Abstract
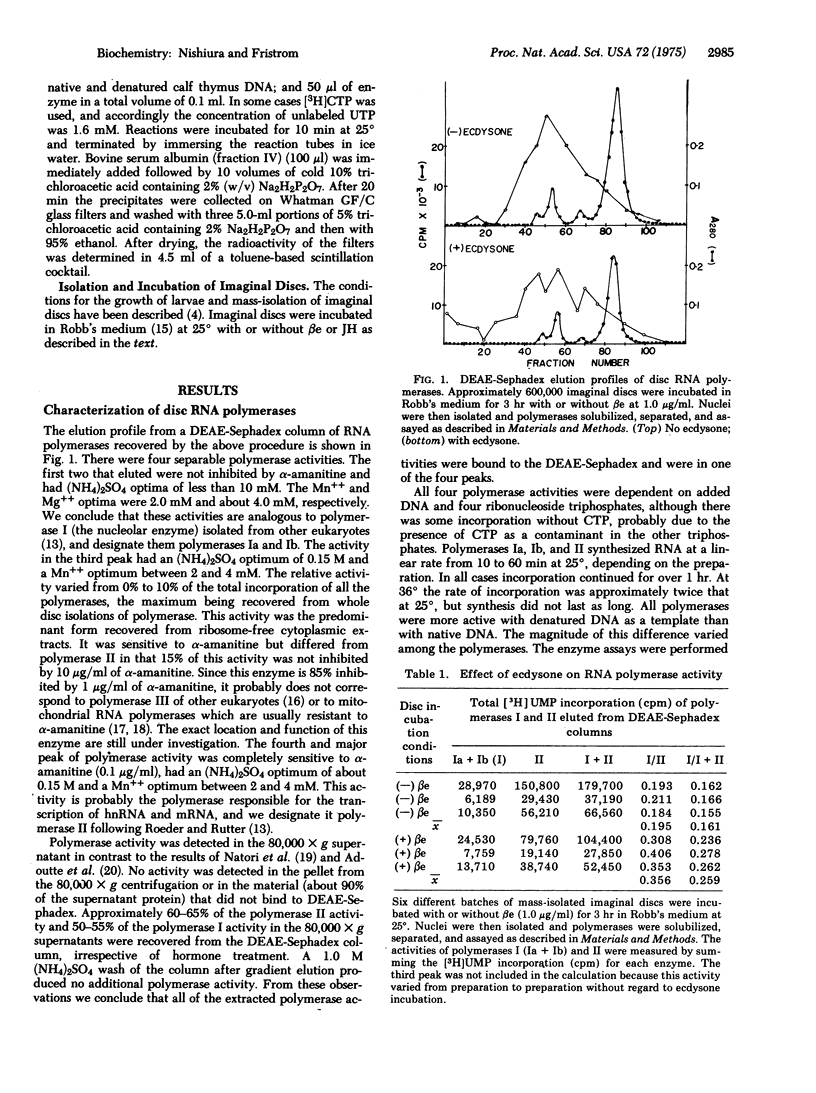
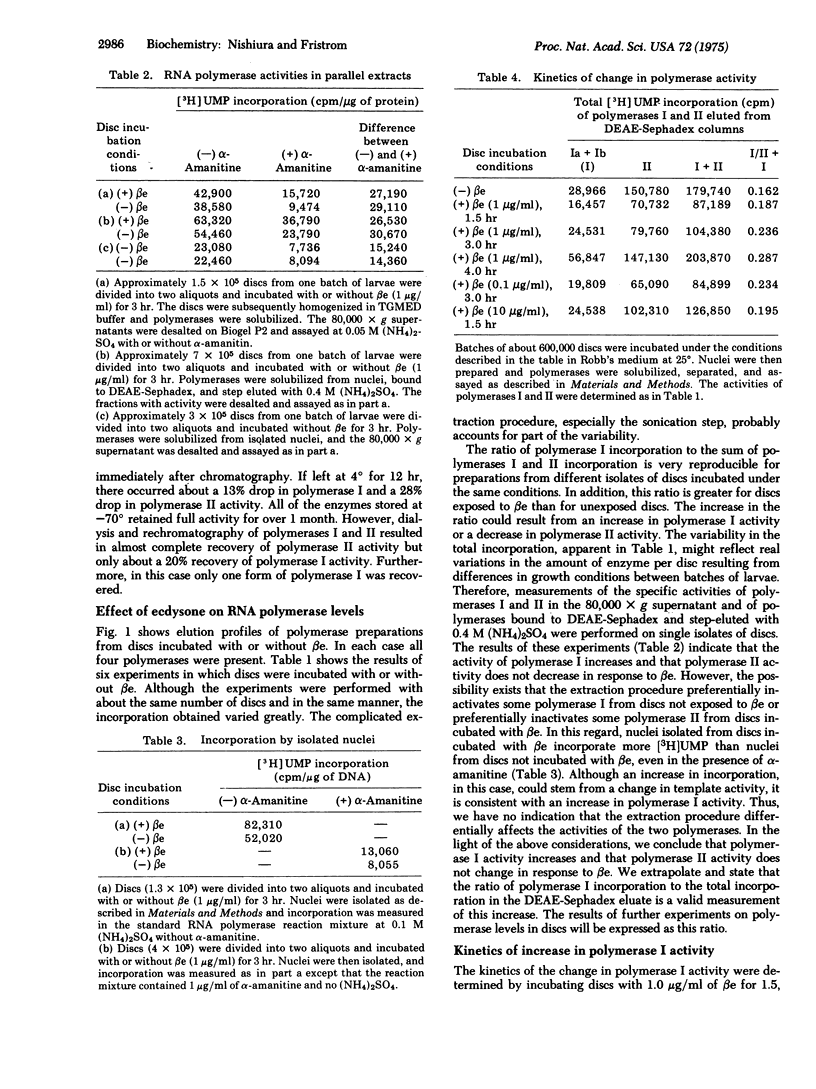
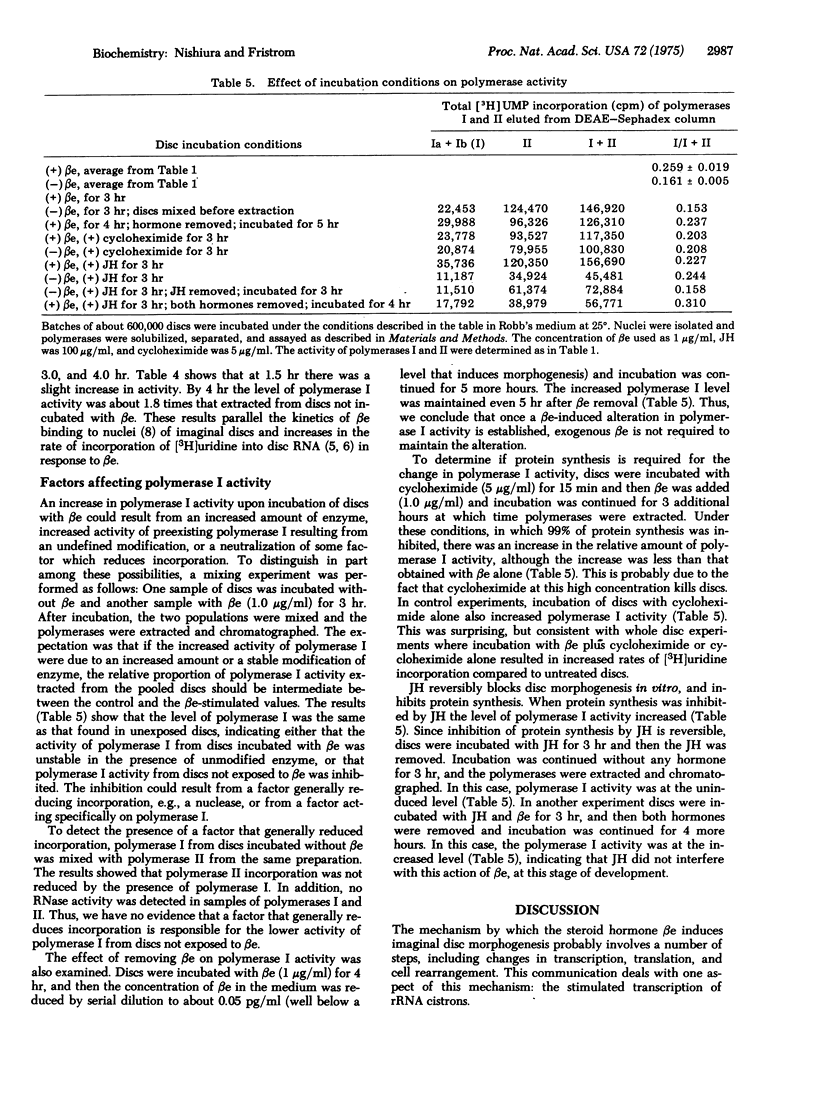
Four chromatographically separable DNA-dependent RNA polymerases (nucleosidetriphosphate:RNA nucleotidyltransferase; EC 2.7.7.6) were partially purified from imaginal discs of Drosophila melanogaster. Their properties are similar to those described for RNA polymerases I and II isolated from other eukaryotes. In vitro incubation of discs with beta-ecdysone, juvenile hormone, or cycloheximide resulted in increased activity of RNA polymerase I. The increase was irreversible with beta-ecdysone incubation and removal but reversible with juvenile hormone incubation and removal. With beta-ecdysone, the rate of the increase in polymerase I activity paralleled the kinetics of ecdysone binding to discs and increases in the rate of precursor incorporation into RNA. A model to explain the increased acticity of RNA polymerase I is presented.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adoutte A., Clément J. M., Hirshbein L. Two methods of extraction and partial purification of Drosophila melanogaster RNA polymerase. Biochimie. 1974;56(3):335–340. doi: 10.1016/s0300-9084(74)80140-9. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Patterns of puffing activity in the salivary gland chromosomes of Drosophila. VI. Induction by ecdysone in salivary glands of D. melanogaster cultured in vitro. Chromosoma. 1972;38(3):255–281. doi: 10.1007/BF00290925. [DOI] [PubMed] [Google Scholar]
- Beebee T. J., Bond R. P. Effect of the exotoxin of Bacillus thuringiensis on normal and ecdysone-stimulated ribonucleic acid polymerase activity in intact nuclei from the fat-body of Sarcophaga bullata larvae. Biochem J. 1973 Sep;136(1):1–7. doi: 10.1042/bj1360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst D. W., Bollenbacher W. E., O'Connor J. D., King D. S., Fristrom J. W. Ecdysone levels during metamorphosis of Drosophila melanogaster. Dev Biol. 1974 Aug;39(2):308–316. doi: 10.1016/0012-1606(74)90242-5. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Chihara C. J., Fristrom J. W. Effects and interactions of juvenile hormone and beta-ecdysone on Drosophila imaginal discs cultured in vitro. Dev Biol. 1973 Nov;35(1):36–46. doi: 10.1016/0012-1606(73)90005-5. [DOI] [PubMed] [Google Scholar]
- Clever U., Romball C. G. RNA and protein synthesis in the cellular response to a hormone, ecdysone. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1470–1476. doi: 10.1073/pnas.56.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congote L. F., Sekeris C. E., Karlson P. On the mechanism of hormone action. 13. Stimulating effects of ecdysone, juvenile hormone and ions on RNA synthesis in fat body cell nuclei from Calliphora erythrocephala isolated by a filtration technique. Exp Cell Res. 1969 Aug;56(2):338–346. doi: 10.1016/0014-4827(69)90023-8. [DOI] [PubMed] [Google Scholar]
- Fristrom J. W., Gregg T. L., Siegel J. The effect of beta-ecdysone on protein synthesis in imaginal discs of Drosophila melanogaster cultured in vitro. Dev Biol. 1974 Dec;41(2):301–313. doi: 10.1016/0012-1606(74)90308-x. [DOI] [PubMed] [Google Scholar]
- Fristrom J. W., Logan W. R., Murphy C. The synthetic and minimal culture requirements for evagination of imaginal discs of Drosophila melanogaster in vitro. Dev Biol. 1973 Aug;33(2):441–456. doi: 10.1016/0012-1606(73)90149-8. [DOI] [PubMed] [Google Scholar]
- Glasser S. R., Chytil F., Spelsberg T. C. Early effects of oestradiol-17 on the chromatin and activity of the deoxyribonucleic acid-dependent ribonucleic acid polymerases (I and II) of the rat uterus. Biochem J. 1972 Dec;130(4):947–957. doi: 10.1042/bj1300947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold M. D., Cohen P. P. Thyroxine-mediated control of ribonucleic acid polymerase activity in liver of Rana catesbeiana. J Biol Chem. 1973 Aug 25;248(16):5854–5860. [PubMed] [Google Scholar]
- Hamilton T. H. Control by estrogen of genetic transcription and translation. Binding to chromatin and stimulation of nucleolar RNA synthesis are primary events in the early estrogen action. Science. 1968 Aug 16;161(3842):649–661. doi: 10.1126/science.161.3842.649. [DOI] [PubMed] [Google Scholar]
- Hamilton T. H., Widnell C. C., Tata J. R. Sequential stimulations by oestrogen of nuclear RNA synthesis and DNA-dependent RNA polymerase activities in rat uterus. Biochim Biophys Acta. 1965 Sep 6;108(1):168–172. doi: 10.1016/0005-2787(65)90129-2. [DOI] [PubMed] [Google Scholar]
- Hanly E. W., Stewart D. J. Separation and characterization of some ribonucleic acids from imaginal discs of Drosophila melanogaster. Mol Gen Genet. 1972;117(4):281–302. doi: 10.1007/BF00333023. [DOI] [PubMed] [Google Scholar]
- Hesselbach B. A., Yamada Y., Nakada D. Isolation of an inhibitor protein of E. coli RNA polymerase from T7 phage infected cell. Nature. 1974 Nov 1;252(5478):71–74. doi: 10.1038/252071b0. [DOI] [PubMed] [Google Scholar]
- Kagawa H., Egberts E. New inhibitors of FNA synthesis in vitro isolated from Escherichia coli. Eur J Biochem. 1974 Nov 1;49(1):293–303. doi: 10.1111/j.1432-1033.1974.tb03834.x. [DOI] [PubMed] [Google Scholar]
- Küntzel H., Schäfer K. P. Mitochondrial RNA polymerase from Neurospora crassa. Nat New Biol. 1971 Jun 30;231(26):265–269. doi: 10.1038/newbio231265a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luck D. N., Hamilton T. H. Early estrogen action: stimulation of the metabolism of high molecular weight and ribosomal RNAs (estradiol-17 -RNA isolation-uterus-ribosomal RNA precursors-actinomycin D). Proc Natl Acad Sci U S A. 1972 Jan;69(1):157–161. doi: 10.1073/pnas.69.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadik S. P., Dharmgrongartama B., Srinivasan P. R. An inhibitory protein of Escherichia coli RNA polymerase in bacteriophage T3-infected cells (core polymerase-sigma factor-host polymerase-phage polymerase-initiation). Proc Natl Acad Sci U S A. 1972 Jan;69(1):162–166. doi: 10.1073/pnas.69.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainwaring W. I., Mangan F. R., Peterken B. M. Studies on the solubilized ribonucleic acid polymerase from rat ventral prostate gland. Biochem J. 1971 Jul;123(4):619–628. doi: 10.1042/bj1230619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson I. S., Anderson K. M. Rat mammary gland nuclear RNA polymerases in late pregnancy and lactation. Biochim Biophys Acta. 1973 Apr 11;299(4):576–587. doi: 10.1016/0005-2787(73)90230-x. [DOI] [PubMed] [Google Scholar]
- Natori S., Takeuchi K., Mizuno D. DNA dependent RNA polymerase from ehrlich ascites tumor cells. IV. A novel protein repressing RNA polymerase II. J Biochem. 1974 Aug;76(2):263–270. doi: 10.1093/oxfordjournals.jbchem.a130568. [DOI] [PubMed] [Google Scholar]
- Phillips J. P., Forrest H. S. Deoxyribonucleic acid-dependent ribonucleic acid polymerase from Drosophila melanogaster embryos. J Biol Chem. 1973 Jan 10;248(1):265–269. [PubMed] [Google Scholar]
- Ponta H., Rahmsdorf H. J., Pai S. H., Herrlich P., Schweiger M. Control of gene expression in bacteriophage T7. Isolation of a new control protein and mechanism of action. Mol Gen Genet. 1974;134(1):29–38. doi: 10.1007/BF00332810. [DOI] [PubMed] [Google Scholar]
- Raikow R., Fristrom J. W. Effects of -ecdysone on RNA metabolism of imaginal disks of Drosophila melanogaster. J Insect Physiol. 1971 Sep;17(9):1599–1614. doi: 10.1016/0022-1910(71)90056-4. [DOI] [PubMed] [Google Scholar]
- Rieber M., Weinstein-Schonfeld F. Appearance of an inhibitor of RNA polymerase activity in thermophilic Mycobacterium phlei exposed to sublethal temperatures. Biochim Biophys Acta. 1974 Aug 29;361(2):236–240. doi: 10.1016/0005-2787(74)90351-7. [DOI] [PubMed] [Google Scholar]
- Robb J. A. Maintenance of imaginal discs of Drosophila melanogaster in chemically defined media. J Cell Biol. 1969 Jun;41(3):876–885. doi: 10.1083/jcb.41.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature. 1969 Oct 18;224(5216):234–237. doi: 10.1038/224234a0. [DOI] [PubMed] [Google Scholar]
- Segall J., Tjian R., Pero J., Losick R. Chloramphenicol restores sigma factor activity to sporulating Bacillus subtilis. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4860–4863. doi: 10.1073/pnas.71.12.4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. G., Fristrom J. W. The effect of beta-ecdysone on protein synthesis in imaginal discs of Drosophila melanogaster cultured in vitro. Dev Biol. 1974 Dec;41(2):314–330. doi: 10.1016/0012-1606(74)90309-1. [DOI] [PubMed] [Google Scholar]
- Tsai M. J., Michaelis G., Criddle R. S. DNA-dependent RNA polymerase from yeast mitochondria. Proc Natl Acad Sci U S A. 1971 Feb;68(2):473–477. doi: 10.1073/pnas.68.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDNELL C. C., TATA J. R. Stimulation of nuclear RNA polymerase during the latent period of action of thyroid hormones. Biochim Biophys Acta. 1963 Jul 30;72:506–508. [PubMed] [Google Scholar]
- Weinmann R., Roeder R. G. Role of DNA-dependent RNA polymerase 3 in the transcription of the tRNA and 5S RNA genes. Proc Natl Acad Sci U S A. 1974 May;71(5):1790–1794. doi: 10.1073/pnas.71.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F. L., Feigelson P. Cortisone stimulation of nucleolar RNA polymerase activity. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2177–2180. doi: 10.1073/pnas.68.9.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]



