Swain et al., 2004b; Swain et al., 2005) which highlights the importance of the choice of stimuli in these experiments as well as not viewing the anterior cingulate as one structure without subdivisions. Perhaps also, 6 s vs. 30 s stimuli have considerably different meanings to new parents and there may be non-linear or multiphasic anterior cingulate responses. Finally, within-group analyses showed that parents activated more to infant crying than laughing in the right amygdala, while non-parent response was greater for infant laughing then crying (Seifritz et al., 2003). These within-group results suggest a potential change in amygdala function with being a parent, although there was no direct comparison of parents to non-parents. Inclusion of psychological measures of parenting parameters will make future studies more practically insightful. These data do, however, represent the first attempts to extend the previous work on parental brain circuits to include gender and experience-dependant aspects of human parenting.
Relevant to parent responses to infant sounds, other fMRI research has been exploring brain responses to emotionally laden human vocalizations, such as having non-parents listen to adult cries and laughter. Some of the brain responses overlap with those found in the parent–infant studies. To reveal emotion circuits, subjects were asked to self-induce happy or sad emotions to correspond with the laughing or crying stimuli, respectively. For pitch detection, subjects were asked to detect pitch shifts. Both conditions led to bilateral activation of the amygdala, insula and auditory cortex with a right-hemisphere advantage in the amygdala, and larger activation during laughing than crying in the auditory cortex with a slight right-hemisphere advantage for laughing. Both of these responses are likely due to acoustic stimulus features. These results suggest that certain brain regions, including the amygdala, activate to emotionally meaningful sounds like laughing and crying independent of the emotional involvement, suggesting the pattern recognition aspect of these sounds is crucial for this activation and that emotional valence might be represented elsewhere in the brain (Sander et al., 2003). Frontal areas may be good candidates for altering emotional valence as suggested by more recent work by Sander and colleagues in which they found a correlation between activity in the orbitofrontal cortex (OFC) in response to angry utterances and an emotional sensitivity scale across a group of young adults (Sander et al., 2005). Sander and colleagues also report a striking gender effect with infant laughing and crying stimuli vs. a control sound activating the amygdala and anterior cingulate of women, while the control stimuli elicited stronger activations in men. Independent of listeners’ gender, auditory cortex and posterior cingulate were more strongly activated by the control stimuli than by infant laughing or crying (Sander et al., 2007). Perhaps the gender-dependent correlates of neural activity in amygdala and anterior cingulate reflect neural predispositions in women for responses to preverbal infant vocalizations, whereas the gender-independent similarity of activation patterns in posterior and anterior cingulate reflects the relatively sensory-based and cognitive levels of neural processing. Related work has used baby face stimuli to underline the role of limbic systems in humans that may be critical for care giving thoughts and behaviors even in non-parents (Glocker et al., 2009; Kringelbach et al., 2008). Again, coordinated responses in drive (amygdala) and regulation (cinglulate) figure prominently in these papers. The question of whether parent brains are actually differentially sensitive to infant stimuli and how gender and kinship modulate these systems will require a direct contrast of men vs. women in parents and non-parents.
In an attempt to further this research on the neurocircuitry underlying emotionally laden parenting thoughts, behaviors and parent–infant attachment, and based in part on the work of Lorberbaum et al. (2002), Swain and colleagues have been gathering data sets on groups of new parents across a range of experience, temperament and parent–infant interaction styles using each parent’s own baby cries and including comprehensive interviews and self-reports (Swain et al., 2003). In this design, parents underwent brain fMRI during 30 s blocks of infant cries generated by their own infant as well as a “standard” cry and control noises matched for pattern and intensity. In addition, they added a longitudinal component with scans and interviews done at 2 time points: 2–4 weeks and 12–16 weeks postpartum. These times were chosen to coincide with the transforming experience of having a baby known to be associated with increased tendency for parents to be highly preoccupied in the early postpartum (Leckman et al., 1999). They hypothesized that parental responses to own baby cries would include specific activations in thalamocortico-basal ganglia circuits believed to be involved in human ritualistic and obsessive–compulsive thoughts and behaviors (Baxter, 2003; Leckman et al., 2004). They also reasoned that emotional alarm, arousal and salience detection centers including amygdala, hippocampus and insula (Britton et al., 2006; LeDoux, 2003) would be activated by baby cry stimuli. The experimental block design was used in order to give parents a chance to reflect on their experience of parenting and, according to our hypothesis, become more preoccupied with their infants’ well being and safety. In a group of first-time mothers (n=9) at 2–4 weeks postpartum, regions that were relatively more active with parent’s own baby cry stimuli compared with other baby cry included the midbrain, basal ganglia, cingulate, amygdala and insula (Swain et al., 2003). Activation of these regions may reflect an increase in arousal, obsessive/anxiety circuits that may be normally temporarily more active for parents and persistently sensitive with some mental illnesses (Swain et al., 2007). Preliminary analysis of connected parenting interview data shows that mothers were significantly more preoccupied than fathers, which was consistent with the relative activations for mothers compared with fathers in the amygdala and basal ganglia (Swain et al., 2004b). In addition, grouping mothers and fathers together across experience and comparing activity at 2–4 weeks vs. 3–4 months postpartum for own baby cry showed that In the group of primiparous mothers, given the same stimuli at 3–4 months postpartum (even though the stimuli were from ~3 months earlier), amygdala and insula activations were not evident; and instead activity had shifted to medial prefrontal cortical and hypothalamic (hormonal control) regions (Swain et al., 2004a; Swain et al., 2004b). This fits well in our initial expectations that parenting over time may reflect changes in regional brain responses as the parent–infant relationship develops: as mother learn to associate their infant cries more with more flexible social behaviors and more mature attachment, there is more regulatory cortical brain activity and less alarm and anxiety-related activity (amygdala and insula) in healthy parents.
Normal parent responses to infant cries are also associated with a several hormones (Fleming et al., 2002), which may serve to help orchestrate appropriate short and long term brain activity and behavioral responses. Among these hormones, oxytocin has emerged to play a critical role across mammalian experimental paradigms to be important to social bonding, parenting in particular (Keverne and Curley, 2004; Lee et al., 2009; Ross and Young, 2009). In recent human studies, it has specifically been connected with parent–infant behavior, as measured in both serum and saliva (Feldman et al., 2010a) and across gender (Feldman et al., 2010b).
Consistent with emerging consistent data across species of the importance of oxytocin in regulating social bonds and parenting in particular, recent parent brain neuroimaging papers are showing the brain circuits that are associated with this hormone. Grouping parents according to vaginal vs. cesarean delivery, brain activity is higher in response to own vs. other baby cry in emotion regulation and limbic regions (Swain et al., 2008). Further considering the importance of oxytocin for parenting, mothers grouped by breastfeeding vs. formula feeding (Kim et al., 2010a) show increases in broadly overlapping regions. This imaging research is bolstered by quantitative studies of maternal serum oxytocin in the postpartum in conjunction with brain imaging by Strathearn and colleagues, which is discussed in the later part along with related papers that use visual stimuli in the next section. In the breastfeeding imaging study, (Kim et al., 2010a), maternal brain responses to their own baby cry was for the first time reported to be correlated with parental sensitivity—an independently rated behavioral measure of parenting, in response (amygdala) and regulation (frontal cortex) regions, suggesting the importance of balanced responses for sensitive parental care giving.
In the first attempt to address the established effects of early-life events on later parental brain function, as elaborated in rodent and non-human primate models (Champagne, 2010; Kaffman and Meaney, 2007; Veenema, 2009) as well as human studies (Lupien et al., 2009; McEwen, 2008), Kim and colleagues studied maternal fMRI responses to baby cry, with mothers grouped according to perceived maternal quality that they themselves experienced (Kim et al., 2010c). Mothers who reported higher maternal care in childhood had greater responses to infant cries in regulatory cortical regions, including emotion regulation areas of the middle and superior frontal gyri, whereas mothers reporting lower maternal care showed increased hippocampal activations—again underlining the likely importance of coordinated responses of these cortical and subcortical regions’ importance for parenting in particular. In this study different cortical regions were found suggesting complexity of cortical regulatory circuits depending on the specific population and behaviors involved. Also, these findings suggest that maternal care in childhood may be associated with long term functional differences in brain regions likely important to parenting and susceptible to chronic stressful in humans just as in other mammals.
4. Parental brains and baby visual stimuli
Several groups are also using baby visual stimuli to activate parental brain circuits (Bartels and Zeki, 2004b; Leibenluft et al., 2004; Nitschke et al., 2004; Noriuchi et al., 2008; Ranote et al., 2004; Strathearn et al., 2009; Strathearn et al., 2008; Strathearn et al., 2005; Swain et al., 2003; Swain et al., 2004a; Swain et al., 2006) with a variety of designs, parent populations and infant age—and again highlighting drive/motivation brain regions responding in concert with cortical regulation regions.
First, investigators have used basic own and other baby photographs as stimuli. In one set of studies, photographs that were taken extremely early 0–2 weeks postpartum, by the parents themselves were shown to groups of mothers and fathers with a block design, 6 pictures continuously shown for 5 s each for blocks of 30 s (Swain et al., 2003; Swain et al., 2006). Photographs were chosen to be ethologically appropriate, such that parents found them potent to evoke their own parental emotions involving motivation and reward. In these studies, own vs. other baby picture contrasts revealed activations in frontal and thalamocortical circuits to at 2–4 weeks postpartum. Specific characterization of these regions according to differences by gender, experience and postpartum time of assessment are underway. Correlations between parent response to own vs. other baby picture and parent behaviors attained from videotapes of interactions with their infant reveals activations in superior temporal lobe, OFC and ventral tegmental areas, may be key to understanding the regulation of parental motivation and reward associated with baby-directed empathy, approach and caring behaviors as well as social bonding.
With the idea that parental love may make use of the same reward and emotion circuits as romantic love (Bartels and Zeki, 2000), Bartels and Zeki used photographs of own, familiar and unfamiliar infants (9 months to 3.5 years of age) as stimuli for parent brains (Bartels and Zeki, 2004b). They measured brain activity in 20 healthy mothers while viewing still face photographs of their own child compared to age-matched photographs of other children. There was increased activity in the midbrain (periaqueductal gray and substantia nigra regions), dorsal and ventral striatum, thalamus, left insula, ORC, sub-, pre-, and supra-genual anterior cingulate, and superior medial prefrontal cortex. There were also increases in the cerebellum, left fusiform, and left occipital cortex, but decreases in the left amygdala. Bartels and Zeki also compared mother brain responses of own child vs. familiar child to the best friend vs. familiar friend in order to control for familiarity and positive affect, and they argue that responses were unique to the own child stimuli. They suggest a variant hypothesis of parent–infant attachment regulation by a push–pull mechanism involving selective activation of motivation and reward systems, but with cortical regions suppressing critical social assessment and negative emotion systems (Bartels and Zeki, 2004b) which may be extended to also foster the orchestration of positive feelings and caring behaviors.
Using a similar approach, but focusing on early stage romantic love, attachment and mate selection (Fisher et al., 2002a; Fisher et al., 2002b), Aron, Fisher and colleagues conducted fMRI studies of brain response to photographs of beloved and familiar individuals (Aron et al., 2005; Fisher et al., 2005). They replicated the findings of Bartels and Zeki’s romantic love findings (Bartels and Zeki, 2000). They also reported that activations specific to the beloved in dopamine-rich areas associated with mammalian reward and motivation areas of the midbrain (right ventral tegmental area) and caudate nucleus (right posterodorsal body and medial parts) correlated with facial attractiveness scores. Furthermore, activation in the right anteromedial caudate was correlated with questionnaire scores that quantified intensity of romantic passion for the individuals whose photographs were used as stimuli. Finally, activity in the left insula-putamenglobus pallidus correlated with trait affect intensity, whereas activity in limbic and cortical regions, including insula, cingulate parietal, inferior temporal and middle temporal cortex was correlated with the length of time in love. Taken together, these studies suggest that romantic love uses subcortical motivation and reward systems to focus thoughts and behaviors on a specific individual, while limbic cortical regions process individual emotional factors. In a related study of romantic relationships using aversive stimuli, Najib, Lorberbaum, Kose and colleagues investigated women whose romantic relationship had ended within the 4 months preceding the experiment. They found that acute grief related to the loss of a romantic attachment figure modulated activity in some of the same areas implicated in social attachment and parenting when they recalled sad thoughts about their loved one (Najib et al., 2004). Decreases in activity in this case were associated with sad thoughts about the loss of the romance compared with neutral thoughts about someone else. This included deactivations in temporal cortex, insula and anterior cingulate/prefrontal cortex. In contrast to the romance-studies which found activations in the anterior cingulate, they also found that romantic grief was consistently associated with deactivations in this region. Finally, they found that activity in the anterior cingulate, insula, and amygdala was inversely related to the level of a subject’s grief.
Returning to a study of parent–child relationship, using photographs of much older children (5–12 years old), mothers viewed pictures of their own vs. other children’s faces during brain fMRI measurements and were asked to press a button to indicate identity (Leibenluft et al., 2004)—but not feelings. Some social cognition regions that were not activated in the Bartels and Zeki (2004b) study were significantly activated in this study, including the anterior paracingulate, posterior cingulate and the superior temporal sulcus which are important for empathy (Saxe, 2006a). This may be explained by the use of much older children, which might involve a different set of circuits relevant to those particular relationships. It may also be that the cognitive task interacts with affective responses to face images in some way (Gray, 2001). In addition, some of the more subcortical activations from Bartels and Zeki (2004b) were not activated in the study by Leibenluft et al. (2004) because of the smaller sample size and the lack of focus on loving thoughts. Differences in child photo affective facial expressions (happy vs. neutral vs. sad) may also constitute a confounding factor. Finally, differences between studies may emerge because of differences in sample populations that need to be controlled. Although all of the studies were of “normative” parent populations, most studies only screened for clinical psychiatric disease. Perhaps different populations may process infant cues in different ways. Studies involving more specific tasks and correlations between brain activations and relationship-specific variables will be able to tease apart the particular roles of different brain regions in different aspects of those relationships.
In another study focusing on parents’ brains using visual stimuli, Nitschke et al. (2004) studied six healthy, primiparous mothers’ brains at 2–4 months postpartum as they viewed smiling pictures of their own and unfamiliar infants. They reported OFC activations that correlated positively with pleasant mood ratings. In contrast, areas of visual cortex that also discriminated between own and unfamiliar infants were unrelated to mood ratings (Nitschke et al., 2004). Perhaps, as they suggest, activity in the OFC—which may vary across individuals—is involved with high order dimensions of maternal attachment. Perhaps the complex aspects of parenting may be quantified using fMRI of frontal brain areas to help predict the risks of mood problems in parents. Continuing with innovative and perhaps more realistic and ethologically appropriate use of videotaped (although silent) infant stimuli, Ranote and colleagues conducted a similar experiment (Ranote et al., 2004). In their study, ten healthy mothers viewed alternating 40 s blocks of their own infant’s video, a neutral video, and an unknown infant. For these women, there was significant activation in the “own” versus “unknown” infant comparison in the left amygdala and temporal pole. They interpreted this circuit as regulating emotion and theory-of-mind regions relating to the ability to predict and explain other people’s behaviors. Certainly, this fits with fMRI experiments on biological motion, which activate similar regions (Morris et al., 2005). It is important to note that all of these visual paradigms used to examine differences between one’s own infant and unfamiliar infants employ a complex set of brain systems necessary for sensory perception, identification, and emotional response. Yet, it now appears from a number of studies, that despite the multisensory complexities of audiovisual stimuli, meaningful analysis of fMRI data is possible. For example, there seems to be a striking inter-subject synchronization among emotions regulating brain areas responding to audiovisual cues during observation of the same scenes of an emotionally powerful movie (Hasson et al., 2004). Also, the intensity with which subjects perceive different features in a movie (color, faces, language, and human bodies) was correlated with activity in separate brain areas (Bartels and Zeki, 2004a). Finally, regional activity between brain areas that are known to be anatomically connected have been shown to be simultaneously active during movie viewing (Bartels and Zeki, 2005). Using infant stimuli, it may be possible to delineate parental emotion regulation under stress. Thinking about the contribution of the infant’s affect to maternal brain function prompted a recent study by Noriuchi et al. (2008) which used silent video clips of their own and other infants in play or separation situations. First, they confirmed the increased activity, associated with recognizing own baby pictures, in certain brain regions including cortical orbitofrontal, anterior insula and precuneus cortical areas as well as subcortical regions including the periaqueductal gray and putamen. These are areas active in arousal and reward learning. Furthermore, they found strong and specific differential responses of mother’s brain to her own-infant’s distress in substantia nigra, caudate nucleus, thalamus, posterior and superior temporal sulcus, anterior cingulate, dorsal regions of OFC, right inferior frontal gyrus and dorsomedial prefrontal cortex. They interpreted OFC and related activations as part of circuits required for the execution of well-learned movements. This could be also be interpreted as activation in emotion regulation and habitual behavioral response systems that are active in a range of normal and abnormal emotion-control states including obsessive–compulsive disorder (Swain et al., 2007). They also found correlations in OFC with own baby-response and happiness as well as to their own distressed baby response in the superior temporal regions. This is consistent with the emerging importance of these areas in social behaviors. Indeed, it seems that movies and other more complex stimuli may be used to stimulate parents’ brains in ways that may be measured and combined with behavioral measures to better understand the functional architecture of parenting brain systems. Taken together, these fMRI experiments with parents and baby visual stimuli also commonly activate emotion/ drive brain structures plus cortical regulation areas, laying the groundwork for testing hypotheses around how these brain structures operate with key hormones, and regulate specific responses to different parenting situations, thoughts and associated parenting behaviors.
Finally, Strathearn and colleagues have been studying healthy, first-time, singleton mother–infant dyads using fMRI and visual infant facial cues of varying affect (smiling, neutral and crying) gathered at 5–10 months postpartum. In one study, 28 healthy right-handed mothers, without a history of psychiatric impairment or child maltreatment, were scanned at 7–17 months postpartum—a period well past the ~3 month postpartum onset of sophisticated social dyadic interactions (Strathearn et al., 2008; Strathearn et al., 2005). They reported that dopamine-associated reward-processing regions of the brain were activated when mothers viewed their own infant’s face compared with an unknown infant’s face. These included the ventral tegmental area/substantia nigra regions, the striatum. In addition, there were frontal lobe responses in emotion processing (medial prefrontal, anterior cingulate, and insula cortex), cognition (dorsolateral prefrontal cortex), and motor/behavioral outputs (primary motor area). Furthermore, happy, but not neutral or sad own-infant faces, activated nigrostriatal brain regions interconnected by dopaminergic neurons, including the substantia nigra and dorsal putamen. Finally, a region-of interest analysis revealed that activation in these regions was related to positive infant affect (happy>neutral>sad) for each own—unknown infant-face contrast. In a sophisticated brain imaging follow-up (Strathearn et al., 2009), 30 first-time mothers, were studied using similar stimuli combined with the Adult Attachment Interview, and peripheral serum oxytocin responses to infant cues. They found that in response to their own infant’s smiling and crying faces during fMRI, mothers with secure attachment showed greater activation of brain reward regions, including the ventral striatum, and the oxytocin-associated hypothalamus/pituitary region. Peripheral oxytocin responses to infant contact provocations at 7 months were also significantly higher in secure mothers, and positively correlated with brain activation in both regions. Insecure/dismissing mothers showed greater insular activation in response to their own infant’s sad faces. These results suggest that individual differences in maternal attachment may be linked with development of the dopaminergic and oxytocinergic neuroendocrine systems, highlighting brain circuits involving the striatum and the hypothalamus.
Along the lines of basic cognitive basic brain imaging work discussed in the introductory section, Lenzi and colleagues have conceptualized empathy as a key part of parenting in a study maternal brain function while observing and imitating faces of their own child and those of someone else (Lenzi et al., 2008). They found that the mirror neuron system, including insula and amygdala were more active during emotional expressions from a mother’s own child, and that insula response correlates with maternal reflective function, conceptualized as a measure of empathy. They also reported that baby joy expressions across identity evoked mostly right limbic and paralimbic areas important to emotional processing, whereas ambiguous expressions elicited responses in left-sided high order cognitive and motor areas, logically reflecting associated cognitive effort.
It now appears that careful use of a variety of baby stimuli can be used to activate parent brains across identity and affect. Furthermore, such responses correlate with psychometrics of parenting, mood, anxiety and attachment parameters as well as across sensibly across hormonal milieus. Thus this promising field promises to provide models of the neuroendocrine circuits that regulate parenting in health and perhaps special populations at risk—discussed after the next section.
5. Parental brain structure
In a pioneering neuroimaging studies of parents, extended to include the dimension of brain structure as well as function, Kim and colleagues have begun to look at brain densities using voxel-based morphometry (Kim et al., 2010b; Kim et al., 2010c). First, this study attempts to address the effects of early-life events on later parenting, as elaborated in rodent and non-human primate models (Champagne, 2010; Kaffman and Meaney, 2007; Veenema, 2009), brain structure as well as functional responses to baby cry vary according to a measure of perceived maternal quality (Kim et al., 2010c). Mothers who reported higher maternal care in childhood showed higher measures of gray matter volume in a range of higher cortical areas, including the superior and middle frontal gyri, orbital gyrus, superior temporal gyrus and fusiform gyrus. This is perhaps not surprising, given that brain structure changes with the environmental demands of learning to juggle (Draganski et al., 2004) or studying (Draganski et al., 2006) over a few months. This work raises the possibility of a range of studies combining structural, functional, as well as endocrine measures to sharpen our understanding of parental behavior and ultimately links with parent and child outcomes.
6. Special parent populations, specific behaviors
In addition to understanding normal human parenting in order to optimize health outcomes, research on parents with health risks from substance abuse and mood disorders to different conditions of birth and infant feeding are needed to improve the recognition and treatment of compromised parenting circumstances. Indeed, recently published follow-up data on the offspring of depressed and anxious mothers who have increased mental health risks (Brown et al., 1987; Heim et al., 1997; Kendler et al., 1993; Sroufe et al., 1999), underscores the significance of work in this area. Clearly, parental wellness (and/or the presence of other attuned care giving adults) has long term positive effects on resiliency and emotional well-being of children as they grow up and for decades later. Furthermore, longitudinal studies of high-risk infants suggest that secure attachment in the perinatal period is associated with a degree of resiliency and protection against the development of psychopathology later in life (Werner, 2004).
Parental mental health problems in the postpartum, such as depression and anxiety, are common and contribute significantly to parent–infant attachment problems. Postpartum depression follows 10% to 15% of all deliveries (Caplan et al., 1989) and more than 60% of patients have an onset of symptoms within the first 6 weeks postpartum (Stowe and Nemeroff, 1995). These common problems, including pre-term delivery, postpartum depression and anxiety and substance abuse have received much less investigative attention and not yet a single published fMRI study (Squire and Stein, 2003), although several groups are close. A growing body of evidence from naturalistic longitudinal studies attests to an adverse impact of postpartum depression, with depressed mothers less sensitively attuned to their infants, less affirming and more negative in describing their infant. These disturbances in early mother–infant interactions were found to predict poorer infant cognitive outcome at 18 months (Murray and Cooper, 2003) and at later time-points, namely 7 years of age (Kim-Cohen et al., 2005). In another recent study (Feldman et al., 2009), infants of control, anxious and depressed mothers were compared with respectively poorer outcomes at 9 months postpartum. Social engagement, maturity of regulatory behaviors and more emotionality were all diminished by mood and anxiety, with associated increases in cortisol reactivity. Interestingly, the effect of major depressive disorder on social engagement and child stress-reactivity were moderated by maternal sensitivity behavioral measures, which have also been reported to be related to cortical parental brain responses to their infant cry (Kim et al., 2010a).
Indeed, certain cortical structures such as the anterior cingulate that are postulated as key to parenting may also mediate the effects of depression on parenting. Previously, the importance of this region in mediating a range of social behaviors as well as parental brain responses to both auditory and visual baby stimuli has been repeatedly highlighted. In addition, this structure and neighbouring medial frontal regions and linked basal ganglia have been also been strikingly linked with mood regulation, including depression and anxiety (Drevets et al., 2008; Gutman et al., 2009; Johansen-Berg et al., 2008; Mayberg et al., 2005). Indeed, the cortical regions involved make sense for both mood regulation and parenting as they are richly connected with motivation and reward regions of the hypothalamus, nucleus accumbens, amygdala, orbitofrontal cortex, dorsomedial frontal cortex and frontal pole. The one study so far linking human maternal brain responses to own baby cry with depressive symptoms showed a correlation in medial frontal cortex—regions that were also differentially sensitive across delivery mode and supporting the controversial link between delivery mode and risk for postpartum depression in new mothers (Swain et al., 2008), though this is just one small study. A related preliminary study of maternal retrospective reports of quality of early experiences related to parental bonding and abuse were significantly related to maternal anterior cingulate cortex response to own vs. other baby face viewing (Barrett et al., 2009). Combining this kind of imaging study with psychotherapies and medications and following outcomes may help individually tailored treatment choices.
It is encouraging that maternal remission from depression within 3 months was associated with significant decreases in the mood symptoms of their children at 7–17 years of age (Weissman et al., 2006), suggesting that research that relates brain function to mood and may help determine treatment is worthwhile. Indeed, in addition to ongoing work on depressed mothers, pilot studies are underway to detect the brain basis of the attachment-based dyadic interventions, such as “circle of security” (Cassidy et al., 2010) for postpartum anxiety and depression involving early time points and young children.
7. Summary and model
Functional MRI experiments on parenting using baby stimuli are now making meaningful and complementary contributions to our understanding of the parental brain. The human parental brain is the concept of a discrete set of interacting brain circuits that serve as substrate for the human transition to parenting, integrate baby as well as internal information, and support key thoughts and behaviors for us to identify and react to baby stimuli. In support of this model, virtually all of the published studies involving infant stimuli to study parent brains with fMRI are summarized and contrasted in Tables 1 and 2 according to baby cry and picture stimuli respectively. This appears to center on the feedback loops involving hypothalamus, midbrain, basal ganglia regions, anterior cingulate, prefrontal cortex, and thalamus—all requiring motivation and reward. More complex planning and social emotional/empathy responses may involve frontal, insular, fusiform and occipital areas. Other important aspects of parenting may be regulated by context and memory processing regions including the hippocampus, parahippocampus and amygdala. Clearly, baby pictures and cries can be used to selectively activate brain circuits related to arousal, mood, and social and habitual behaviors according to identity and affect of baby, as well as and group differences such as parental status, early-life experience, delivery, etc. The maturing state of literature, however, is made complex as different groups have used a mixture of stimuli including baby cries, laughter and child pictures examining varied postpartum time windows, baby affects and stimuli presentations focusing on cognitive and social aspects of brain function. The parental brain is also a moving target, subject to adaptive plastic changes associated with postpartum, so richly affected by the baby. However, a clearer picture of the specificity of different brain areas’ roles is emerging, as researchers link brain responses in respective circuits to specific aspects of parenting by adding sophisticated interviews, naturalistic assessments of parent–infant interaction and bonding, as well as more sophisticated brain-imaging analyses.
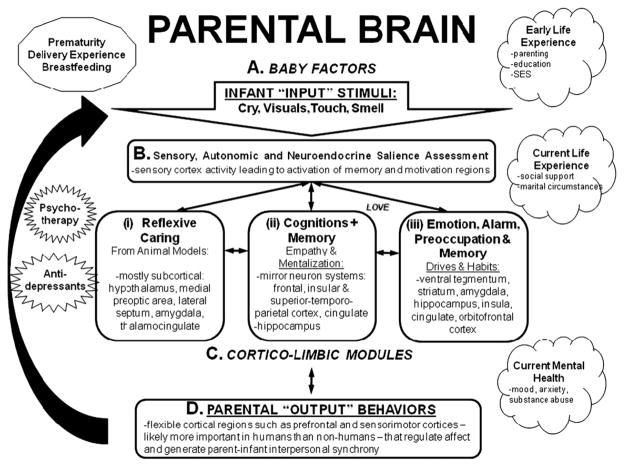
Based on brain imaging of parents to this point and informed by animal work (Swain et al., 2007), the following model is presented (Fig. 1) to account for parenting behaviors, organize future discussion and plan future experiments. First, key parenting sensory signals, including cry, visuals as well as touch and smell from baby (A), must be organized in sensory cortices which appraise the input and interact with subcortical memory and motivation structures (B). Ultimately, sufficient motivation will activate corticolimbic modules (C), that have delineated as (i) reflexive caring impulses, such as those studied in preclinical animal models and requiring little or no cortical input such as licking, grooming and nursing in which the hypothalamus, MPOA, and other limbic and thalamocingulate circuits are of primary importance (Numan and Insel, 2003). In humans, this might be case as the caring endophenotype (Panksepp, 2006) and so amenable to further study. In addition, (ii) cognitive circuits would be brought online in the parent brain, including those that regulate “mirroring”, empathy, planning and further cognitive flexibility, including the inferior frontal, insular and superior–temporoparietal cortical regions. These regions might allow accurate modeling of the baby’s mind to predict their needs and plan behavior (Baron-Cohen and Wheelwright, 2004; Decety and Grezes, 2006; Pelphrey et al., 2004; Saxe, 2006a). Finally, (iii) other alarm/ emotion–preoccupation anxiety systems might be activated to increase arousal and regulate parental worries and habitual responses in coordination with memory systems. These emotional circuits might include the ventral tegmental area, striatum, amygdala, insula, cingulate cortex and OFC (Mayes et al., 2005; Swain et al., 2007). These reflexive, cognitive and emotional modules would interact with each other in the experience of parental love and attachment formation, and work together to generate coordinated hormonal, autonomic and behavioral output required for parenting (D) via motor cortex and hypothalamus. The output would also feed back to sensory systems (B) during dynamic interactions with the infant to generate new input from baby (A). Other critical factors to be added include current circumstances such as delivery and breastfeeding, early-life experience for which we have brain imaging evidence of modifying brain function in response to infant stimuli (Kim et al., 2010a; Kim et al., 2010c; Swain et al., 2008).
Fig. 1.
Human parental circuits. Brain regions expected to be important to human parenting. This based on human and animal studies. Please see text for description.
In summary, this review is an attempt to synthesize our current understanding of parent–infant bonding, chiefly from the perspective of the parent’s brain physiology. The parent–infant bond is so central to the human condition, contributes to risks for mood and anxiety disorders, and the potential for resiliency and protection against the development of psychopathology throughout life, not to mention the far-reaching aspects of human attachment across individual behaviors and between cultures. Efforts to characterize this reciprocal interaction between caregiver and infant and to assess its impact have provided a powerful theoretical and empirical framework in the fields of social and emotional development. On the one hand, the complex nature of the phenomena and experimental approaches leads to the consideration of many overlapping brain systems of sensory, emotion, and cognition to support behavior, but on the other hand a relatively small set of brain regions seem to be robustly involved, including basal ganglia and related cortex for emotion and drive (including striatum, amygdala, hypothalamus and hippocampus), and regulatory cortical regions of anterior cingulate, medial frontal and orbitofrontal cortices.
8. Future directions
Future studies will add integrated and increasingly ethologically valid baby stimuli such as movies and perhaps even different sensory systems, such as the olfactory system, as well as more measures of parent responses to expand and refine our understanding of the parental brain. These approached will require careful consideration and study of how these patterns of brain activation may differ between attachment groups or across mental health measures. Do mothers with insecure patterns of attachment, for instance, respond differently to their infant cues? Are depressed mothers neglectful because they are unresponsive to these cues, do they fail to receive reward signals in the brain or are they overwhelmed with alarm signals or preoccupation? Longitudinal research designs are also necessary to answer these questions, although this raises methodological issues with respect to using fixed stimuli to compare cross time-point, or using stimuli matched to the age of infant that may not be directly compared across time. Some of these problems may be addressed with meta-analyses once enough studies have been published. In addition, it will be important to clarify the associated roles of different neuroendocrine pathways, such as by measures of cortisol and oxytocin, as well as different genetic variations in mediating parenting brain activations. Some key questions that have been partly addressed are summarized in Box 1 to summarize some data and encourage further work.
Box 1. Summary of questions addressed emerging data and future experiments to explore.
| Ongoing and future questions | Current scientific evidence | References |
|---|---|---|
| 1. Does it matter if the stimuli are derived from the parents’ own child vs. another child? | There is strong evidence from many studies using picture and cry response data that own vs. other brain activity is significant. The links between such responses and meaningful behavioral measures such as behavioral measures require more work. | Circuits (Bartels and Zeki, 2004b; Kim et al., 2010a; Kim et al., 2010c; Leibenluft et al., 2004; Nitschke et al., 2004; Noriuchi et al., 2008; Ranote et al., 2004; Strathearn et al., 2009; Strathearn et al., 2008; Strathearn et al., 2005; Swain et al., 2003; Swain et al., 2004a; Swain et al., 2006; Swain et al., in press; Swain et al., 2008) |
| 2. Do fathers differ from mothers? | Mothers and fathers have been grouped together to look for common parental brain responses suggesting commonalities across sex. | Seifritz et al. (2003); Swain et al. (in press) |
| Preliminary data on differences between responses in mothers and fathers has been presented as abstract and is in preparation. | Swain et al. (2003); Swain et al. (2008) | |
| 3. Does experience matter (first vs. subsequent children)? | Preliminary data presented in abstract form is in preparation for publication. | Swain et al. (2003); Swain et al. (2006) |
| 4. Do parents with differing attachment styles respond differently to infant stimuli? | Attachment style and reflective function has only been formally assessed in one imaging study so far showing direct correlation with insula response to own infant face. | Lenzi et al. (2008) |
| Related measures of early-life experience correlate with brain cortical structure and function, supporting the importance of attachment style for parental brain and behavior. | Barrett et al. (2009); Kim et al. (2010c) | |
| 5. How does peripartum depression alter these patterns of response? | Only one study with direct correlation between own baby cry response and depression in medial frontal cortex. Other previous reports (4) suggest link worth exploring. | Swain et al. (2008) |
Helpful approaches to these questions will include systematic studies of well characterized, but different, populations of parents using a range of infant stimuli paradigms and psychometric tools. As in other areas of cognitive neuroscience, there will be debates about whether to use more ethologically correct, but poorly controlled, stimuli versus more tightly controlled, but less natural stimuli. Both types of experiments will be needed to tease apart the basic apparatus of baby responsiveness and bond formations as well as the parts of the circuit that are actually at work in normal day-to-day-parenting. This work will also require the study of both parents and infants to understand how their interactions contribute to their bond and infant outcomes. Other modalities of study for confirmation and the benefits of the temporal resolution of different techniques include the use of evoked potentials, and he potential flexibility of dense array electroencephalography nets or functional near infrared spectroscopy that might allow subjects more mobility and simultaneous parent–infant studies.
Studies are underway such that differences in parental response patterns will be reported in specific clinical populations, such as those with postpartum depression and substance abuse in the near future. This may lead to future assessments of parent mental health risk and resilience profiles using standardized imaging techniques and to improvements in the detection, treatment and prevention of mental illness that interferes with parenting.
Acknowledgments
The authors would like to acknowledge the generous support of colleagues, research assistants and research participants at our respective institutions: JES was supported by grants from the Institute for Research on Unlimited Love (unlimitedloveinstitute.org), the National Alliance for Research on Schizophrenia and Depression (narsad.org), the Klingenstein Third Generation Foundation, Yale Center for Risk, Resilience and Recovery, Associates of the Yale Child Study Center and the Department of Psychiatry of the University of Michigan. Dr. Swain would especially like to acknowledge the inestimable collegial support of many, including Drs. Robert T. Constable, Ruth Feldman Pilyoung Kim, James F. Leckman, Jeffrey Lorberbaum, Linda C. Mayes, Robert T. Schultz and Lane Strathearn.
Abbreviations
- fMRI
functional magnetic resonance imaging
- OFC
orbitofrontal cortex
- ACT
activation
- DEACT
decativation
Glossary for Tables 1 and 2
- Activations and deactivations
measured by functional magnetic resonance imaging, satisfied significance criteria of random effects analysis at p<0.05 or fixed effects analysis at p<0.001at a minimum
- T
Tesla (unit of magnetic field strength)
- Blocks
periods of stimulus exposure and fMRI data acquisition
- Events
brief exposures to infant stimuli during fMRI experiments
- other cry
cry of an unfamiliar baby
- own cry
cry of the subject’s own baby
- MPOA
medial preoptic area
- BNST
bed nucleus of the stria terminalis
- DLPFC
dorsolateral prefrontal cortex
References
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J Neurophysiol. 2005;94:327–37. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J AutismDevDisord. 2004;34:163–75. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Barrett J, Hall G, Wonch K, Ali N, Gonzalez A, Steiner M, et al. Quality of early parental care reported by mothers is related to anterior cingulate response to own infant stimuli: preliminary findings. Neuroimage. 2009;47:S181-S. [Google Scholar]
- Bartels A, Zeki S. The neural basis of romantic love. NeuroReport. 2000;11:3829–34. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. Functional brain mapping during free viewing of natural scenes. Hum Brain Mapp. 2004a;21:75–85. doi: 10.1002/hbm.10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004b;21:1155–66. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. Brain dynamics during natural viewing conditions—a new guide for mapping connectivity in vivo. Neuroimage. 2005;24:339–49. doi: 10.1016/j.neuroimage.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Baxter LR., Jr Basal ganglia systems in ritualistic social displays: reptiles and humans; function and illness. Physiol Behav. 2003;79:451–60. doi: 10.1016/s0031-9384(03)00164-1. [DOI] [PubMed] [Google Scholar]
- Bodini B, Iacoboni M, Lenzi GL. Acute stroke effects on emotions: an interpretation through the mirror system. Curr Opin Neurol. 2004;17:55–60. doi: 10.1097/00019052-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I. Neural correlates of social and nonsocial emotions: an fMRI study. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Brown GW, Bifulco A, Harris TO. Life events, vulnerability and onset of depression: some refinements. Br J Psychiatry. 1987;150:30–42. doi: 10.1192/bjp.150.1.30. [DOI] [PubMed] [Google Scholar]
- Buchheim A, Erk S, George C, Kachele H, Kircher T, Martius P, et al. Neural correlates of attachment trauma in borderline personality disorder: a functional magnetic resonance imaging study. Psychiatry Res. 2008;163:223–35. doi: 10.1016/j.pscychresns.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Buchheim A, Erk S, George C, Kachele H, Ruchsow M, Spitzer M, et al. Measuring attachment representation in an fMRI environment: a pilot study. Psychopathology. 2006;39:144–52. doi: 10.1159/000091800. [DOI] [PubMed] [Google Scholar]
- Caplan HL, Cogill SR, Alexandra H, Robson KM, Katz R, Kumar R. Maternal depression and the emotional development of the child. Br J Psychiatry. 1989;154:818–22. doi: 10.1192/bjp.154.6.818. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA. 2003;100:5497–502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy J, Ziv Y, Stupica B, Sherman LJ, Butler H, Karfgin A, et al. Enhancing attachment security in the infants of women in a jail-diversion program. Attach HumDev. 2010;12:333–53. doi: 10.1080/14616730903416955. [DOI] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic influence of social experiences across the lifespan. Dev Psychobiol. 2010;52:299–311. doi: 10.1002/dev.20436. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. Int J Psychophysiol. 2009;73:88–94. doi: 10.1016/j.ijpsycho.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, Molfese PJ, Mayes LC. Social exclusion in middle childhood: Rejection events, slow-wave neural activity, and ostracism distress. Soc Neurosci. 2010;12:1–13. doi: 10.1080/17470919.2010.500169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Grezes J. The power of simulation: imagining one’s own and other’s behavior. Brain Res. 2006;1079:4–14. doi: 10.1016/j.brainres.2005.12.115. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–2. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26:6314–7. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–81. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent–infant contact. Psychoneuroendocrinology. 2010a;35:1133–41. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. The cross-generation transmission of oxytocin in humans. Horm Behav. 2010b;58:669–76. doi: 10.1016/j.yhbeh.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J Am Acad Child Adolesc Psychiatry. 2009;48:919–27. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Fisher H, Aron A, Brown LL. Romantic love: an fMRI study of a neural mechanism for mate choice. J Comp Neurol. 2005;493:58–62. doi: 10.1002/cne.20772. [DOI] [PubMed] [Google Scholar]
- Fisher H, Aron A, Mashek D, Li H, Strong G, Brown LL. The neural mechanisms of mate choice: a hypothesis. Neuro Endocrinol Lett. 2002a;23(Suppl 4):92–7. [PubMed] [Google Scholar]
- Fisher HE, Aron A, Mashek D, Li H, Brown LL. Defining the brain systems of lust, romantic attraction, and attachment. Arch Sex Behav. 2002b;31:413–9. doi: 10.1023/a:1019888024255. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Corter C, Stallings J, Steiner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Horm Behav. 2002;42:399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodi AM, Lamb ME. Child abusers’ responses to infant smiles and cries. ChildDev. 1980;51:238–41. [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. Aunifying view of the basis of social cognition. Trends Cogn Sci. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Glocker ML, Langleben DD, Ruparel K, Loughead JW, Valdez JN, Griffin MD, et al. Baby schema modulates the brain reward system in nulliparous women. Proc Natl Acad Sci USA. 2009;106:9115–9. doi: 10.1073/pnas.0811620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR. Emotional modulation of cognitive control: approach–withdrawal states double-dissociate spatial from verbal two-back task performance. J Exp Psychol Gen. 2001;130:436–52. doi: 10.1037//0096-3445.130.3.436. [DOI] [PubMed] [Google Scholar]
- Gutman DA, Holtzheimer PE, Behrens TE, Johansen-Berg H, Mayberg HS. A tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry. 2009;65:276–82. doi: 10.1016/j.biopsych.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303:1634–40. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- Heim C, Owens MJ, Plotsky PM, Nemeroff CB. The role of early adverse life events in the etiology of depression and posttraumatic stress disorder. Focus on corticotropin releasing factor. Ann NY Acad Sci. 1997;821:194–207. doi: 10.1111/j.1749-6632.1997.tb48279.x. [DOI] [PubMed] [Google Scholar]
- Hein G, Singer T. I feel how you feel but not always: the empathic brain and its modulation. Curr Opin Neurobiol. 2008;18:153–8. doi: 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Neurobiology of imitation. Curr Opin Neurobiol. 2009;19:661–5. doi: 10.1016/j.conb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–83. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry. 2007;48:224–44. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Neale MC, Heath AC, Eaves LJ. The prediction of major depression in women: toward an integrated etiologic model. Am J Psychiatry. 1993;150:1139–48. doi: 10.1176/ajp.150.8.1139. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Curley JP. Vasopressin, oxytocin and social behaviour. Curr Opin Neurobiol. 2004;14:777–83. doi: 10.1016/j.conb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Moffitt TE, Taylor A, Pawlby SJ, Caspi A. Maternal depression and children’s antisocial behavior: nature and nurture effects. Arch Gen Psychiatry. 2005;62:173–81. doi: 10.1001/archpsyc.62.2.173. [DOI] [PubMed] [Google Scholar]
- Kim P, Feldman R, Leckman JF, Mayes LC, Swain JE. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. J Child Psychol Psychiatry. 2010a doi: 10.1111/j.1469-7610.2011.02406.x. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Xin W, Swain JE. The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behavioral Neuroscience. 2010b;124(5):695–700. doi: 10.1037/a0020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Newman MA, Feldman R, Swain JE. Perceived quality of maternal care in childhood and structure and function of mothers’ brain. Dev Sci. 2010c;13:662–73. doi: 10.1111/j.1467-7687.2009.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Lehtonen A, Squire S, Harvey AG, Craske MG, Holliday IE, et al. A specific and rapid neural signature for parental instinct. PLoS ONE. 2008;3:e1664. doi: 10.1371/journal.pone.0001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Feldman R, Swain JE, Eicher V, Thompson N, Mayes LC. Primary parental preoccupation: circuits, genes, and the crucial role of the environment. J Neural Transm. 2004;111:753–71. doi: 10.1007/s00702-003-0067-x. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Mayes LC, Feldman R, Evans DW, King RA, Cohen DJ. Early parental preoccupations and behaviors and their possible relationship to the symptoms of obsessive–compulsive disorder. Acta Psychiatr Scand Suppl. 1999;396:1–26. doi: 10.1111/j.1600-0447.1999.tb10951.x. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–38. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS., III Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88:127–51. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers’ neural activation in response to pictures of their children and other children. Biol Psychiatry. 2004;56:225–32. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Pantano P, Macaluso E, Iacoboni M, Lenzi GL, et al. Neural basis of maternal communication and emotional expression processing during infant preverbal stage. Cereb Cortex. 2008;19:1124–33. doi: 10.1093/cercor/bhn153. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Dubno JR, Horwitz AR, Nahas Z, Teneback CC, et al. Feasibility ofusing fMRI to study mothers responding to infant cries. Depress Anxiety. 1999;10:99–104. doi: 10.1002/(sici)1520-6394(1999)10:3<99::aid-da2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, et al. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51:431–45. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- MacLean PD. The triune brain in evolution : role in paleocerebral functions. New York: Plenum Press; 1990. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, et al. Neural correlates of social exclusion during adolescence:understanding the distress of peer rejection. Soc Cogn Affect Neurosci. 2009;4:143–57. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Swain JE, Leckman JF. Parental attachment systems: neural circuits, genes, and experiential contributions to parental engagement. Clin Neurosci Res. 2005;4:301–13. [Google Scholar]
- McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism. 2008;57(Suppl2):S11–5. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JP, Pelphrey KA, McCarthy G. Regional brain activation evoked when approaching a virtual human on a virtual walk. J Cogn Neurosci. 2005;17:1744–52. doi: 10.1162/089892905774589253. [DOI] [PubMed] [Google Scholar]
- Murray L, Cooper PJ. The impact of postpartum depression on child development. In: Goodyer I, editor. Aetiological mechanisms in developmental psychopathology. Oxford: Oxford University Press; 2003. [Google Scholar]
- Najib A, Lorberbaum JP, Kose S, Bohning DE, George MS. Regional brain activity in women grieving a romantic relationship breakup. Am J Psychiatry. 2004;161:2245–56. doi: 10.1176/appi.ajp.161.12.2245. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage. 2004;21:583–92. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: mother’s response to infant’s attachment behaviors. Biol Psychiatry. 2008;63:415–23. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. New York: Springer-Verlag; 2003. [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci. 2002;5:900–4. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Olds DL, Kitzman H, Cole R, Robinson J, Sidora K, Luckey DW, et al. Effects of nurse home-visiting on maternal life course and child development: age 6 follow-up results of a randomized trial. Pediatrics. 2004;114:1550–9. doi: 10.1542/peds.2004-0962. [DOI] [PubMed] [Google Scholar]
- Olds DL, Sadler L, Kitzman H. Programs for parents of infants and toddlers: recent evidence from randomized trials. J Child Psychol Psychiatry. 2007;48:355–91. doi: 10.1111/j.1469-7610.2006.01702.x. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Emotional endophenotypes in evolutionary psychiatry. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:774–84. doi: 10.1016/j.pnpbp.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Grasping the intentions of others: the perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. J Cogn Neurosci. 2004;16:1706–16. doi: 10.1162/0898929042947900. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, Michelich CR, Allison T, McCarthy G. Functional anatomy of biological motion perception in posterior temporal cortex: an FMRI study of eye, mouth and hand movements. Cereb Cortex. 2005;15:1866–76. doi: 10.1093/cercor/bhi064. [DOI] [PubMed] [Google Scholar]
- Perlman SB, Pelphrey KA. Regulatory brain development: balancing emotion and cognition. Soc Neurosci. 2010;5:533–42. doi: 10.1080/17470911003683219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purhonen M, Kilpelainen-Lees R, Paakkonen A, Ypparila H, Lehtonen J, Karhu J. Effects of maternity on auditory event-related potentials to human sound. NeuroReport. 2001a;12:2975–9. doi: 10.1097/00001756-200109170-00044. [DOI] [PubMed] [Google Scholar]
- Purhonen M, Paakkonen A, Ypparila H, Lehtonen J, Karhu J. Dynamic behavior of the auditory N100 elicited by a baby’s cry. Int J Psychophysiol. 2001b;41:271–8. doi: 10.1016/s0167-8760(01)00139-8. [DOI] [PubMed] [Google Scholar]
- Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JF, Appleby L. The neural basis of maternal responsiveness to infants: an fMRI study. NeuroReport. 2004;15:1825–9. doi: 10.1097/01.wnr.0000137078.64128.6a. [DOI] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–47. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander D, Grandjean D, Pourtois G, Schwartz S, Seghier ML, Scherer KR, et al. Emotion and attention interactions in social cognition: brain regions involved in processing anger prosody. Neuroimage. 2005;28:848–58. doi: 10.1016/j.neuroimage.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Sander K, Brechmann A, Scheich H. Audition of laughing and crying leads to right amygdala activation in a low-noise fMRI setting. Brain Res Brain Res Protoc. 2003;11:81–91. doi: 10.1016/s1385-299x(03)00018-7. [DOI] [PubMed] [Google Scholar]
- Sander K, Frome Y, Scheich H. FMRI activations of amygdala, cingulate cortex, and auditory cortex by infant laughing and crying. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Curr Opin Neurobiol. 2006a;16:235–9. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Saxe R. Why and how to study theory of mind with fMRI. Brain Res. 2006b;1079:57–65. doi: 10.1016/j.brainres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int JDev Neurosci. 2005;23:125–41. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, et al. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry. 2003;54:1367–75. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Soltis J. The signal functions of early infant crying. Behav Brain Sci. 2004;27:459–90. 443–58. discussion. [PubMed] [Google Scholar]
- Squire S, Stein A. Functional MRI and parental responsiveness: a new avenue into parental psychopathology and early parent–child interactions? Br J Psychiatry. 2003;183:481–3. doi: 10.1192/bjp.183.6.481. [DOI] [PubMed] [Google Scholar]
- Sroufe LA, Carlson EA, Levy AK, Egeland B. Implications of attachment theory for developmental psychopathology. Dev Psychopathol. 1999;11:1–13. doi: 10.1017/s0954579499001923. [DOI] [PubMed] [Google Scholar]
- Stowe ZN, Nemeroff CB. Women at risk for postpartum-onset major depression. Am J Obstet Gynecol. 1995;173:639–45. doi: 10.1016/0002-9378(95)90296-1. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–66. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Montague PR. An fMRI study of maternal mentalization: having the baby’s mind in mind. Neuroimage. 2005;26:S25. [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Constable RT, Schultz RT. The neural circuitry of parent–infant attachment in the early postpartum. Puerto Rico: American College of Neuropsychopharmacology; 2003. [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Constable RT, Schultz RT. Brain circuitry and psychology of human parent–infant attachment in the postpartum. International Academy of Child and Adolescent Psychiatry and Allied Professions,16th World Congress; 2004a; p. 365. [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Constable RT, Schultz RT. Neural substrates and psychology of human parent–infant attachment in the postpartum. Biol Psychiatry. 2004b;55:153S. [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Schultz RT. Early human parent–infant bond development: fMRI, thoughts and behaviors. Biol Psychiatry. 2005;57:112S. [Google Scholar]
- Swain JE, Leckman JF, Mayes LC, Feldman R, Schultz RT. Own baby pictures induce parental brain activations according to psychology, experience and postpartum timing. Biol Psychiatry. 2006;59:126S. [Google Scholar]
- Swain JE, Leckman JF, Mayes LCRF, Constable RT, Schultz RT. First-time mothers and fathers show differential activation of the right orbital frontal and insular cortices in response to listening to their own infant’s cries. Biological Psychiatry. submitted for publication. [Google Scholar]
- Swain JE, Leckman JF, Mayes LCRF, Hoyt E, Kang K, Kim P, David D, Nguyen S, Constable RT, Schultz RT. Functional brain activations of parents listening to their own baby-cry change over the early postpartum. Developmental Psychobiology. In press. [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent–infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48:262–87. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Mayes LC, Leckman JF. The development of parent–infant attachment through dynamic and interactive signaling loops of care and cry. Behav Brain Sci. 2004c;27:472–3. [Google Scholar]
- Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, Leckman JF. Maternal brain response to own baby-cry is affected by cesarean section delivery. J Child Psychol Psychiatry. 2008;49:1042–52. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio N, Massioui FE, Crivello F, Joliot M, Renault B, Mazoyer B. Functional anatomy of human auditory attention studied with PET. Neuroimage. 1997;5:63–77. doi: 10.1006/nimg.1996.0252. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn Sci. 2007;11:153–7. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Veenema AH. Early life stress development aggression neuroendocrine neurobiological correlates what can we learn animal models? Front Neuroendocrinol. 2009;30:497–518. doi: 10.1016/j.yfrne.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Pilowsky DJ, Wickramaratne PJ, Talati A, Wisniewski SR, Fava M, et al. Remissions in maternal depression and child psychopathology: a STAR*D-child report. Jama. 2006;295:1389–98. doi: 10.1001/jama.295.12.1389. [DOI] [PubMed] [Google Scholar]
- Werner EE. Journeys from childhood to midlife: risk, resilience, and recovery. Pediatrics. 2004;114:492. doi: 10.1542/peds.114.2.492. [DOI] [PubMed] [Google Scholar]