Abstract
We hypothesized that a deficiency in the descending serotonergic input to spinal cord may underlie postnatal muscle hypertonia after global antenatal hypoxic-ischemic injury in a rabbit model of cerebral palsy. Neurotransmitter content was determined by HPLC in the spinal cord of newborns with and without muscle hypertonia after fetal global hypoxic-ischemic brain injury and naïve controls. Contrary to our hypothesis, serotonin levels in both cervical and lumbar expansions and norepinephrine in cervical expansion were increased in hypertonic kits relative to non-hypertonic kits and controls, with unchanged number of serotonergic cells in caudal raphe by stereological count. Serotonergic fiber length per unit of volume was also increased in hypertonic kits’ cervical and lumbar spinal cord, both in dorsal and ventral horns. Gene expression of serotonin transporter was increased and 5-HTR2 receptors were decreased in hypertonic kits relative to controls in cervical and lumbar cord. Intrathecal administration of nonselective serotonin receptor inhibitor methysergide decreased muscle tone in hypertonic kits only. Conversely, intrathecal administration of serotonin solution increased muscle tone only in non-hypertonic kits. We speculate that maturation of serotonergic system in spinal cord may be directly affected by decreased corticospinal connectivity after antenatal hypoxic-ischemic brain injury.
Keywords: serotonin, muscle hypertonia, perinatal brain injury, hypoxia-ischemia
INTRODUCTION
The incidence of cerebral palsy (CP) remains unchanged despite many improvements in perinatal practice in recent decades (Pakula et al. 2009, Perlman 2006). Although the anatomical pattern of brain injury in CP patients is well categorized, functional changes in motor control in CP are poorly understood. Progress in understanding these changes has been hampered by the lack of perinatal animal models with overt motor deficits. We have developed a model of fetal hypoxia-ischemia (H-I) that results in pronounced motor deficits in newborn rabbit kits, including muscle hypertonia (Derrick et al. 2004), which strikingly resembles the human CP phenotype. This model presents a unique opportunity to investigate pathophysiology of CP since functional and structural changes in the rabbit brain and spinal cord can be followed longitudinally from initial H-I insult to the onset of motor deficits.
The loss of descending corticospinal input has been implicated (although never directly tested in an experimental animal model or human patients) in origin of muscle hypertonia and spasticity in CP patients (Sanger 2003), similar to mechanisms involved in spinal cord injury. In response to decreased descending input of biogenic amines after spinal cord injury, spinal serotonin receptors has been recently found to become constitutively active and restore large persistent calcium currents in motoneurons (Murray et al. 2010). Resulted increase of spinal motoneuron excitability may lead to pathological spasticity. Descending corticospinal connections in CP patients, however, are typically not completely interrupted (Hoon et al. 2009) and it is unclear whether analogous pathophysiological mechanisms take place after antenatal brain injury.
Depletion of serotonin levels in the brain has been reported in the rabbit antenatal H-I (Vasquez-Vivar et al. 2009) and perinatal inflammation model of CP (Kannan et al. 2010), along with injury to the brain serotonergic system after H-I injury in neonatal rats (Reinebrant et al. 2013, Buller et al. 2012). We hypothesized that a deficiency in the serotonergic input may underlie hypertonia in rabbits following antenatal H-I, as has been suggested in adult spastic conditions (Dentel et al. 2013). We measured levels of spinal monoamine neurotransmitters (serotonin, epinephrine, norepinephrine, dopamine and metabolites), number of serotonergic neurons in brain stem nuclei projecting to the spinal cord, the fiber length per unit of volume of spinal serotonergic projections, and the expression of serotonin receptors and transporters, in newborn controls and kits with and without muscle hypertonia after antenatal H-I. Effect of serotonin and a 5HT receptor antagonist on muscle tone was assessed by in vivo intrathecal administration of the drugs.
METHODS
Animal Model
All animal procedures were approved by the Institutional Animal Care and Use Committee of NorthShore University HealthSystem. The surgical procedure has been previously described (Derrick et al. 2004). In vivo global H-I of fetuses was induced by sustained 40-min uterine ischemia at 22 days gestation (E22, 70% of term gestation at 31.5 days) in timed pregnant New Zealand white rabbits (Myrtle’s Rabbits, Thompson Station, TN). This procedure models acute placental insufficiency at a premature gestation. Briefly, dams were anesthetized with intravenous fentanyl (75 μg/kg/hr) and droperidol (3.75 mg/kg/hr), followed by spinal anesthesia using 0.75% bupivacaine. A balloon catheter was introduced into the left femoral artery and advanced into the descending aorta to above the uterine and below the renal arteries. The balloon was inflated for 40 minutes causing uterine ischemia and subsequent global fetal H-I. At the end of H-I, the balloon was deflated, resulting in uterine reperfusion and reperfusion-reoxygenation to the fetal brains and spinal cords. The catheter was removed, the femoral artery reconstructed, and the dam returned to her cage and allowed to deliver spontaneously. In the sham control group the surgical procedures were identical except the balloon was not inflated.
Surviving rabbit kits were subjected to neurobehavioral testing at P1 (10 days after H-I), including assessment of sensory and motor deficits as described before (Derrick et al. 2004). Based on neurobehavioral assessment, the rabbit kits of either sex were divided into 3 groups: sham control, hypertonic (muscle tone score >2 at least in one limb) and non-hypertonic kits. Hypertonia was manually assessed in each limb separately and the affected kits were further subdivided into groups with hypertonia of all limbs, forelimbs or hind limbs.
Measurements of neurotransmitters
Newborn rabbit kits on day 1 of life (P1), including 7 sham controls, 8 hypertonic kits and 5 non-hypertonic kits after H-I were anesthetized with Ketamine -Xylazine mixture and euthanized by decapitation. For developmental profile study, naïve control E25 fetuses (n=5), P1kits (n=7), P18 kits (n=5) and rabbit dams (n=4) were used. The dams were euthanized with intracardiac pentobarbital injection. Spinal cords were quickly dissected and tissue samples of spinal cervical and lumbar expansions were frozen on dry ice and stored at −80°C until analysis. Frozen tissue was homogenized in 0.1N hydrochloric acid and centrifuged at 13,000 rpm for 10 min. An aliquot of the supernatant was removed for protein assay and the remaining was incubated with 0.1 M perchloric acid for 30 min on ice following centrifugation 20,000 rpm for 15 min. Neurotransmitters in the clarified supernatant were measured by HPLC with electrochemical detection using an analytical column synergy Polar RP (Phenomenex 4.6×250.0 mm) eluted with 0.075 M sodium dihydrogenphosphate, 0.75 mM Triethylamine, 1.5 mM sodium octanesulfonate, 10% acetonitrile pH 3.0 at flow rate of 1.0 ml/min. The system consisted of an ESA automated injection system, an ESA 582 pump, and a CoulArray detector (ESA, Chelmsford, MA). Data collection and analysis was performed using a ESA Coularray 3.06 software. Authentic samples of dopamine, 3,4-Dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), serotonin (5-HT), norepinephrine, epinephrine (10–100 ng/ml) were used as standards. Contents of neurotransmitters were calculated as ng/mg protein.
Real time PCR for measuring rabbit SERT, and 5-HT2 receptor mRNA steady status
Rabbit 5-HT2 receptor PCR target sequence was based on the common part of alignment of human (NM_000868), mice (NM_008312) and rat (NM_012765) 5-HT2 receptor sequences. Rabbit SERT sequence was based on NCBI Reference Sequence: XM_002718904.1. The primer sequences are: for SERT, forward primer: 5′-TTTCCGGATTCGTCATCTTC-3′, and reverse primer, 5′-CAAGCCCAGTGTGATTAGCA-3′, for 5HTR2, forward primer: 5′-ATGGTGAACCTGAGGAATGG-3′, and reverse primer, 5′-CGTCTGGGAATTTAAAGCGTC-3′. Sequences were then submitted to Applied Biosystems website to order custom made Taqman primer and probers set. 2 g of total RNA was reverse transcribed to cDNA using high capacity RT cDNA kit (ABI) in a total volume of 20 l. Real time PCRs were carried in ABI StepOne machine with default program. The reaction system contained 2X ABI Taqman master mix and 1 l of cDNA and 1 l of primer/probe set, adding water to total volume of 20 l. 18s rRNA was used as a internal control. Relative quantification (RQ) values are presented.
Immunostaining
Newborns P1 control rabbit kits (n=6) and with hypertonia (n=6) after H-I were anesthetized with Ketamine -Xylazine mixture on day 1 of life (P1) and transcardially perfused with saline, followed by 10% buffered formalin. The brains and spinal cord were removed, post-fixed and cryoprotected in 30% sucrose. Serial sections 40 μm thick were cut on a cryostat and mounted onto poly-lysine-coated slides (Sigma Aldrich, St Louis, MO, USA). The sections were blocked with 3% goat serum followed by incubation with the primary antibodies rat anti-serotonin (MAB352, Millipore, Billerica, MA, USA) for 48 hours at 4°C. Concentration of the anti-serotonin antibody was 1:50 for spinal cord and 1: 200 for forebrain sections. This was followed by incubation with biotinylated secondary goat anti-rat IgG antibodies (1:200; Vector, Burlingame, CA, USA) for 1 hour at room temperature and Avidin biotin complex for 1 hour. Color was developed using 3,3′-diaminobenzidine (Sigma Aldrich).
Stereological quantification of serotonergic fiber length in the spinal cord
The axonal projections stained with anti-serotonin antibodies were visualized under a microscope (Leica Microsystems, Wetzler, Germany) attached to a motorized stage (Ludl Electronic Products, Hawthorne, NY). Spherical probes were used to estimate the total length of serotonin-immunoreactive fibers in the spinal cord in Stereo-Investigator software (MBF Bioscience, Williston, VT, USA). Serotonin immunostaining produce strong signal and axon course is clearly visible and can be unambiguously reconstructed (Kannan et al. 2010). Serotonin-immunoreactive fibers were counted bilaterally in gray matter in dorsal and ventral horns separately in cervical (C1–C7) and lumbar expansions (L1–L4) for each kit on five 40-μm coronal sections that were 200 μm apart. The minimum average thickness of the sections post-processing was measured to be 15 μm and sampling grid of 250 × 250 μm2 was used. For all the samples, the virtual sphere was maintained at a radius of 10 μm and guard zones were 2 μm. The length of the serotonin-immunoreactive fibers inside the volume of 1 μm3 in the spinal cord was calculated by dividing the total length (L) by the total measured volume.
Stereological serotonergic cell count in caudal raphe nuclei
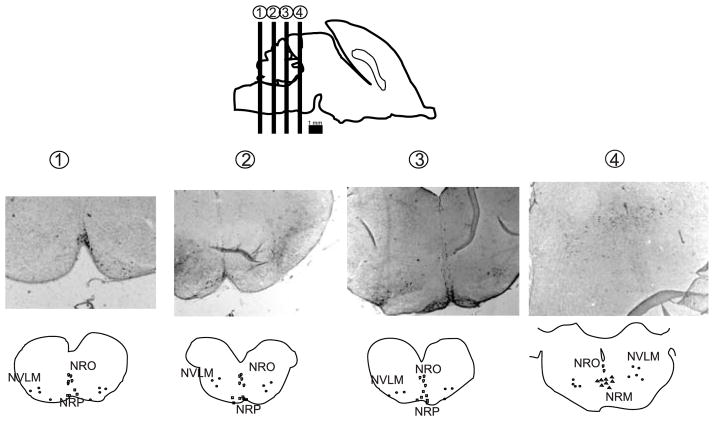
An optical fractionator probe in StereoInvestigator was used to obtain an unbiased estimate of the total number of serotonergic neurons in the caudal cluster of raphe nucleus, projecting to the spinal cord (Bowker et al. 1982). Boundaries of each nuclei in the caudal raphe nuclei cluster, consisting of nucleus raphe obscurus (NRO), nucleus raphe pallidus (NRP), nucleus raphe magnus (NRM), and caudal and rostral ventrolateral medulla (NVLM) were defined using anatomical landmarks and cell morphology as described previously (Bjarkam et al. 1997). The caudal raphe cluster spanned from hypoglossal nuclei in medulla, caudally, to inferior colliculus in caudal pons, rostrally, as illustrated in Figure 1. The boundaries of each raphe nuclei in each section were defined at × 2.5 magnification by drawing a contour around the majority of neurons stained with anti-serotonin antibody with compact aggregation. Diffusely stained and solitary neurons outside the boundaries were excluded from the count. The sections were further visualized under a 40x objective with a sampling grid 200 × 200 μm2, with uniform random sampling (X=500 and Y=500). The average section thickness ranged between 15 and 18 μm, and the guard zone was calculated as 2 μm with dissector height=10 μm. The total number of cells was determined by a stereological formula. The coefficient of error of the stereological estimation for each animal ranged from 0.05 to 0.15.
Figure 1.
Serotonin immunoreactive neuron cell bodies were counted in the caudal cluster of the raphe nuclei: nucleus raphe obscurus (NRO), nucleus raphe pallidus (NRP), nucleus raphe magnus (NRM), and caudal and rostral ventrolateral medulla (NVLM). Locations of the different nuclei in the caudal raphe are shown on histological sections and schematic drawings.
Manipulations of serotonin and laboratory muscle tone measurements
Solutions of non-selective 5-HT receptor antagonist methysegide, 80 μg 40μL (Hammond & Yaksh 1984) and serotonin hydrochloride, 100 μmol 40μL (Crick & Wallis 1991) (both drugs from Sigma Alrdich, St. Louis, MO, USA) dissolved in saline. These agents were slowly injected in the epidural space at L2–L3 lumbar region of non-sedated P2 kits. 27 kits after antenatal H-I with hypertonia of either forelimbs or hind limbs or both, or without hypertonia were randomly assigned into 3 groups, 9 kits each, (2 drug groups plus saline vehicle group). Pelvic bones were used as anatomical landmarks. Muscle tone measurements were performed twice for each kit: before and 20 min after intrathecal drug injection.
Muscle tone was measured by a custom torque-displacement apparatus (Drobyshevsky et al. 2012) in forelimbs and hind limbs of non-sedated kits on right side. Wrist, elbow, knee and ankle joint were passively stretched by 40–45 degrees range in sinusoidal motion 0.8 Hz frequency. Special provisions were made to avoid active muscle contraction and to ensure passive properties measurement. Joint stiffness (slope of torque-stretch angle curve) and averaged complex modulus (Lee et al. 2004) were derived as indexes of muscle tone. The technique provides quantitative, sensitive, and reproducible muscle tone measurements that were used to assess effect of drug manipulation on muscle tone. Results were sorted based on the first tone measurements by presence of hypertonia in individual limbs using a joint stiffness threshold of 0.09 g*cm/deg, obtained in naïve control P2 kits. Number of measured limbs was between 6 and 10 for each combination of drug/hypertonia groups.
Statistical analysis
Differences in mean values were tested using two-tailed t-test with Bonferroni correction for multiple comparisons or ANOVA with post-hoc Duncan group difference test. Differences with p-values less 0.05 were considered significant. Data are presented as means ± standard error of means.
RESULTS
Serotonin in the spinal cord is increased in hypertonic kits
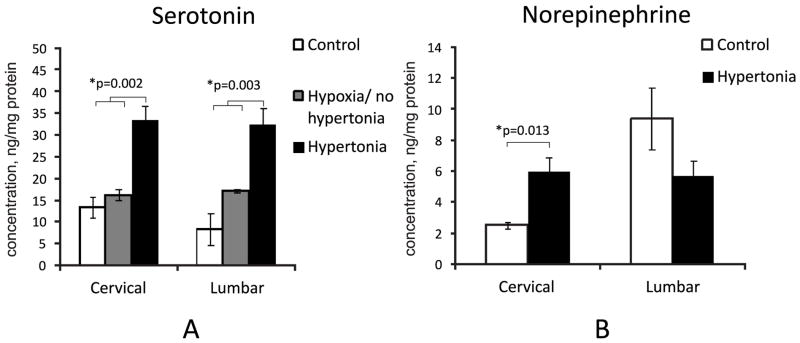
Serotonin concentration was significantly larger, by more than threefold, in P1 kits with hypertonia after antenatal H-I than in non-hypertonic and naïve controls (Figure 2A), both in cervical and lumbar expansions. There was also a significant increase in norepinephrine in the cervical spinal cord of hypertonic animals but not in the lumbar cord (Figure 2B).
Figure 2.
A Increased levels of serotonin in cervical and lumbar spinal cord in hypertonic P1 kits after E22 40′ H-I. B. Increased norepinephrine was found in cervical spinal cords in hypertonic P1 kits. Neurotransmitters were measured by electrochemical detection on HPLC.*-p<0.05 ANOVA
Measurements of neurotransmitters and neuromodulators in P1 control and rabbit kits after E22 H-I in cervical and lumbar spinal cord expansions are presented in Table 1. Serotonin content was significantly increased in cervical and lumbar spinal cord in kits with hypertonia of all limbs and with hypertonia of hind limbs only. In cases when forelimbs were unaffected and hind limbs affected, the increase of serotonin was larger in lumbar relative to cervical expansion. Similarly, norepinephrine was elevated in both groups of hypertonic kits, in cervical cord only. No kits with selective or predominant hypertonia of only forelimbs, although sometimes observed in this model, were included in the present study.
Table 1.
Concentrations of neurotransmitters in spinal cord of newborn P1 controls and rabbit kits after antenatal H-I measured with electrochemical detection HPLC and expressed in ng/mg of protein. Statistically significant differences from control values in corresponding segmental level are indicated in bold font (t-test with Bonferroni correction). Number of animals in control group n=7, hypoxia/no hypertonia n=5, hypertonia n=8 (including hypertonia all limbs n=4, hypertonia hind limbs only n =4). Data are presented as mean±SEM.
| Norepinephrine | Epinephrine | Dopamine | DOPAC | 5HT | HVA | |
|---|---|---|---|---|---|---|
| Cervical: control | 2.51±0.22 | 0.00±0.00 | 0.18±0.03 | 0.32±0.07 | 13.36±2.43 | 4.39±0.26 |
| Cervical: hypoxia/no hypertonia | 6.61±0.76 | 0.55±0.05 | 0.34±0.06 | 16.19±1.28 | 3.13±0.40 | |
| Cervical: hypertonia all limbs | 5.32±1.56 | 0.02±0.01 | 0.37±0.19 | 0.43±0.12 | 38.22±5.19 | 8.39±1.05 |
| Cervical: hypertonia hind limbs only | 4.83±0.84 | 0.01±0.01 | 0.44±0.10 | 0.30±0.09 | 28.72±2.45 | 5.31±1.00 |
| Lumbar: control | 9.37±2.49 | 3.95±1.39 | 0.13±0.06 | 0.09±0.06 | 8.30±3.70 | 0.89±0.08 |
| Lumbar: hypoxia/no hypertonia | 6.13±0.33 | 0.60±0.06 | 0.48±0.16 | 17.13±0.32 | 3.33±0.24 | |
| Lumbar: hypertonia all limbs | 5.82±2.07 | 7.54±5.02 | 0.20±0.08 | 0.24±0.13 | 31.56±7.38 | 3.79±1.51 |
| Lumbar: hypertonia hind limbs only | 5.52±0.79 | 0.01±0.01 | 0.60±0.06 | 0.22±0.03 | 33.21±3.92 | 6.00±0.93 |
Note: norepinephrine data are missing from the hypoxic/no hypertonia group due to inadvertent omission of standards during HPLC analysis
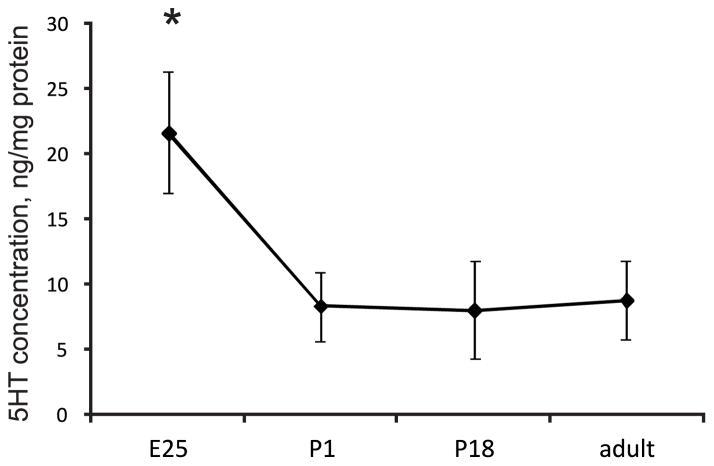
Serotonin in the spinal cord trends to decreases with postnatal development
To test whether abnormally high serotonin in the spinal cord in hypertonic kits can be attributed to a deviation from the normal pattern of maturation, we measured spinal neurotransmitters in naïve control animals during perinatal development and adults. Serotonin content in lumbar spinal cord decreased around term from E25 to P1 kits (Figure 3).
Figure 3.
Developmental decrease in serotonin in the lumbar spinal cord in control kits. *-p=0.044 ANOVA, E25 group differs from the rest of groups on post-hoc test.
Gene expression of serotonin receptors is decreased and serotonin transporter is increased in spinal cord of hypertonic kits
To examine further the physiological implications of abnormally high serotonin in the spinal cord in hypertonic kits, we looked at mRNA expression of 5-HTR2 receptors. These receptors are a subclass of 5-HT receptors that regulate excitability of motor neurons in spinal cord (Harvey et al. 2006). We found that the 5-HTR2 mRNA expression was not statistically different in cortex and midbrain but was decreased in spinal cord in hypertonic vs. control kits (Figure 4A). At the same time, the expression of serotonin transporter SERT, that transports the neurotransmitter serotonin from synaptic cleft back into presynaptic neurons and is a major regulator of serotonin concentration in synapses, was unchanged in cortex and midbrain but was increased in cervical and lumbar spinal cord of hypertonic animals (Figure 4B).
Figure 4.
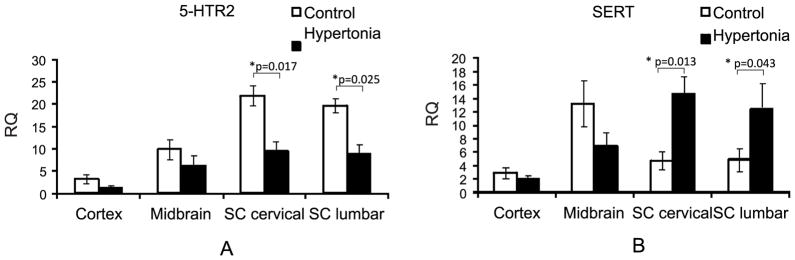
Gene expression of 5-HTR2 receptors (A) was decreased, while expression of serotonin transporter was increased (B) in P1 hypertonic kits after H-I vs. controls (n=5,5) determined by RT PCR. *- p<0.05, t-test control vs. hypertonia.
Serotonergic terminal density is increased in hypertonic kits’ spinal cord
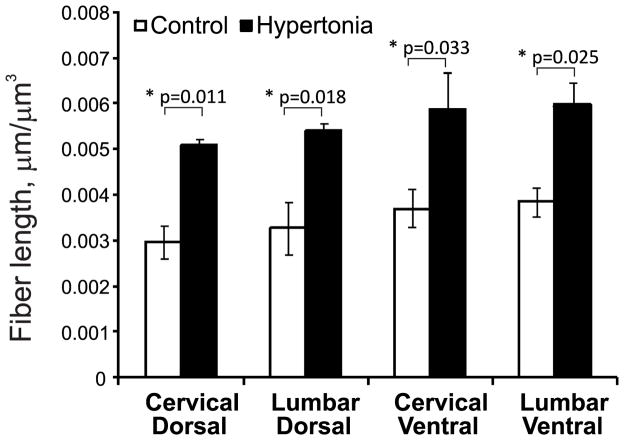
To examine whether the source of elevated serotonin in the spinal cord was neuronal or vascular in origin, we stained spinal cord sections with anti-serotonin antibody and performed quantitative analysis of serotonin-immunoreactive axons. Representative pictures of serotonergic fibers in control and hypertonic kits are presented on Figure 5. Serotonin immunoreactive fibers appeared as a fine mesh with beaded fibers. Stereological estimation revealed a significantly larger fiber length per unit of volume of serotonin-immunoreactive fibers in hypertonic kits in both dorsal and ventral horns, at both cervical and lumbar levels (Figure 6), compared to controls.
Figure 5.
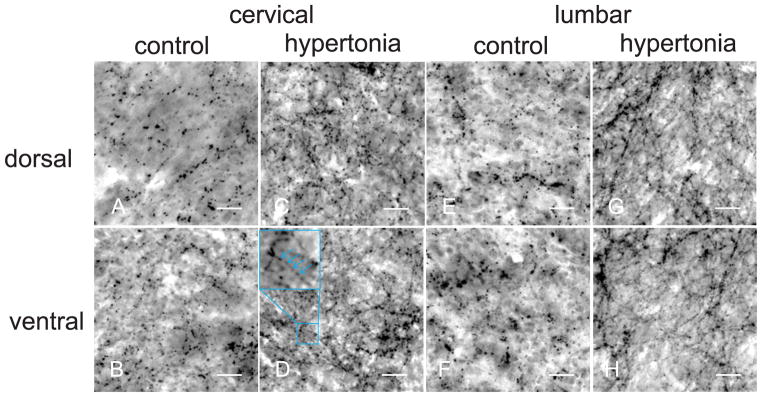
Serotonergic fibers in dorsal (first row) and ventral (second row) horns in spinal cord of control (A,C,E,G) and hypertonic kits (B,D,F,H). Density of serotonergic fibers was visibly higher in hypertonic group. Course of serotonergic fibers could be reconstructed following the beaded lines on anti-serotonin immunostaining. Objective 40x, scale bar 25 μm.
Figure 6.
Stereological estimation of serotonergic fiber length per unit of volume in spinal cord in P1 rabbit kits after E22 H-I. Serotonergic fiber length per unit of volume were longer in ventral and dorsal horns both in cervical and lumbar cords of hypertonic kits, estimated using spherical ball probe. *-p<0.05, t- test control vs. hypertonia
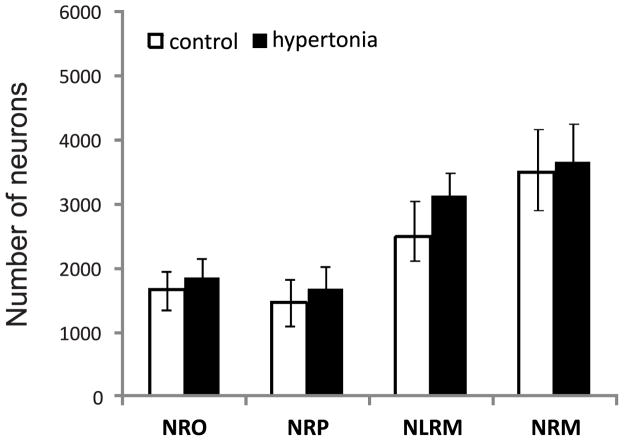
To elucidate whether the higher larger fiber length per unit of volume of spinal serotonergic fibers in hypertonic kits is due to an increase in the number of serotonergic brainstem neurons, we performed stereological counts of neurons in caudal clusters of the raphe nucleus, which project to the spinal cord (Bowker et al. 1982). Counts of serotonin immunoreactive neuron cell bodies in the caudal raphe are shown in Figure 7. No significant differences were found between control and hypoxia/hypertonia groups in ether NRO, NRP, NRLM or NRM nuclei.
Figure 7.
Stereological neuron counts of caudal raphe nuclei in P1 control and hypertonic kits after E22 H-I. NRO – nucleus raphe obscures, NRP – nucleus raphe pallidus, NRLM – ventrolateral medulla, NRM – nucleus raphe magnus.
Manipulations of serotonin
To explore physiological implications of alterations in serotonergic systems after antenatal H-I, we intrathecally administered solutions of nonselective serotonin receptor inhibitor methysergide, 5-HT, or saline vehicle to P2 kits after E22 antenatal H-I. Changes in muscle tone before and after the drugs administration were recorded using a torque –displacement apparatus. There was no significant change in tone from the baseline after injection of saline vehicle. Intrathecal administration of methysegide significantly decreased muscle tone in wrist, knee and ankle in hypertonic group relative to vehicle (saline) as shown on Figure 8A. It should be noted that there was large variation in response in kits with hypertonic ankle: 3 kits had dramatic decrease of tone > 0.3 g*cm/deg, while 3 kits had no or little change. There were no significant changes in non-hypertonic joints.
Figure 8.
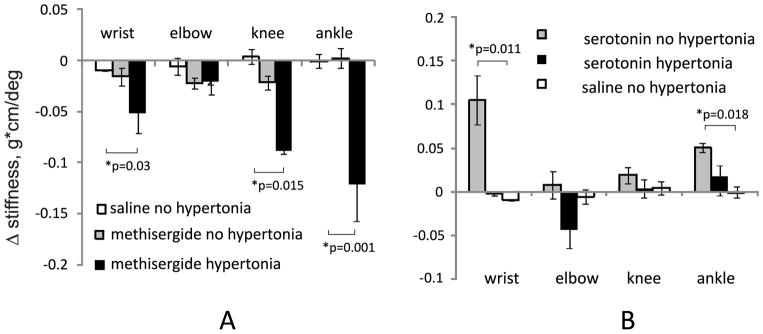
Muscle tone change after intrathecal administration of methysergide (A) or serotonin (B) in P2 kits after antenatal H-I. Muscle tone was measured using a torque-displacement apparatus, before and 20 min after the drug administration. *-p<0.05, t-test vs. saline vehicle group
We next tested whether H-I had changed the conditions for getting hypertonia and whether the absence of hypertonia in H-I kits could be explained by a relative deficiency of serotonin. Intrathecal administration of serotonin significantly increased muscle tone only in non-hypertonic wrists and ankles relative to saline vehicle (Figure 8B).
DISCUSSION
The main finding of the study is the link between elevated levels of serotonin in the spinal cord and hypertonia of newborn rabbit kits following antenatal global hypoxia-ischemia at E22 (Figure 2). Based on the results of the current study, we have to reformulate our original thinking. Increased and not decreased serotonin in the spinal cord may contribute to muscle hypertonia in newborns. This constitutes a novel pathophysiological mechanism for the development of motor deficits in CP: This hypothesis is supported by a large increase in lumbar serotonin in kits with hypertonia in only hind limbs (Table 1). Increased levels of serotonin in the spinal cord were associated with a larger amount of serotonergic projections in both dorsal and ventral horns at cervical and lumbar levels (Figures 5 and 6), suggesting that the origin of the elevated serotonin in the spinal cord is neuronal and not vascular. There was an associated finding of elevated norepinephrine in the cervical spinal cord alone.
In contrast to the spinal cord where elevated serotonin was observed in the current study, we had previously shown decreased serotonin levels in the brain in our antenatal H-I model (Vasquez-Vivar et al. 2009). In an endotoxin inflammatory perinatal rabbit model and using an enzyme-linked immunosorbent assay, decreased serotonin was found in cortical and hippocampal brain homogenates of P1 rabbit kits following lipopolysaccharide exposure at E28 (Kannan et al. 2010) of cerebral palsy in rabbits. Similar decreases in cortical serotonin have been reported in a rat model of perinatal H-I (Buller et al. 2012). In this model, a selective, significant decrease in numbers of 5-HT-positive neurons was noted in rostral raphe nuclei (dorsal raphe), while there was no change in the caudal raphe nuclei projecting to spinal cord, such as raphe magnus (Reinebrant et al. 2010), similar to observation in a mouse model of neonatal asphyxia (Takeuchi et al. 1992). Recently, increased levels of spinal serotonin, similar to our study, have been reported after unilateral carotid ligation and hypoxia in neonatal mice (Bellot et al. 2014). Rodent models of perinatal brain injury, however, do not produce overt motor deficits and muscle hypertonia as in our rabbit model. A limitation of this study, as well as the cited studies, is that the neurotransmitter levels represent total tissue content, and may not reflect changes at the synaptic cleft.
One implication of our findings is that H-I could stimulate a proliferation of neurons in the caudal raphe which project to the spinal cord. The speculation is that this in turn could increase serotonin concentrations in the spinal cord. However, the absence of any difference between H-I and control animals in numbers of serotonergic neurons in the caudal raphe does not support this contention. An alternative explanation for the larger fiber length per unit of volume of serotonergic projections and higher levels of serotonin in the spinal cord could involve altered maturation of sensory-motor networks in spinal cord due to decreased cortico-spinal connectivity after antenatal H-I. This hypothesis is supported by the normal trend during maturation towards decreasing levels of serotonin in the spinal cord (Figure 3). It has been shown previously that during normal perinatal development there is a transient increase, followed by a decrease, in spinal serotonergic projections (Okado et al. 1992). It has been suggested that this process is regulated by the functional activity of descending cortico-spinal projections that mature during this same period. Similar processes also occur when the arrival of descending pathways to the spinal cord brings about a reorganization and paring back of sensory afferents, while injury to corticospinal tracts causes abnormal expansion of sensory afferents (Clowry 2007). The development of descending pathways and proprioceptive afferents is tightly coordinated (Chakrabarty & Martin 2011). Spinal serotonergic projections may be regulated and pared back postnatally by similar mechanisms. We have also shown that injury to descending corticospinal fibers takes place after antenatal H-I (Drobyshevsky et al. 2007) and this injury may decrease the number of serotonergic projections to the spinal cord. Further work will elucidate whether observed abnormal changes in spinal neurotransmitters are transient (Rajaofetra et al. 1989) and whether such abnormalities in maturation in the spinal cord are associated with decreased corticospinal efferent control (Drobyshevsky et al. 2013) after antenatal H-I injury to the brain and possibly to spinal cord.
The physiological implications of altered spinal monoamine neurotransmitters in the rabbit model of CP are unclear, considering the different effects of monoamines in ventral and dorsal spinal cord (Heckman et al. 2009). We found increased serotonergic fiber larger fiber length per unit of volume both in dorsal and ventral horns, though the increase in the latter appeared to be larger (Figures 5, 6). Raphe pallidus and raphe obscurus project predominantly to the ventral horn, hypothetically acting as a gain-setting system, while raphe magnus projects to the dorsal horn, regulating sensory transmission (Holstege & Kuypers 1987). Therefore increased serotonin in the spinal cord may affect spinal motor circuits in multiple ways. The physiological relevance of the observed disturbances in monoamines levels, serotonin transporters, and receptors in hypertonic kits was confirmed by the differential effect of serotonin and serotonin receptor inhibitors on muscle tone. Intrathecal application of non-selective serotonin receptor inhibitor methysergide decreased muscle tone only in hypertonic limbs and not in non-hypertonic kits. This suggests that increased serotonin is important for pathological increases in muscle tone and not the maintenance of normal tone. This was confirmed by the increased muscle tone found only in non-hypertonic limbs by serotonin application (Figure 8B). However, the large variability in serotonin levels and changes in muscle tone with serotonin modulation (Figure 8), suggests that this explanation may be too simple for the causation of postnatal hypertonia in the rabbit model of cerebral palsy. Elevated levels of norepinephrine after H-I may also play a role in muscle hypertonia that could be elucidated with specific antagonists. At a minimum, we cautiously conclude that disturbances in serotonergic pathways in spinal cord may play a key role in regulation of abnormal muscle tone after antenatal H-I. The reasons for the observed increase in SERT and decrease in 5-HTR2 gene expression in the current study are currently unclear and may constitute adaptive changes to the elevated serotonin concentration. The changes in serotonin receptors and transporter after antenatal H-I occurred in the opposite direction to those observed in spinal cord injury (Lee et al. 2007), suggesting that mechanism of hypertonia and spasticity in CP may be different from direct spinal cord injury. Further studies are needed to elucidate the complex effect of elevated spinal monoamines on the properties of motor neurons and spinal reflexes, and help identify potential therapeutic targets.
Abnormalities in spinal neurotransmitters in the early period of CP evolution after the brain injury has great clinical significance, because in human infants this period often lasts weeks to months before the development of hypertonia. Rabbits develop hypertonia more quickly after birth than human infants, and therefore we cannot exclude an inter species difference in the mechanisms. If found to be true for humans, the proposed novel pathophysiological mechanism of hypertonia after antenatal H-I due to the abnormal increases in serotonin in the spinal cord opens new therapeutic targets and avenues for intervention. Ability to prevent hypertonia and the aberrant increase in spinal excitability (Sanger 2003) would greatly facilitate efficiency of the rehabilitation interventions and improve motor recovery.
CONCLUSION
Elevated levels of serotonin in the spinal cord were linked to hypertonia of newborn rabbit kits following antenatal global hypoxia-ischemia at E22. The neuroanatomic, biochemical and physiological evidences suggest a novel pathophysiological mechanism of increased muscle tone in CP.
Acknowledgments
NIH NS 43285(ST), NS051402 (ST), FAPESP São Paulo Research Foundation, Brazil (AD).
Abbreviations
- CP
cerebral palsy
- H-I
hypoxia-ischemia
- 5-HT
serotonin
Footnotes
The authors have no conflicts of interest to declare.
References
- Bellot B, Peyronnet-Roux J, Gire C, Simeoni U, Vinay L, Viemari JC. Deficits of brainstem and spinal cord functions after neonatal hypoxia-ischemia in mice. Pediatr Res. 2014;11:42. doi: 10.1038/pr.2014.42. [DOI] [PubMed] [Google Scholar]
- Bjarkam CR, Sørensen JC, Geneser FA. Distribution and morphology of serotonin-immunoreactive neurons in the brainstem of the New Zealand white rabbit. The Journal of Comparative Neurology. 1997;380:507–519. [PubMed] [Google Scholar]
- Bowker RM, Westlund KN, Sullivan MC, Coulter JD. Organization of descending serotonergic projections to the spinal cord. Prog Brain Res. 1982;57:239–265. doi: 10.1016/S0079-6123(08)64132-1. [DOI] [PubMed] [Google Scholar]
- Buller KM, Wixey JA, Reinebrant HE. Disruption of the serotonergic system after neonatal hypoxia-ischemia in a rodent model. Neurol Res Int. 2012;2012:650382. doi: 10.1155/2012/650382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty S, Martin JH. Co-development of proprioceptive afferents and the corticospinal tract within the cervical spinal cord. Eur J Neurosci. 2011;34:682–694. doi: 10.1111/j.1460-9568.2011.07798.x. [DOI] [PubMed] [Google Scholar]
- Clowry GJ. The dependence of spinal cord development on corticospinal input and its significance in understanding and treating spastic cerebral palsy. Neuroscience & Biobehavioral Reviews. 2007;31:1114–1124. doi: 10.1016/j.neubiorev.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Crick H, Wallis DI. Inhibition of reflex responses of neonate rat lumbar spinal cord by 5-hydroxytryptamine. Br J Pharmacol. 1991;103:1769–1775. doi: 10.1111/j.1476-5381.1991.tb09861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentel C, Palamiuc L, Henriques A, et al. Degeneration of serotonergic neurons in amyotrophic lateral sclerosis: a link to spasticity. Brain. 2013;136:483–493. doi: 10.1093/brain/aws274. [DOI] [PubMed] [Google Scholar]
- Derrick M, Luo NL, Bregman JC, et al. Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: a model for human cerebral palsy? J Neurosci. 2004;24:24–34. doi: 10.1523/JNEUROSCI.2816-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyshevsky A, Derrick M, Luo K, Zhang LQ, Wu YN, Takada SH, Yu L, Tan S. Near-term fetal hypoxia-ischemia in rabbits: MRI can predict muscle tone abnormalities and deep brain injury. Stroke. 2012;43:2757–2763. doi: 10.1161/STROKEAHA.112.653857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyshevsky A, Derrick M, Wyrwicz AM, Ji X, Englof I, Ullman LM, Zelaya ME, Northington FJ, Tan S. White matter injury correlates with hypertonia in an animal model of cerebral palsy. J Cereb Blood Flow Metab. 2007;27:270–281. doi: 10.1038/sj.jcbfm.9600333. [DOI] [PubMed] [Google Scholar]
- Drobyshevsky A, Jiang R, Lin L, Derrick M, Luo K, Back SA, Tan S. Unmyelinated White Matter Injury After Fetal Hypoxia Is Associated With Postnatal Hypertonia. Ann Neurol. 2013 doi: 10.1002/ana.24115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond DL, Yaksh TL. Antagonism of stimulation-produced antinociception by intrathecal administration of methysergide or phentolamine. Brain Res. 1984;298:329–337. doi: 10.1016/0006-8993(84)91432-x. [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol. 2006;96:1158–1170. doi: 10.1152/jn.01088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Mottram C, Quinlan K, Theiss R, Schuster J. Motoneuron excitability: The importance of neuromodulatory inputs. Clin Neurophysiol. 2009;120:2040–2054. doi: 10.1016/j.clinph.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege JC, Kuypers HGJM. Brainstem projections to spinal motoneurons: An update. Neuroscience. 1987;23:809–821. doi: 10.1016/0306-4522(87)90160-6. [DOI] [PubMed] [Google Scholar]
- Hoon AH, Jr, Stashinko EE, Nagae LM, et al. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol. 2009;51:697–704. doi: 10.1111/j.1469-8749.2009.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S, Saadani-Makki F, Balakrishnan B, Dai H, Chakraborty PK, Janisse J, Muzik O, Romero R, Chugani DC. Decreased cortical serotonin in neonatal rabbits exposed to endotoxin in utero. J Cereb Blood Flow Metab. 2010 doi: 10.1038/jcbfm.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Chen JJ, Ju MS, Lin CC, Poon PP. Validation of portable muscle tone measurement device for quantifying velocity-dependent properties in elbow spasticity. J Electromyogr Kinesiol. 2004;14:577–589. doi: 10.1016/j.jelekin.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Lee JK, Johnson CS, Wrathall JR. Up-regulation of 5-HT2 receptors is involved in the increased H-reflex amplitude after contusive spinal cord injury. Exp Neurol. 2007;203:502–511. doi: 10.1016/j.expneurol.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, et al. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med. 2010;16:694–700. doi: 10.1038/nm.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okado N, Sako H, Homma S, Ishikawa K. Development of serotoninergic system in the brain and spinal cord of the chick. Prog Neurobiol. 1992;38:93–123. doi: 10.1016/0301-0082(92)90036-e. [DOI] [PubMed] [Google Scholar]
- Pakula AT, Van Naarden Braun K, Yeargin-Allsopp M. Cerebral palsy: classification and epidemiology. Phys Med Rehabil Clin N Am. 2009;20:425–452. doi: 10.1016/j.pmr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Perlman JM. Summary proceedings from the neurology group on hypoxic-ischemic encephalopathy. Pediatrics. 2006;117:S28–33. doi: 10.1542/peds.2005-0620E. [DOI] [PubMed] [Google Scholar]
- Rajaofetra N, Sandillon F, Geffard M, Privat A. Pre- and post-natal ontogeny of serotonergic projections to the rat spinal cord. J Neurosci Res. 1989;22:305–321. doi: 10.1002/jnr.490220311. [DOI] [PubMed] [Google Scholar]
- Reinebrant HE, Wixey JA, Buller KM. Neonatal hypoxia-ischaemia disrupts descending neural inputs to dorsal raphe nuclei. Neuroscience. 2013;248C:427–435. doi: 10.1016/j.neuroscience.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Reinebrant HE, Wixey JA, Gobe GC, Colditz PB, Buller KM. Differential effects of neonatal hypoxic-ischemic brain injury on brainstem serotonergic raphe nuclei. Brain Res. 2010;1322:124–133. doi: 10.1016/j.brainres.2010.01.065. [DOI] [PubMed] [Google Scholar]
- Sanger TD. Pathophysiology of Pediatric Movement Disorders. J Child Neurol. 2003;18:S9–S24. doi: 10.1177/0883073803018001S0401. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Fujiwara K, Ishimura K, Aoki S, Okano S, Yamazoe I, Yoshioka H, Sawada T. Effects of neonatal asphyxia on the serotonin neuron system in the developing brain studied by immunohistochemistry. Dev Neurosci. 1992;14:394–402. doi: 10.1159/000111688. [DOI] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Whitsett J, Derrick M, Ji X, Yu L, Tan S. Tetrahydrobiopterin in the prevention of hypertonia in hypoxic fetal brain. Ann Neurol. 2009;66:323–331. doi: 10.1002/ana.21738. [DOI] [PMC free article] [PubMed] [Google Scholar]