Abstract
Restoration of neuronal functions by outgrowths regenerating at ~1mm/d from the proximal stumps of severed peripheral nerves takes many weeks or months, if it occurs at all, especially after ablation of nerve segments. Distal segments of severed axons typically degenerate in 1–3 days. The purpose of this study was to show that Wallerian degeneration could be prevented or retarded and lost behavioral function restored following ablation of 0.5 – 1 cm segments of rat sciatic nerves in host animals. This is achieved using 0.8 – 1.1cm microsutured donor allografts treated with bioengineered solutions varying in ionic and polyethylene glycol (PEG) concentrations (modified PEG-fusion procedure), being careful not to stretch any portion of donor or host sciatic nerves. Our data show that PEG-fusion permanently restores axonal continuity within minutes as initially assessed by action potential conduction and intracellular diffusion of dye. Behavioral functions mediated by the sciatic nerve are largely restored within 2 – 4 wk as measured by the Sciatic Functional Index (SFI). Increased restoration of sciatic behavioral functions after ablating 0.5 – 1 cm segments is associated with greater numbers of viable myelinated axons within, and distal to, PEG-fused allografts. Many such viable myelinated axons are almost-certainly spared from Wallerian degeneration by PEG-fusion. PEG-fusion of donor allografts may produce a paradigm-shift in the treatment of peripheral nerve injuries.
Keywords: axotomy, axonal regeneration, polyethylene glycol, Wallerian degeneration
Introduction
Behavioral recovery after severing PNS axons in mammals and invertebrates
Rapid and effective repair of peripheral nerve injuries to restore lost behavioral functions, especially after ablation of ≥ 0.5 – 2 cm segments, remains an unattained goal of clinicians and neuroscientists (Birch et al., 1998; Allan, 2000; Bittner et al., 2000, 2012; Campbell, 2008).
Severed mammalian peripheral axons naturally regenerate by slow (~1mm/d) outgrowths from severed proximal stumps while their severed distal stumps usually degenerate within 1 – 3d (Wallerian degeneration) (Ramon y Cajal, 1928; Campbell, 2008). Regenerating axons often do not remake functional connections after a simple transection, and behavioral recovery is rarely fully restored. Even the best current techniques to re-appose cut ends by microsutures through the epineurium or tubular conduits do not re-establish axonal continuity, produce rapid functional recovery or prevent Wallerian degeneration (Campbell, 2008). Furthermore, behavioral recovery is much worse and sometimes non-existent if the cut ends cannot be directly re-apposed after loss of a segment of a PNS nerve in humans (Allan, 2000) and rats (Bittner et al., 2012). In contrast to the slow and often inadequate regeneration-repair of severed mammalian peripheral nerves, severed PNS (and some CNS) axons in many phyla naturally, rapidly and effectively restore lost behaviors by outgrowths from proximal stumps that functionally activate or specifically fuse with their own surviving distal axonal segments so that the two severed axonal halves function as one continuous axon within days after lesioning (Hoy et al., 1967; reviewed by Bittner et al., 2000).
Development of PEG-fusion procedures to repair singly crushed or cut axons
Polyethylene glycol (PEG) as an artificial membrane fusogen was initially used to rapidly and permanently reconnect (PEG-fuse) the severed proximal and distal ends of giant invertebrate axons (Krause and Bittner, 1990; Lore et al, 1999). We and others subsequently described cellular pathways that induce sealing (repair) of plasmalemmal holes or axonal transections and identified many substances such as calcium and methylene blue that promote or inhibit, respectively, vesicle formation and membrane repair (Krause et al., 1994; Steinhardt et al, 1994; Spaeth et al, 2012). Consequently, PEG-fusion success was greatly enhanced if the cut ends were opened and vesicle formation at cut ends inhibited by calcium-free hypotonic salines containing methylene blue or melatonin (Krause and Bittner, 1990; Lore et al, 1999; Britt et al., 2010).
A PEG-fusion technique consisting of a well-defined sequence of bioengineered solutions could rapidly and permanently (12 weeks postoperatively) restore up to 80% of lost behavioral function as measured by the Sciatic Functional Index (SFI) in rats with singly crushed sciatic nerves severed and repaired in calcium-free salines (Britt et al, 2010). By using microsutures to closely appose cut axonal ends that separate by several mm, lost behavioral function could be similarly restored when all procedures were again performed in calcium-free salines (Bittner et al., 2012). Sciatic nerves cut in calcium-containing solutions or extracellular fluids could also be repaired, although the animals were only tested for action potential through-conduction immediately postoperatively (Bittner et al., 2012) or followed for 1 week behaviorally (Rodriguez-Feo et al., 2013). In the course of these procedures, we noted that careful trimming of cut axonal ends in Ca-free hypotonic salines enhanced their close apposition and the PEG-fusion of cut ends. However, cut-end trimming increased the separation between cut ends and stretching or pulling on nerves greatly reduced behavioral recovery after their PEG-fusion. Furthermore, crushes made in calcium-containing salines were infrequently repairable because the crushed segment had to be cut out (ablated) and the nerve stretched for PEG-repair with microsutures.
Development of PEG-fusion procedures to repair ablated axonal segments
Repair of 0.5cm segments of rat sciatic nerves ablated in calcium-containing extracellular fluids could be achieved by re-inserting the segment (an autograft) and repairing both cut ends with microsutures and PEG-fusion (Sexton et al., 2012). At three post-operative days, animals with PEG-fused autografts had (1) action potentials conducting across the autograft, (2) significantly more sensory and motor axons surviving within, and distal to, the autograft, and (3) significantly better SFI scores compared to negative control animals with autografts that were not PEG-fused. However, microsuturing during PEG-fusion stretched axons in the donor autograft and host sciatic nerve and PEG-fused autografts might pull apart or otherwise fail after 3d. Furthermore, in almost any clinical setting, an ablated nerve segment is not recoverable for subsequent use as an autograft. Autografts taken from other nerves to repair a major nerve are also often associated with significant donor site morbidity (Birch et al., 1998; Allan 2000; Campbell 2008) and are much smaller in diameter than the sciatic nerves, making PEG-fusion repair difficult or impossible. Clinicaly, there has been increased interest in using nerve allografts to avoid donor site morbidity. In contrast, sciatic nerves (potential allografts) stored for 4 days ex-vivo at 6 – 9°C exhibited good PEG-fusion repair un-stretched ex vivo (Marzullo et al, 2001). Finally, in a speculative pilot study using allografts stored for 2 days at 7°C for six rats having micro-sutured, un-stretched PEG-fused sciatic nerves at 7 post-operatve days, we observed significant behavioral recovery compared to six negative control animals. At 7 postoperative days, we also observed retardation of Wallerian degeneration within, and distal to the allograft, in three PEG-fused animals compared to the same regions of the sciatic nerve in three negative control animals.
Given these findings, we developed a modified PEG-fusion procedure that uses 0.8 –1.1 cm long sciatic allografts excised in calcium-containing fluids from donor Sprague-Dawley rats and stored at 7°C for 30min – 4 hr. The donor allograft is placed in a host Sprague-Dawley rat having a complete ablation of a 0.5–1cm long segment of its sciatic nerve in calcium-containing extracellular fluids. All severed axonal ends are carefully trimmed in calcium-free salines and microsutured to enhance close apposition of un-stretched cut axonal ends that are opened, and vesicle formation inhibited, by a hypotonic calcium-free saline containing methylene blue. PEG is then applied in a hypotonic calcium-free saline to induce axolemmal continuity. PEG is washed off with a calcium-containing isotonic saline to seal any remaining axolemmal discontinuities (Britt et al., 2010; Bittner et al., 2012).
Our data collected from two separate laboratories show that axolemmal function and axoplasmic continuity is rapidly (within minutes) restored in animals with successful PEG-fusion of allografted, un-stretched, sciatic nerves. Significant behavioral function is restored within 3–14d and is correlated with increased axonal viability of myelinated sensory and motor axons within, and distal to, the allograft, i.e., Wallerian degeneration is prevented or retarded. Furthermore, greater restoration of behavioral function is correlated with greater numbers of viable axons within, and distal to, PEG-fused allografts. Finally, this modified PEG-fusion procedure developed in an animal model uses concepts, techniques, and chemical substances that should be readily translatable to clinical procedures (Campbell, 2008; Williams et al., 2008; Burch, 2011).
Materials and Methods
Study design
We used 250–300 g Sprague-Dawley rats. Sprague-Dawley rats are not genetically homogeneous, i.e., there is genetic variation but not to the extent that might occur between different species. We used two groups of animals. One group received all PEG fusion procedures described below (PEG-fused animals) and the other group all procedures except application of PEG to closely apposed cut ends (negative control animals). All animals in both groups were initially tested for through-conduction of action potentials from the upper to lower thigh before and after lesioning and allograft repair. For the main study, at VU, 12 “negative control” rats received donor allografts that were not PEG-fused as did 6 negative control rats at UT; 13 rats received donor allografts that were PEG-fused at VU and 6 PEG-fused animals were produced at UT. VU animals were behaviorally tested at 3 and 7d postoperatively to determine whether there were any initial effects of PEG-fusion. UT animals were behaviorally tested at 3, 7, 14, 21, 28, 35 and 42 d postoperatively to confirm short-term effects and determine longer-term effects and sciatic nerves taken for histological analyses at 42 postoperative days. In a pilot study at VU, six PEG-fused and six negative control animals were behaviorally tested at 7 postoperative days and sciatic nerves taken from three of these animals for histological analyses.
We used 5 or more animals per group because power analyses and our previous data (Britt et al, 2010; Bittner et al, 2012; Rodriguez-Feo et al, 2013) had shown that such sample sizes allowed us to detect SFI differences of 15 – 20% at p < 0.05 with Student’s T-test. We used a larger number (12 – 13) of animals maintained for 1 wk to identify a statistically significant initial effect with more certainty. Once that was determined, power analyses of the data and our previously published data showed that we could use a smaller number (6) of animals to follow the effects for 6wk. We chose 6wks because 42 days should be beyond tissue rejection times and because previous studies on singly cut nerves had shown a plateau in behavioral recovery at 6wks that was maintained for 6 – 12wk thereafter (Bittner et al., 2012). Whether or not animals received PEG was determined by a pre-determined schedule. Animals were tested by those blinded to the conduct of the experiment. All experimental procedures were approved by and performed in accordance with the standards set forth by the Institutional Animal Care and Use Committees at VU and UT.
Surgical procedures for the modified PEG-fusion protocol
Female Sprague-Dawley rats were anesthetized with inhaled isoflurane (2%) and the left hind limb shaved with clippers. A three cm incision was made parallel, and just posterior, to the femur of the left leg. Using micro-scissors, a clean transection was made through the biceps femoris, parallel to the muscle fibers in order to minimize muscle damage under a surgical microscope at 10–30x (VU) or a dissecting microscope at 10–30x magnification (UT). CAPs were recorded in isotonic extracellular fluid by stimulating the entire sciatic nerve in the upper thigh and recording action potentials extracellularly from the entire sciatic nerve in the lower thigh.
A 0.5 – 1cm segment of sciatic nerve was removed (ablated) and replaced with a slightly longer segment of sciatic nerve from a donor Sprague-Dawley rat in calcium-containing extracellular fluid, sometimes washed with calcium-containing isotonic saline. This procedure took 15 – 30 min. The donor segment was excised in calcium-containing extracellular fluids, occasionally washed with calcium free-containing isotonic saline, and stored at 7°C for 30min – 4 hr in Plasma-lyte A® (Baxter: Deerfield, IL), which is calcium free and isotonic. The exposed, severed nerve in the host animal was then rinsed in Plasma-lyte A® (Baxter: Deerfield, IL). The donor segment was positioned carefully to maintain fascicle orientation and sized to insure that neither host nor donor nerves would be stretched at any step of the PEG-fusion procedure. Sciatic nerve ends of both donor and host animal were neatly trimmed flush to the epineural tissue and a hypotonic solution of 1% Methylene Blue (Acros Organics; Morris Plains, NJ) in sterile distilled water was applied to coaptation sites. Using standard microsurgical techniques, the allograft was sutured into place using 9–0 Ethilon (Ethicon, Sommerville, NJ) so that all cut ends were apposed (touching) without stretching the proximal, allograft, or distal axons. Once both ends were approximated and held in place by microsutures, a hypotonic 50% by weight solution of PEG (3.35kD molecular weight, Sigma-Aldrich; St. Louis, MO) in sterile water was applied to the coaptation sites for 1 minute in PEG-fused animals. Negative control animals did not receive PEG.
The wound was then flushed with isotonic Lactated Ringer (Hospira; Lake Forest, IL), which contains calcium, and tested for through-conduction of CAPs stimulated proximal to the allograft and recorded distal to the allograft. The cut thigh muscles were re-apposed by horizontal mattress sutures. The skin was re-approximated using a running subcuticular 5–0 monocryl suture (Ethicon, Sommerville, NJ). All animals received a subcutaneous injection of ketoprofen (5mg/kg) and were allowed to recover from anesthesia under careful monitoring
Electrophysiological testing
Extracellular recordings of Compound Action Potentials (CAPs) are a measure of axonal continuity between the stimulating and recording electrodes (Lore et al, 1999; Bittner et al., 2012). All CAPs were obtained using a Powerlab Data Acquisition System (AD Instruments; Colorado Springs, CO) interfaced with LabChart 7 (AD Instruments; Colorado Springs, CO). The stimulating dual terminal electrode was placed under the sciatic nerve, proximal to the allograft. The recording electrode was placed under the sciatic nerve, distal to the allograft. CAPS were recorded prior to nerve segment removal (pre-injury) and immediately after application of isotonic Ca2+-containing saline (post-PEG-fusion for experimental animals).
Intra-axonal dye diffusion across lesion sites
To assess morphological continuity in PEG-fused and negative control sciatic nerves, intra-axonal dye diffusion was observed for 12 sciatic nerves excised from the animal immediately after surgery at UT. After confirmation of physiological continuity through CAP conduction, a 3–4cm segment of sciatic nerve, including the graft site, was excised from the animal within 2hr. The proximal end of the sciatic nerve segment was placed within a watertight ring of petroleum jelly (Vaseline) containing Plasma-lyte A® and 50μl of 2kDa Texas red dextran (Lore et al., 1999; Bittner et al, 2012; Spaeth et al, 2012). The remainder of the nerve, including the proximal and distal lesion sites of the graft, was bathed in Plasmalyte A outside of the ring. The nerve was refrigerated for about 24hr at 4°C to allow time for dye to diffuse intracellularly through the length of the nerve. Nerves were examined for intra-axonal diffusion of fluorescent dye beyond the graft site using a Leica SP2 AOBS Confocal Microscope. Images were then stitched together using Adobe Photoshop in order to visualize the diffusion of dye through the entire length of the nerve.
Behavioral testing using the Sciatic Functional Index (SFI)
The SFI is a well-accepted measure of sciatic function that includes both appropriate sensory feedback and precise motor control, particularly for distal muscle masses that determine lower leg and toe position and movement (Mediniceli et al., 1982; Bittner et al, 2000, 2012; Britt et al, 2010). Behavioral assessments were at 3d and then weekly intervals up to 6wk post-operatively. Animals were not tested earlier to allow adequate recovery time from the effects of ketoprofen administration at the time of surgery. Rats were trained to walk up an inclined beam to a cage. After these habituation trials, the rats navigated the beam to the cage without hesitation. For each trial run, a strip of white receipt paper was secured to the wooden beam for data collection. The hind limbs were inked such that blue designated the surgical limb and red the un-operated limb before the animals were placed on the end of the beam farthest from the cage.
As previously reported (Medinaceli et al., 1983; Britt et al., 2010; Bittner et al,. 2012) the SFI was scored as follows: Three consecutive footprints from each limb were used to measure normal print length (NPL), normal toe spread (NTS), normal intermediary toe spread (NIS), experimental print length (EPL), experimental toe spread (ETS), and experimental intermediary toe spread (EIS). Intermediary toe spread was measured from toes 2 – 4, toe spread toe 1–5, and print length from the heel to end of toe 3. SFI scores were then calculated using mean values by:
Behavioral function was assessed by those blind by to the treatment. SFI scores of −100 or less indicate complete loss of sciatic nerve function and scores of ± 10 indicate normal sciatic nerve function.
Histology
For toluidine blue stains at UT of viable myelinated axons, rats were deeply anesthetized with ketamine/xylazine and perfused transcardially with 0.1M sodium cacodylate buffer, pH 7.4, followed by the same buffer containing 2% paraformaldehyde and 3% glutaraldehyde. Sciatic nerves were dissected and pinned into a Sylgard-coated dish and fixed overnight in the same fixative. The following day, nerves were washed in cacodylate buffer, trimmed into regions of interest, stained en bloc in 1% osmium tetroxide with 1% ferrocyanide in cacodylate buffer, washed in water, stained en bloc in 1% uranyl acetate in water, then washed and stored in water. Nerves were dehydrated through graded alcohols, exchanged to acetone, and embedded in Hard Plus Resin 812 (Electron Microscopy Sciences). Thick sections were cut on a Leica Ultracut UCT ultramicrotome and stained with toluidine blue, coverslipped, and imaged.
For immunohistochemical stains at VU as previously described (Sexton et al., 2012; Rodriguez-Feo et al, 2013), rats were deeply anesthetized with ketamine/xylazine and perfused transcardially with PBS. Nerves were pinned into a Sylgard dish with 4% paraformaldehyde overnight, then dehydrated and embedded in paraffin. Immunohistochemical staining was performed using commercial antibodies specifically directed against carbonic anhydrase II (Abcam, Cambridge, MA) to identify viable myelinated sensory axons and choline acetyltransferase (Millipore, Temecula, CA) to identify viable myelinated motor axons. For carbonic anhydrase II marker, heat mediated target retrieval was performed in 1X Target Retrieval Buffer, pH 9.0 (DAKO, Carpenteria, CA). Endogenous peroxidases and non-specific background were blocked by subsequent incubations in 3% H2O2 (Fisher, Suwanee, GA) in TBS-T and serum-free Protein Block (RTU, DAKO). For choline acetyltransferase marker, heat mediated target retrieval was performed in 1X Target Retrieval Buffer (DAKO). Endogenous peroxidases were blocked and non-specific background, secondary, and tertiary labeling of target was accomplished by use of Vector’s ABC Elite Goat IgG kit (Vector Laboratories, Burlingame, CA) (Sexton et al., 2012). Visualization was achieved with DAB+ chromogen (DAKO). Slides were counterstained with Mayer’s hematoxylin, dehydrated through a series of alcohols and xylenes, and then coverslipped with Acrytol Mounting Media (Surgipath, Richmond, IL).
For light microscopic observations at UT, slides were imaged at 10x or 20x on a Zeiss Axiovert A.1 or 200M with a digital Axiocam. Images for axon counts were montaged in Photoshop and counted in ImageJ. Viable myelinated axons within the sciatic epineural sheath that had an intact, non-collapsed myelin sheath and axoplasm with no dense staining were then counted manually. [Coagulated degenerating axoplasm stains densely.] At VU, all slides were examined using an Olympus Vanox-T AH-2 light microscope (Olympus, Center Valley, PA) interfaced to Pixera Pro 600 HS digital camera (Pixera Corporation; Santa Clara, CA). Digital photos were captured at 10x and 20x using Viewfinder V3.0.1 (Pixera Corporation; Santa Clara CA). For each nerve processed a cross section was photographed proximal to the site of injury, from the allograft, and distally from the sciatic nerves. Axon counts were made manually. For axonal counts, counters used 10x images of sciatic nerve cross sections. Viable myelinated axons within the sciatic epineural sheath that had an intact, non-collapsed myelin sheath and no dense-staining axoplasm were then counted manually in ImageJ (http://imagej.nih.gov/ij/) using the cell counter plugin.
Statistical Tests
We performed all statistical analysis using GraphPad Prism5 for PC (GraphPad Software, San Diego, CA) and R Studio,. Comparisons between mean and SE values for SFI scores on a given postoperative day (or between experimental and negative control axon counts at 7 days for pilot data) were made using a two tailed Student’s T-test. For comparison of axonal counts, we also used an ANOVA. All p-values were two-tailed and significance was determined at p < 0.05, p<0.01, and p<0.001, typically indicated in graphs by symbols *, **, and ***, respectively.
Results
Electrophysiological and morphological evidence of rapid restoration of axonal continuity
Our electrophysiological data obtained at both VU and UT for both the main and the pilot study consistently showed that CAPs generated by stimulating the entire intact sciatic nerve in the upper thigh were conducted to the lower thigh in all animals sampled prior to any experimental procedure (Fig. 1A: Pre Injury traces). These CAPs, produced a twitch of the muscles they innervated in the calf and foot. This through-conduction of CAPs was lost after ablating a 0.5–1cm segment in the mid-thigh and was not restored by inserting a 0.8 – 1.1cm microsutured donor allograft (Fig. 1A), unless that allograft was also PEG-fused (Fig. 1A: PEG Post Repair). Within minutes, such PEG-fusion of both ends of the allograft restored through-conducting CAPs from upper thigh to lower limb, as well as twitching of muscles in the calf and foot. CAP amplitudes after PEG-fusion were typically not as large when compared to CAPs initially recorded from the intact nerve (Fig. 1A, B). CAP amplitudes were 0 mv (CAPs could not be detected) in negative control animals, i.e., if the donor allograft was not PEG-fused. These data suggest that PEG-fusion restored axolemmal continuity to some, but not all, sciatic axons from proximal to distal of the allograft. While PEG-fusion restores electrophysiological continuity to some axons, PEG-fusion almost-certainly does not specifically reconnect the distal and proximal portions of sensory with sensory axons and motor with motor axons, much less specific motor or specific sensory axons.
Fig. 1. Electrophysiological assessment of sciatic nerve function shortly after allograft repair with and without PEG-fusion.
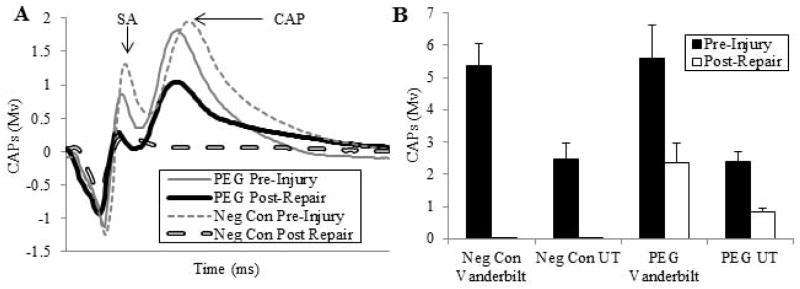
A. Representative CAP (mV) recordings from a negative control (dashed lines) and a PEG fused (solid lines) allograft pre-injury (thinner lines) and within 5 min after ablation of a 1 cm segment, insertion of a 1 cm donor segment without (negative control: thicker dashed line) or with PEG-fusion (thicker solid line) of both severed ends microsutured to the proximal or distal ends of the host sciatic nerve. SA = arrow points to peak of stimulus artefact. CAP: arrow points to peak amplitude of compound action potential
B. CAPs (mV, mean ± SE) recorded pre-injury and immediately post-repair plotted for 4 groups: Negative controls recorded at VU (n=12), and UT (n=6), PEG-fused at VU (n=13) and UT (n=6).
Our morphological data taken ex vivo begun within 2hr and examined 1d after allograft insertion showed that fluorescent dye intracellularly-loaded (Lore et al., 1999; Britt et al., 2010; Bittner et al., 2012; Spaeth et al., 2012) proximal to a microsutured allograft did not diffuse across the allograft (Fig. 2A), unless the allograft was also PEG-fused (Fig. 2B). These results were the same for each of 6 negative control and 6 PEG-fused sciatic nerves sampled at UT. These results suggest that PEG-fusion rapidly restores axoplasmic and axolemmal morphological continuity from proximal to distal of the allograft for many sciatic axons.
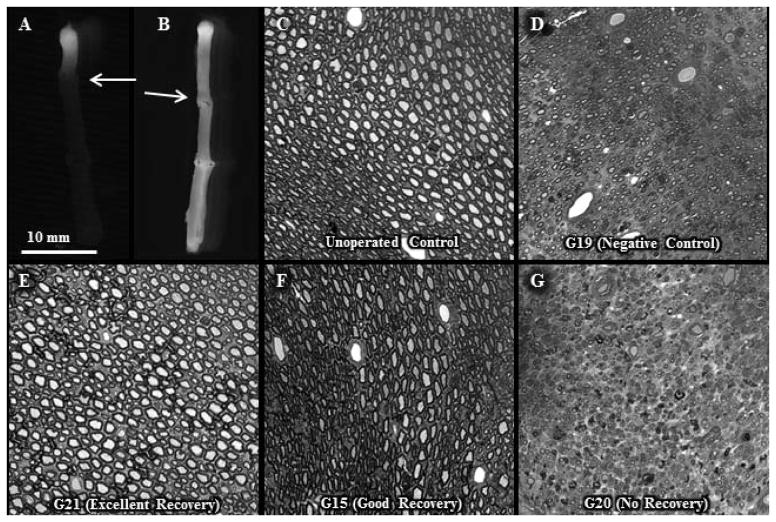
Fig. 2. Morphological evidence of myelinated axon viability with and without allograft PEG-fusion 1d or 6w postoperatively.
A, B. Intra-axonal dye diffusion of Texas Red at 1d postoperatively in a negative control (A) or PEG-fused (B) sciatic nerve. Arrow: site of proximal cut end of host sciatic nerve microsutured proximal end of donor sciatic allograft. Note that dye does not diffuse from the proximal segment of the host nerve into the donor allograft in the negative control sciatic nerve, but does diffuse into the allograft in the PEG-fused nerve.
C – G. Viable myelinated axons in toluidine-blue, plastic embedded sections of sciatic nerves viewed at 20x for an un-operated control (C) and the mid-allograft region at 6wk postoperatively for a negative control (D, animal # G-19) and PEG-fused sciatic nerves showing excellent (E, animal G-21), good (F, animal G-15) or no (G, animal G-20) behavioral recovery at 6wk postoperatively.
SFI-assessed behavioral function rapidly restored
While electrophysiological and morphological measures of restored axonal functions post-severance injury are important, behavioral measures are the most critical assessments of restored axonal function (Bittner et al, 2000, 2012), because regenerating axonal outgrowths or PEG-fused (repaired) axons may never establish or maintain functional connections. SFI scores at 7 post-operative days for the pilot study at VU were significantly better (p < 0.05) for 6 negative control animals (65 ± 8 SE) versus −90 ± 8 SE for six animals with PEG-fused sciatic allografts. SFI measurements for the main study (Fig. 3) at 3d and 1wk at VU and from 3d – 6wk at UT were initially performed on all animals before they received any operative procedure to establish a baseline for each animal; i.e., each animal served as its own control. The baseline SFI scores of individual animals varied from −23 to + 16 with an average of 0.8 for all animals (dotted line in Fig. 3A) and +3.7 for UT animals (dotted line in Fig. 3B). After a sciatic nerve was ablated, the SFI scores for negative control animals varied between −119 and −81 with a mean value of −99.5 ± 3.7SE at 3 days (n=18) and showed no significant trend of recovery or decline 1–6wk post-operatively (Fig. 3A). In contrast, SFI measurements of PEG-fused animals showed significant (p < 0.05 to p< 0.001) behavioral recovery at 3d and 1wk at both UT (n=6) and VU (n=13). SFI scores for negative control animals at UT and VU did not differ significantly (p > 0.05, Student’s T-test) from each other. That is, data from both laboratories were very similar (Fig. 3A). Behavioral recovery as measured by the SFI for PEG-fused animals at 1w was not significantly better compared to 3d at UT or VU.
Fig. 3. Behavioral assessments of sciatic nerve function with and without allograft PEG-fusion.
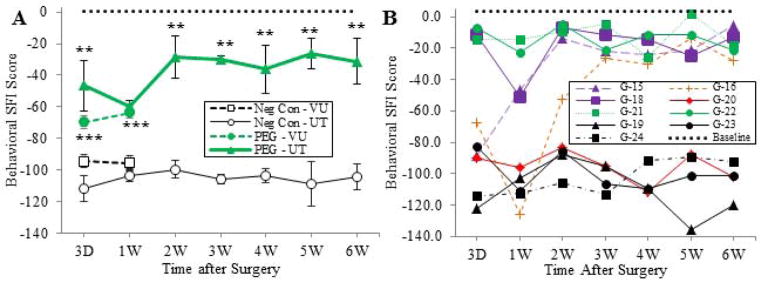
A. SFI score (mean ± SE) assessed at 3d to 6wk post-operatively at VU and UT. Groups and symbols as follows; unfilled circle: negative control UT, unfilled square: negative control VU, green filled triangle: PEG fused UT, green filled circle: PEG fused VU. The average baseline SFI value for all UT and VU animals is indicated by a horizontal dotted line. Significance comparisons between PEG-fused versus negative control SFI values at each time point for each data point calculated using Student’s T-test (two tailed) indicated by *, **, *** for p < 0.05, 0.01, and 0.001 respectively, in this and other figures.
B. SFI data for individual negative control and PEG -fused animals at UT. Negative control animals (G-19, G-23, and G-24) showing no behavioral recovery indicated by black filled symbols. Behavioral recovery during first two weeks for PEG-fused animals were classified as excellent (green symbols: G-21and G-22), good (purple symbols: G-15 and G-18), poor (orange symbols: G-16) or no (red symbols: G-20) behavioral recovery. PEG fused animals classified as excellent showed −30 or better SFI scores at 3d that were maintained for 6wk. PEG-fused animal classified as good showed −30 or better SFI scores at 2 weeks that were maintained for 6wk. The PEG-fused animal classified as poor showed −30 or better SFI scores at 3 weeks that were maintained for 6wk. The PEG-fused animal classified as having no behavioral recovery (animal G-20) showed SFI scores of −80 to −110 from 3d – 6wk with no improvement. These SFI values were within the range of values observed for negative control animals (black filled symbols). The average baseline SFI value for all UT animals is indicated by a horizontal dotted line.
SFI measures of behavioral recovery for most (5/6) PEG-fused animals at UT in the main study dramatically increased by 2 – 3 wk post-operatively and then plateaued through 6wk (Fig. 3A,B). As described in the legend of Figure 3B individual PEG-fused animals were classified as showing excellent (animals G-18, G-21, G-22), good (G-15), poor (G-16) or non-existent (G-21) behavioral recovery as defined by their pattern of SFI scores. The mean SFI’s of PEG-fused animals were not significantly different at 2wk compared to 3 – 6wk (Fig. 3A). The mean SFI’s for PEG-fused animals at 2 – 6wk were significantly better (p < 0.05 – p <0.001) than negative controls for each time point (Fig. 3A). On average, PEG-fused animals recovered about 70% of the SFI behavioral score attained by un-operated animals. The UT data for one PEG-fused animal (G-20) showed no behavioral recovery, possibly because the PEG-fusion procedure was unsuccessful for this animal, consistent with few viable axons observed distal to the proximal severance site (see below). All other (5/6) PEG-fused animals showed much more SFI behavioral recovery of ~80% to baseline values compared to un-operated animals having little or no recovery, with two PEG-fused animals showing 90–95% recovery. Qualitative visual observations of movements of successfully PEG-fused rats in their cages were often very difficult to distinguish from movements of un-operated rats at 2–6wk postoperatively.
Increased numbers of viable myelinated axons at six weeks after injury
For the main study prior to fixation, nerves were assessed for gross anatomical features using a dissecting microscope at 10–50X. Suture sites on all negative control and most PEG-fused nerves showed some enlargement that was more prominent in negative controls. The sciatic nerve from PEG-fused animal G21 (excellent SFI recovery) was distinguishable from an un-operated nerve only by the presence of sutures. The nerve from PEG-fused animal G20 (no SFI recovery) was the most enlarged at the suture site and throughout the allograft.
For the main study, the morphological status of myelinated sciatic axons at 6wk postoperatively was assessed using several histological techniques. The number of total viable myelinated axons in toluidine-blue, plastic embedded sections viewed at 10x for the mid-allograft region of PEG-fused sciatic nerves with excellent SFI recovery at 6wk postoperatively was less than the number of total viable axons in un-operated sciatic nerves, but more than the negative control sciatic nerves (Figs. 2C – G, 4A). The number of viable myelinated axons in animals having PEG-fused sciatic allografts associated with excellent (G-21) or good behavioral recovery (G-15) as measured by the SFI was greater than the number of viable myelinated axons in a PEG-fused sciatic nerve resulting in no behavioral recovery (G-20). In fact, the PEG-fused animal showing no behavioral recovery had fewer surviving axons in the allograft than the negative control animal (Figs. 2C – G, 4A). We saw evidence of tissue rejection such as lymphocytic infiltration, tissue destruction, or vessel injury in negative controls or unsuccessful PEG-fusions at 6wk, but not in animals with successful PEG-fusion (Figs. 2C–G).
Fig. 4. Number of viable myelinated axons with and without allograft PEG-fusion 6w postoperatively.
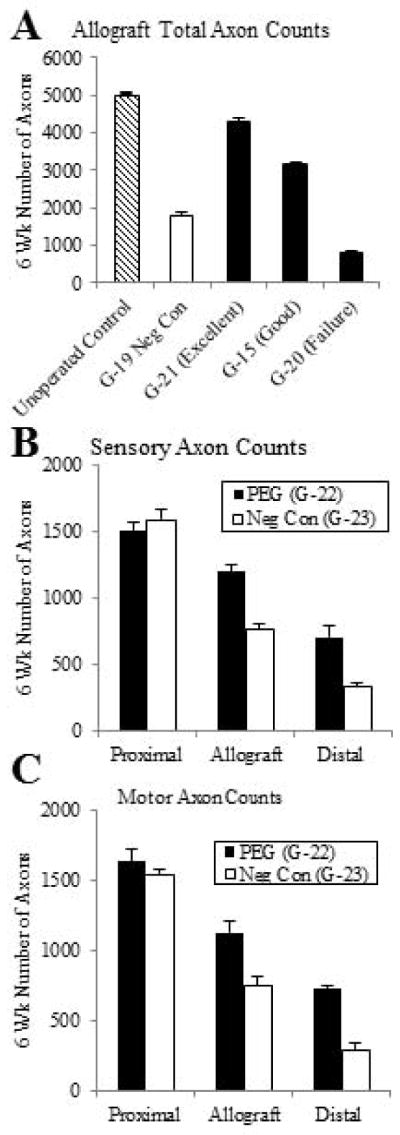
A. Total viable myelinated axon counts (mean ± SE, n=4) from a representative cross-section of sciatic nerves from the allograft region of un-operated control, negative control, and three PEG-fused allografts showing excellent, good or no SFI behavioral recovery 6wk postoperatively.
B, C. Sensory (B) or motor (C) viable myelinated axon counts (mean ± SE, n=6) at 6wk postoperatively from a representative cross section from each of proximal, allograft, and distal regions of PEG-fused and negative control animals.
For the pilot study at VU, the sciatic nerves of three PEG-fused animals and three negative control animals were taken for histological analyses of total axonal number. The number of viable myelinated sciatic axons proximal the allograft (985 ± 61 SE) for the PEG-fused animals was not significantly different (p = 0.57 by Student’s T-test) from the number for the negative controls (1072 ± 128). As reported for the main study, the PEG-fused animals had more viable axons (634 ± 85 SE) within the allograft compared to the negative controls ( 331 ± 92; p = 0.072). Distal to the allograft, the three animals with PEG-fused sciatic nerves had significantly more (p = 0.0034) viable myelinated axons (393 ± 35) compared to the negative control animals (119 ± 27). The total number of viable myelinated axons decreased from the proximal sciatic nerve to the allograft and from the allograft to the distal sciatic nerve in both control and PEG-fused animals. However, within the allograft segment or distal to the allograft, the number of viable axons in PEG-fused animals was higher than those in control animals.
For the main study, counts of myelinated sensory axons stained for carbonic anhydrase II in paraffin-embedded cross-sections (Figs. 4B, 5) showed no obvious difference proximal to the allograft in a PEG-fused animal (G-22) exhibiting excellent SFI behavioral recovery compared to counts for a negative control animal (G-23) showing little or no behavioral recovery. As observed for total axon number in the pilot study, the number of viable sensory axons was greater for the allograft and distal cross sections of a PEG-fused sciatic nerve compared to a negative control; the number of sensory axons decreased in the allograft and further decreased distal to the allograft, but the decrease was less in all cases for the PEG-fused animal (Figs. 4B, 5A, C, E). Comparable results were found for counts of motor axons stained for choline acetyltransferase in paraffin-embedded cross-sections for a PEG-fused animal (G-22) compared to its negative control (G-23) (Figs. 4C, 5B, D, F). As shown in Figure 6A, SFI scores of individual animals at 1wk postoperatively (Fig. 3B) had a significant correlation (R2 >0.995, p < 0.05) to their viable axon counts in the allograft made at 6wk postoperatively (Figs. 2, 4A). As shown in Figure 6B, SFI scores averaged for each animal for all postoperative weeks 1–6 (Fig. 3B) had a positive correlation to viable axon counts in the allograft made at 6wk postoperatively (Figs. 2, 4A).
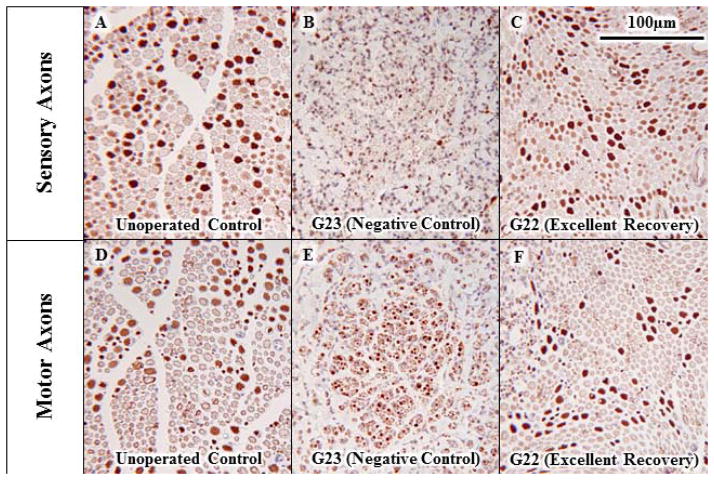
Fig. 5. Morphological evidence of myelinated sensory or motor axon viability with and without allograft PEG-fusion 6wk postoperatively.
Viable sensory (A, C, E) or motor (B, D, F) myelinated axons in paraffin-embedded sections of sciatic nerves viewed at 20x stained for carbonic anhydrase II (A, C, E) or choline acetyltransferase (B, D, F) for an un-operated control (A, B) and distal to the allograft 6wk postoperatively for a negative control (C, D) and PEG-fused sciatic nerves showing typical (E, F) behavioral recovery
Fig. 6. Correlation of behavioral recovery with surviving axons in allografts with and without successful PEG-fusion at UT.
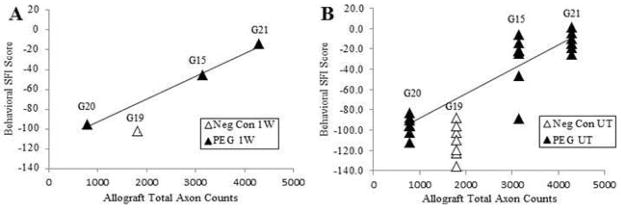
A. SFI scores at 1w for each animal as listed versus number of surviving myelinated axons in allografts at 6wk postoperatively. R2 = 0.9946, p < 0.05. G19 = negative control animal not PEG-fused.
B. SFI scores at 1–6wk individually plotted for each animal as listed versus number of surviving myelinated axons in allografts at 6wk postoperatively.
Discussion
Repair and recovery after clinical repair of peripheral nerve injuries
Peripheral nerve injuries in humans are common and often devastating as they frequently lead to poor recovery of sensory function in the affected extremity. Even worse can be the loss of motor function after proximal injury as axonal outgrowth is slow, and irreversible muscle atrophy often occurs before re-innervation, even with appropriate repair. (Allan, 2000; Campbell, 2008; Williams and Dellon, 2008; Burch, 2011). The cell bodies of PNS nerves lie within the spinal cord or dorsal root ganglion and their axons can extend great lengths (centimeters in rats, meters in humans) to reach their synaptic targets. Transection of these axons leads to distal axonal (Wallerian) degeneration within days (Ramon y Cajal, 1938; Burch, 2011). Functional recovery after nerve injury depends upon slow proximal outgrowth at ~1mm/d, requiring months to years for potentially appropriate re-innervation to distal-most targets. Since muscles lose the ability to be re-innervated effectively over time, functional recovery has a greatly reduced chance of occurring (Fu and Gordon, 1995). Current clinical nerve repair techniques make no attempt to limit Wallerian degeneration (Burch, 2011; Menorca et al, 2013.
The success of subsequent nerve repair following PNS nerve transection in a human extremity depends on a number of variables such as mechanism of injury, timing of repair, associated muscle, skin, vascular and bone injury, repair technique of the surgeon such as stretching or drying the nerve, postoperative rehabilitation, age of the patient, and patient coping mechanisms (Williams and Dellon, 2008; Farber et al., 2013; Menorca et al., 2013). More-distal nerve injuries have better results than more-proximal injuries, but successful sensory recovery is typically equated only to protective sensation, not full recovery (Bertelli et al., 2009; 2011). The most critical determinants of recovery are distance of the injury site from the target organ, the length of nerve damaged by the injury (defect length or gap), and stretching the nerve during repair. For example, a sharp clean nerve injury with a short nerve defect typically has a better recovery than a severe traction rupture nerve injury that produces a long segment of nerve injury. The outgrowing axons lose their way, or get trapped in scar, preventing proper outgrowth (Menorca et al., 2013). Current strategies use conduits or nerve grafts to bridge nerve gaps (Bertelli et al., 2009, 2011; Farber et al., 2013). These nerve repair strategies often yield poor results and functional recovery can be limited (Bertelli et al., 2009, 2011; Taras et al., 2011; Huag et al., 2013). Nerve autografts have been employed to shorten the effective distance from the re-innervation and/or repair site to the target, but appropriate donor autografts are not always available (Williams and Dellon, 2008: Cho et al., 2012). In brief, current PNS nerve repair techniques often produce very limited functional recovery, especially for more proximal injuries in humans that involve large nerve gaps that cannot be closely apposed without stretching the nerve. For example, the loss of function associated with nerve resection for oncologic purposes can be devastating and prevent limb salvage when tumors involve major peripheral nerves (Campbell, 2008).
Results of PEG-fused sciatic allografts
Our data suggest that PEG-fusion of allograft tissues might greatly reduce many of these problems outlined above and clinically produce nerve recovery within weeks and prevent or retard much Wallerian degeneration. For example, our data reported herein consistently show that action potential (CAP) conduction and dye diffusion in sciatic nerves from upper to lower thigh is much better for PEG-fused that for negative control animals and PEG-fused sciatics are similar to unoperated animals, especially for intraaxonal dye diffusion. are similar to unoperated, intact sciatic axons. Total axon counts in the allograft are higher for PEG-fused animals compared to negative controls at 6wk (main study) and at 1 wk (pilot study), as are counts of sensory and motor axons for the main study for different animals than those used for total axon counts. In brief, the electrophysiological, morphological and behavioral data presented herein are consistent with hypotheses that (1) PEG-fusion of allografts rapidly (within minutes) restores electrophysiological, axolemmal, and axoplasmic continuity to many sciatic axons. (2) PEG-fusion of allografts maintains the viability of many sciatic axons (motor and sensory) for at least 1hr – 6wk, perhaps permanently, thereby preventing Wallerian degeneration of many of those axons. (3) Greater restoration of through-conducting CAPs immediately after PEG-fusion of allografts is associated with greater SFI behavioral recovery at 1wk, but not at 6wk. (4) Greater numbers of surviving axons in the allograft are associated with greater SFI behavioral recovery.
Our data from PEG-fused allografts are consistent with previously published data on PEG-fusion of sciatic nerves after a 1mm long crush severance (Britt er al., 2010) or cut-severance (Bittner et al, 2012; Rodriguez-Feo et al., 2013) producing ~ 1mm separation of the cut ends that were then re-apposed by microsutures after which moderate (15–30 SFI units) but significant SFI-assessed behavioral recovery was noted at 3d – 1wk. Behavioral recovery then greatly increased starting at 2 – 3 wk postoperatively, then plateaus at 4 – 6 postoperative weeks (Britt et al., 2010; Bittner et al, 2012) and is maintained for at least 12 postoperative weeks. PEG-fused autografts also produced moderate but significant behavioral recovery at 1 – 3d (Sexton et al, 2013). The SFI-measured behavioral recovery of 70–80% in two weeks or six weeks for PEG-fused allografts following ablations in calcium-containing salines in the current study is at least as good (in fact, slightly better) than SFI behavioral recovery at two weeks (40%) or six weeks (50–60%) for PEG-fused sciatic nerves following single cuts or crushes in calcium-free salines. We suspect this enhanced recovery using allografts is due to cleanly re-cutting the cut ends in calcium-free salines and avoiding any stretching of sciatic axons. In any event, our PEG-fusion procedure uses concepts, techniques, and chemical substances that should be readily translatable to clinical procedures.
Morphological data from these previous studies are consistent with the hypothesis that PEG-fusion of cut axonal ends eliminates or retards Wallerian degeneration of some severed axons. In this study, as in a previous study (Sexton et al., 2012), the total number (or number of sensory axons or number of motor axons) of viable myelinated axons in an allograft or autograft is greater for PEG-fused versus negative control animals. The same result holds for the number of viable axons distal to the allograft or autograft.
As described in the Introduction, the cellular/molecular mechanism underlying retardation of Wallerian degeneration and behavioral recovery is rapid fusion-repair of open ends of severed axons produced by a sequence of bioengineered solutions varying in their composition of ionic strength, calcium, PEG, and MB as previously published (Lore et al., 1999; Britt et al., 2010; Bittner et al., 2012; Spaeth et al., 2012; Sexton et al, 2012). However, we do not know how such PEG-fusion mechanisms at the cellular/molecular level produce such rapid and dramatic behavioral recovery at the systems level. The simplest explanation would be that we are reconnecting appropriate proximal and distal portions of transected sensory and motor axons. However, we recognize that there is no readily-conceivable way that we are selectively reconnecting distal and proximal portions of severed motor or sensory axons, much less individually-identifiable axons, in simple transections, much less for ablations and insertions of donor allografts. For example, we do not know whether (1) distal portions of sensory or motor axons are re-specified by the PEG-fused proximal portion; or, (2) distal portions of sensory or motor axons re-specify spinal connections made by the PEG-fused proximal segment; or, (3) higher CNS sensory or motor centers are highly plastic and can quickly learn how to reuse altered sensory and motor peripheral connections; or (4) collateral outgrowths from surviving distal and motor axons rather quickly remake a set of new peripheral connections.
We note that these unanswered questions on behavioral mechanisms at the systems level that are obvious for PEG-fused allografts are also questions that have not been answered (and often not asked) for behavioral recovery by outgrowth from surviving proximal stumps in sciatic and other PNS preparations. That is, unanswered questions for regeneration by outgrowth at the systems level include how many (if any) outgrowing motor axons reinnervate their original muscle fibers, grow down sensory sheaths, the relative importance of collateral sprouting vs spinal or brain plasticities. However, we do attempt to maintain proximal and anatomic orientation during the procedure and we suspect that functional axonal groups/fascicles are typically aligned leading to our observed functional outcomes.
Significance and implications of our data
Whatever the systems-level mechanisms responsible for the behavioral recovery, our data show that PEG-fusion of allografts following ablation of sciatic nerve segments can produce rapid, dramatic, and long-lasting recovery of sciatic nerve function. On a few animals (data not shown), we have also observed that sciatic allografts can be stored for several days at 7ºC and then successfully PEG-fused as measured by CAP through-conduction. These data are consistent with previous findings that Wallerian degeneration of the distal portions of cut-severed sciatic or tail nerves in rats can be retarded for 5 – 7d in vivo by cooling the body part to 13 – 25ºC or by cyclosporine injections (Sea et al., 1995; Sunio and Bittner, 1997). Furthermore, rat sciatic or spinal axons can be maintained ex vivo for 5 – 7d by cooling to 6–9º C and then successfully PEG-fused (Marzullo et al, 2001). We do not know why non-processed allografts that are successfully PEG-fused as measured by SFI behavioral recovery show no obvious evidence of tissue rejection by six weeks. It is possible that fused cell membranes share MHC I molecules and thus down regulate host recognition of allografted sciatic nerves. We have not yet carefully examined this issue.
Consideration of all these data suggest that a well-specified use of microsutures, allografts, and PEG-fusion procedures might produce a paradigm shift in the clinical treatment of traumatic injuries to peripheral nerves for which the gold-standard for simple cuts has been microsuturing the severed ends (Allan, 2000; Campbell, 2008; Williams and Dellon, 2008; Menorca et al., 2013). The ability to PEG-fuse severed axons in allografts to restore behavioral deficits within weeks after ablation of long segments of major peripheral nerves might dramatically alter functional outcomes for patients with mutilated extremity injuries and potentially even change the types of injuries for which salvage is attempted after extensive traumatic injury or oncologic resection. It is perhaps even reasonable to consider the future establishment of tissue banks for peripheral nerve allografts. Finally, since the loss of function following traumatic injury to spinal cords is primarily due to loss of axonal continuity within spinal white matter (Bittner at al., 2000), it might even be possible that PEG-fusion, perhaps combined with peripheral or spinal nerve allografts, would restore behavioral function lost after spinal injury much better than any currently-used procedure.
Acknowledgments
Supported by a DOD grant OR120216 to WPT and an NIH grant R01 NS081063 to GDB and TS and a Lone Star Paralysis Grant to GDB. We thank Dr. Robert Grossfeld, Professor of Biology, North Carolina State University, for careful reading and insightful comments on drafts of this manuscript. We thank Dr. Kevin Sexton, MD, for data on an allograft pilot study.
References
- Allan CH. Functional results of primary nerve repair. Hand Clinics. 2000;16:67–71. [PubMed] [Google Scholar]
- Bertelli JA, Ghizoni MF. Results of c5 root grafting to the musculocutaneous nerve using pedicled, vascularized ulnar nerve grafts. J Hand Surg. 2009;34A:1821–1826. doi: 10.1016/j.jhsa.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Bertelli JA, Ghizoni MF. Very distal sensory nerve transfers in high median nerve lesions. J Hand Surg. 2011;36A:387–393. doi: 10.1016/j.jhsa.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Birch R, Bonney G, Wynn-Parry CB. Clinical aspects of nerve injury. Surgical Disorders of the Peripheral Nerves. 1998;2:71–87. [Google Scholar]
- Bittner GD, Keating CP, Kane JR, Britt JM, Spaeth CS, Fan JD, Zuzek A, Wilcott RW, Thayer WP, Winograd JM, Gonzalez-Lima F, Schallert T. Rapid, effective and long-lasting behavioral recovery produced by microsutures, methylene blue and polyethlene glycol after complete cut of rat sciatic nerves. J Neurosci Res. 2012;90:967–980. doi: 10.1002/jnr.23023. [DOI] [PubMed] [Google Scholar]
- Bittner GD, Schallert T, Peduzzi JD. Degeneration, trophic interactions, and repair of severed axons. The Neuroscientist. 2000;6:88–109. [Google Scholar]
- Britt JM, Kane JR, Spaeth CS, Zuzek A, Robinson GL, Gbanaglo MY, Estler CJ, Boydston EA, Schallert T, Bittner GD. Polyethylene glycol rapidly restores axonal integrity and improves the rate of motor behavior recovery after sciatic nerve crush injury. J Neurophysiol. 2010;104:695–703. doi: 10.1152/jn.01051.2009. [DOI] [PubMed] [Google Scholar]
- Burch R. Green operative hand surgery. Vol. 6. Elsevier; Churchill Livingstone: 2011. Nerve repair; pp. 1035–107. [Google Scholar]
- Campbell WW. Evaluation and management of peripheral nerve repair. Clinical Neurophys. 2008;119:1951–1965. doi: 10.1016/j.clinph.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Cho MS, Rinker BD, Weber RV, Chao JD, Ignari JV, Brooks D, Buncke GM. Functional outcome following nerve repair in the upper extremity using processed nerve allografts. J Hand Surg. 2012;37A:2340–2349. doi: 10.1016/j.jhsa.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Farber SJ, Glaus SW, Moore AM, Hunter DA, Mackinnon SE, Johnson PJ. Supercharge nerve transfer to enhance motor recovery: a laboratory study. J Hand Surg. 2013;38A:466–477. doi: 10.1016/j.jhsa.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug A, Bartels A, Kotas J, Kunesch E. Sensory recovery 1 year after bridging digital nerve defects with collagen tubes. J Hand Surg. 2013;38A:90–97. doi: 10.1016/j.jhsa.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Hoy RR, Bittner GD, Kennedy D. Regeneration in Crustacean Motorneurons: Evidence for Axonal Fusion. Science. 1967;156:251–252. doi: 10.1126/science.156.3772.251. [DOI] [PubMed] [Google Scholar]
- Krause TL, Bittner GD. Rapid Mophological Fusion of Severed Myelinated Axons by Polyethylene Glycol. PNAS. 1990;87:1471–1475. doi: 10.1073/pnas.87.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause TL, Fishman HM, Ballinger ML, Bittner GD. Extent and Mechanism of Sealing in Transected Giant Axons of Squid and Earthworms. J Neurosci. 1994;14:6638–6651. doi: 10.1523/JNEUROSCI.14-11-06638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lore AB, Hubbell JA, Bobb DS, Jr, Ballinger ML, Loftin KL, Smith JW, Smyers ME, Garcia HD, Bittner GD. Rapid induction of functional and morphological continuity between severed ends of mammalian or earthworm myelinated axons. J Neurosci. 1999;19:2442–2454. doi: 10.1523/JNEUROSCI.19-07-02442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzullo TC, Britt JS, Stavisky R, Bittner GD. Cooling enhances in vitro survival and fusion repair of severed axons taken from the peripheral and central nervous system of rats. Neuroscience Letters. 2001;327:9–12. doi: 10.1016/s0304-3940(02)00378-6. [DOI] [PubMed] [Google Scholar]
- Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982;77:634–643. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- Menorca R, Fussell TS, Elfar JC. Nerve physiology: mechanisms of injury and recovery. Hand Clin. 2013;29:317–330. doi: 10.1016/j.hcl.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal S. In: Degeneration and regeneration of the nervous system. May RM, translator. Oxford University Press; London: 1928. [Google Scholar]
- Rodriguez-Feo CL, Sexton KW, Boyer RB, Pollins AC, Cardwell NL, Nanney LB, Shack RB, Mikesh MA, McGill CH, Driscoll CW, Bittner GD, Thayer WP. Blocking the P2X7 receptor improves outcomes after axonal fusion. J Surgical Res. 2013;184:705–13. doi: 10.1016/j.jss.2013.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sea T, Ballinger ML, Bittner GD. Cooling of peripheral myelinated axons retards Wallerian degeneration. J Neurophys. 1995;133:85–95. doi: 10.1006/exnr.1995.1010. [DOI] [PubMed] [Google Scholar]
- Sexton KW, Pollins AC, Cardwell NL, Del Corral GA, Bittner GD, Shack RB, Nanney LB, Thayer WP. Hydrophilic polymers enhance early functional outcomes after nerve autografting. J Surgical Res. 2012;177:392–400. doi: 10.1016/j.jss.2012.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaeth CS, Boydston EA, Figard LA, Zuzek A, Bittner GD. A Model for Sealing Plasmalemmal Damage in Neurons and Other Eukaryotic Cells. J Neurosci. 2010;30:15790–15800. doi: 10.1523/JNEUROSCI.4155-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaeth CS, Robinson T, Fan JD, Bittner GD. Cellular mechanisms of plasmalemmal sealing and axonal repair by polyethylene glycol and methylene blue. J Neursosci Res. 2012;90:955–966. doi: 10.1002/jnr.23022. [DOI] [PubMed] [Google Scholar]
- Steinhardt RA, Bi G, Alderton JM. Cell Membrane Resealing by a Vesicular Mechanism Similar to Neurotransmitter Release. Science. 1994;263:390–393. doi: 10.1126/science.7904084. [DOI] [PubMed] [Google Scholar]
- Sunio A, Bittner GD. Cyclosporin A retards the Wallerian degeneration of peripheral mammalian axons. Exp Neurol. 1997;146:46–56. doi: 10.1006/exnr.1997.6484. [DOI] [PubMed] [Google Scholar]
- Taras JS, Jacoby SM, Lincoski CJ. Reconstruction of digital nerves with collagen conduits. J Hand Surg. 2011;36A:1441–1446. doi: 10.1016/j.jhsa.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Williams EH, Dellon AL. Upper extremity nerve repair tips and techniques: a master skills publication. ASSH. 2008:75–88. [Google Scholar]