Abstract
The aim of this article is to evaluate current literature on investigation and management of traumatic optic neuropathy (TON), propose recommendations for diagnosis and management, and explore novel future treatments. TON, though uncommon, causes substantial visual loss. Without clear guidelines, there is much ambiguity regarding its diagnosis and management. Investigation and treatment (conservative, medical, surgical, and combined) vary widely between centers. Electronic databases PubMed, MEDLINE, PROSPERO, CENTRAL, and EMBASE were searched for content that matched “Traumatic optic neuropathy.” Articles with abstracts and full text available, published in the past 10 years, written English and limited to human adults, were selected. All study designs were acceptable except case reports and case series with fewer 10 patients. All abstracts were then evaluated for relevance. References of these studies were evaluated and if also relevant, included. A total of 2,686 articles were retrieved and 43 examined for relevance. Of these, 23 articles were included. TON is a clinical diagnosis. Visual-evoked potential is useful in diagnosis and prognosis. Computed tomography demonstrates canal fractures and concomitant injuries. Magnetic resonance images should be reserved for select and stable patients. Conservative treatment is appropriate in mild TON. Steroids are of questionable benefit and may be harmful. Surgery should be reserved for patients with radiological evidence of compression and individualized.
Keywords: traumatic optic neuropathy, oculofacial trauma, corticosteroid therapy, optic nerve decompression, neuroprotection and neuroregeneration
The optic nerve (ON) comprises axons of retinal ganglion cells (RGCs) and support cells. At 50 mm in length, it consists the following four segments: intraocular (1 mm), intraorbital (24 mm), intracanalicular (9 mm), and intracranial (16 mm). The ON may be injured in trauma, resulting in visual loss and this is known as traumatic optic neuropathy (TON). This occurs from either direct or indirect trauma and both primary and secondary mechanisms of damage have been proposed.1 2 3 4
In direct trauma, stress is applied directly to the ON and is often when orbital fracture fragments lacerate the it or when mechanical contusion/concussion.5 The ON commonly sustains indirect trauma, where stress is transmitted through the oculofacial soft tissues and skeleton. The resultant coup–contrecoup forces damage the nerve at transitions between mobile and fixed segments. Commonly, this occurs at the junction of the intraorbital and intracanalicular segments. This results in compression and disruption of pial vessels within the canal, limiting vascular supply of the ON.6 7
In a study of 42 patients with TON,8 the frequency of site of injury was: intracanalicular (71.4%) > orbital apex (16.7%) > both (11.9%). This is supported by static loading studies which demonstrate that force applied to the superior orbital rim is transferred and concentrated on the orbital roof and optic canal.8 The next most common site is within the anterior cranial fossa where the intracranial ON lies close to the falciform dural fold.9
Primary damage occurs when there is an immediate disruption (direct trauma) or shearing (indirect trauma) of RGC axons. The inflammation and vascular dysfunction that follows gives rise to secondary damage. Though the pathophysiologies of both mechanisms differ greatly, patients often have elements of both.
A 5-year British study of TON in the general population reported 121 cases.10 Of those, 79% were male with a median age of 31 years and significantly, 21% were younger than 18 years. Common etiologies were falls (26%), motor vehicle accidents (21%), and assaults (21%). In the trauma setting, a 20-year study at the largest level 1 trauma center in Canada reported 0.4% of all trauma (injury severity score [ISS] > 12) patients had TON.11 Of those, 76% were male with a median age of 33.5 years. Significantly, all patients with TON had head injuries (two-third had a significant head injury—Head injury Abbreviated Injury Score [HAIS] ≥ 4). However, only 2.3% of patients with head injury suffered concomitant TON. The most common etiology was motor vehicle accidents (63%) but patients with falls (second most common etiology) were most likely to develop TON.
A retrospective study on TON in pediatric patients (< 18 years; mean age, 11.6 years; 43 affected eyes) yielded similar results to adult studies.12 Overall, 60% were males, common etiologies included motor vehicle accidents (62%) and sports injuries (22%). About 78% of cases were because of blunt trauma.
Iatrogenic TON is an understudied cause of postoperative blindness. It can occur during orbital surgery,13 Le Fort I osteotomies,14 maxillofacial fracture fixation,15 16 and endoscopic sinus surgery.17 18 19 In patients with chronic sinusitis, the ON may protrude and complicate sphenoidal sinus surgery. Among 260 patients with chronic inflammatory sinus disease, ON protrusion occurred in 28% of cases. Overall, 12% of ON indented the sinus wall and 8% of them coursed through it.20
Risk factors for TON include loss of consciousness and injury to the superolateral orbital region.21 Spontaneous improvement in vision can occur and time from injury to the presentation should be documented. Concomitant head injury can leave the patient obtunded, making assessment difficult and delaying the diagnosis.22 Decreased visual acuity (VA) is observed with 40 to 60% of patients presenting with light perception or worse.21 23 This is associated with a relative afferent pupillary defect (RAPD), except in cases of symmetrical bilateral TON.24 There may be impaired color vision and variable visual field defects.
Initial fundoscopy is of value in detecting patients with anterior optic nerve injury with associated optic nerve head swelling and adjacent retinal hemorrhages and to rule out preexisting optic neuropathy (optic nerve head pallor) or retinopathy/maculopathy as a preexisting cause of visual loss and RAPD. However, in cases of posterior injuries (more common), a normal fundus is seen. In a study of 27 patients, 89% had a normal fundus.25 ON avulsions may be visualized on funduscopy as well. Optic atrophy may develop but is only evident after 4 to 6 weeks.26
At present, no clear indications or contraindications for choice of imaging or treatment of TON have been proposed. In addition, treatment (corticosteroids and surgery) largely minimizes damage but in the future, neuroprotection and neuroregeneration may be possible.
Materials and Methods
A search of PubMed, MEDLINE (Medical Literature Analysis and Retrieval System Online), PROSPERO (International prospective register of systematic reviews), CENTRAL (the Cochrane library), and EMBASE (Elsevier) electronic databases for publications with content matching the term “traumatic optic neuropathy” was performed. Articles with abstracts and full text available, published in the past 10 years, written in English, and limited to human adults (older than 18 years), were selected. All study designs were acceptable except case reports and series with fewer than 10 patients. All abstracts were evaluated for relevance. References of these studies were evaluated as well and if relevant, included. Articles on the investigation, management (conservative, medical, surgical, and combined), and future treatment (neuroprotection and neuroregeneration) of TON were included.
Results
The electronic database search yielded 2,689 articles (PROSPERO, 0; CENTRAL,7; PubMed/MEDLINE, 2,682; and Embase,0). Of these, 43 articles had available abstract and full text, were written in English, not older than 10 years, and were limited to human adults. Of these articles, nine were found to be relevant. The references of these articles were evaluated, and further 14 articles were added. Hence, a total of 23 articles were reviewed in this study. This has been summarized in Fig. 1.
Fig. 1.
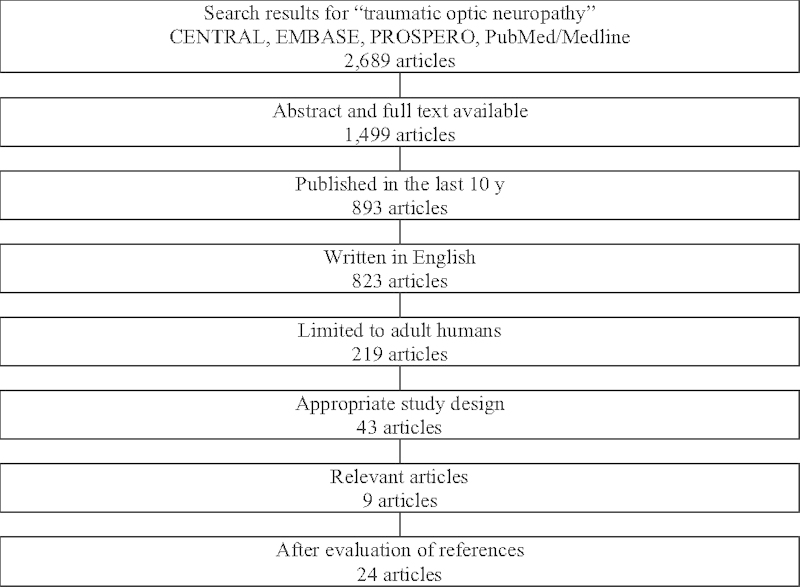
Literature search results.
The details of these studies have been summarized in Table 1. There were zero randomized controlled clinical trials, one meta-analysis, seven prospective studies, six retrospective studies, five reviews, three case series, and one expert opinion which resulted in a mean level of evidence of 2.8. The level of evidence was determined according to the definitions stated in Table 2.
Table 1. Included studies.
| No. | Author | Year | Study design | Sample size | Level of evidence |
|---|---|---|---|---|---|
| 1 | Steinsapir et al | 2011 | Review | 3 | |
| 2 | Warner and Eggenberger | 2010 | Review | 3 | |
| 3 | Steinsapir and Goldberg | 2005 | Review | 3 | |
| 4 | Ropposch et al | 2013 | Retrospective | 42 | 3 |
| 5 | Agrawal et al | 2013 | Review | 3 | |
| 6 | Yu-Wai-Man and Griffiths | 2013 | Meta-analysis | 1 | |
| 7 | Lee et al | 2010 | Retrospective | 24 | 3 |
| 8 | Kubal | 2008 | Expert opinion | 5 | |
| 9 | Lee et al | 2004 | Retrospective | 23 | 3 |
| 10 | Yeh and Foroozan | 2004 | Review | 3 | |
| 11 | Holmes and Sires | 2004 | Prospective | 11 | 2 |
| 12 | Bodanapally et al | 2013 | Prospective | 12 | 2 |
| 13 | Li et al | 2014 | Prospective | 28 | 2 |
| 14 | Shi et al | 2013 | Prospective | 54 | 2 |
| 15 | Ustymowicz et al | 2009 | Prospective | 72 | 2 |
| 16 | Rajiniganth et al | 2003 | Prospective | 44 | 2 |
| 17 | Li et al | 2008 | Prospective | 237 | 2 |
| 18 | Wang et al | 2008 | Retrospective | 46 | 3 |
| 19 | Chen et al | 2007 | Case series | 30 | 4 |
| 20 | Yang et al | 2006 | Case series | 12 | 4 |
| 21 | Yang et al | 2012 | Retrospective | 96 | 3 |
| 22 | Chen et al | 2004 | Case series | 11 | 4 |
| 23 | Yang et al | 2004 | Retrospective | 42 | 3 |
Table 2. Levels of evidence.
| Level I: Evidence based on large randomized, controlled trials or meta-analysis of randomized, controlled trials |
| Level II: Evidence based on controlled study without randomization |
| Level III: Evidence based on nonexperimental descriptive studies |
| Level IV: Evidence based on case-series |
| Level V: Evidence based on expert opinion |
Of these 23 articles, 8 articles investigated the choice of imaging, 14 articles investigated choice of treatment, and 1 explored future treatments of TON. To supplement the review of these articles and substantiate or refute their claims, wherever appropriate, the authors have cited relevant studies outside the inclusion criteria. These include older trials on TON, studies on the efficacy and safety of intravenous corticosteroids in acute trauma, evaluation of surgical approaches and techniques as well as experimental animal model studies that explore possible neuroprotective and neuroregenerative strategies.
Discussion
Investigation
Investigations are performed for diagnosis, prognosis, and preoperative planning. Some institutions include neuroimaging in the management of all patients with TON, whereas others reserve it for severe cases or before considering treatment.27 28 29 To further compound this, there are many modalities available to choose from.
Investigations not only evaluate the ON but can diagnose concomitant (intraocular, extraocular, orbital, and craniomaxillofacial) injuries. These include intracranial hemorrhage, fractures of the craniomaxillofacial skeleton, disruption of the globe, retro orbital hemorrhages, periorbital hematomas, and other traumatic neuropathies.
In a study of 24 patients with TON,30 computed tomography (CT)/magnetic resonance imaging (MRI) of the brain and/or orbit revealed that 19 patients (79.2%) presented with more than one bony fracture of the skull and 7 patients (29.2%) had intracranial bleeding. However, none of the patients had evidence of ON compression. The most common associated eye injuries seen were periorbital hematomas (91.7%) and subconjunctival hemorrhage (83.3%) which are benign and do not threaten life nor sight. Hematomas may not always be insignificant as they can compress the ON if in the right position and of sufficient size.31 In a retrospective analysis of 354 cases of maxillofacial trauma, 2.25% were diagnosed with TON. The results of which suggested associations between TON and zygomatic, Le Fort II, and cranial bone fractures and radiological evidence of blood in the posterior ethmoidal cells. Some studies question the utility of neuroimaging as there is no consistent correlation between the finding of an optic canal fracture, the severity of visual loss, and prognosis for visual recovery.29 32
CT scans are the most versatile and appropriate radiological investigation in cases of ocular and orbital trauma.33 34 35 They allow visualization of intraocular lesions including foreign bodies and subluxated lenses as well as extraocular lesions such as fractures. An ultrasound cannot be performed in an open globe injury (whereas a flat tire sign can be seen on a CT scan) and an MRI is contraindicated when a ferromagnetic foreign body is suspected. A CT scan is adequate for most foreign bodies except small pieces of glass and wood. In addition, in trauma, time is precious (CT scans are much faster than MRI scans) and multiple CT scans will already be performed (head, face, neck, etc.), making it easy to include an additional CT scan of the orbits.
Visual-evoked potential (VEP) has been described as an adjunctive investigation particularly useful in patients who are obtunded from their initial injury,36 those with unreliable pupillary responses and in cases of bilateral TON. It offers an objective assessment of the electrophysiological health of the ON and visual pathway. Both pattern reversal and flash VEP testing have been described as accurate in predicting VA in the literature36 37 with a reduction of amplitude and latency in the flash VEP being of particular importance.37 The presence as well as amplitude of VEP has been demonstrated to be predictive of long-term outcome. When the VEP is absent, recovery of vision is unlikely. VEP testing should be performed bilaterally, using the normal side as a control. When the VEP amplitude is within 50% of the normal side, the patient might have a favorable outcome.33 36
Though it is useful in assessing the severity as well as visual recovery potential of the patient, it is logistically difficult to move the required equipment to the bedside, especially in the case of a patient with multiple life-threatening injuries being resuscitated in the accident and emergency. A portable VEP, if available, may be used to overcome this. Another shortcoming would be that in patients with concomitant injury to the visual centers of the brain, abnormal VEP results might be obtained when the ON is in fact normal and undamaged.
In the literature, reduction in diffusion tension imaging (DTI) parameters (fractional anisotropy, mean diffusivity, axial diffusivity, and radial diffusivity) have been correlated with ON damage.37 38 39 In another study investigating ON regeneration following intravitreal implantation of a peripheral nerve graft in rats, Mn(2 + )-enhanced MRI (MEMRI) and DTI findings were correlated with immunohistochemical evidence of axon regeneration.40 41
Several studies have employed optical coherence tomography (OCT) to document thinning of the retinal nerve fiber layer (RNFL) following trauma to the ON.42 43 44 It takes time for this thinning to occur, and in the acute trauma scenario it may not be possible to sit the patient up to perform the OCT, reducing its utility in diagnosis. However, it may be useful for documenting the progression of damage over time and can be employed as part of the long-term follow-up after the injury. When comparing RNFL thinning to macular thinning, the former thinned to a greater degree and at a faster rate.39
In an ultrasound Doppler study of the central retinal artery (CRA) following TON, hemodynamics was significantly reduced.45 Specifically, peak systolic velocity (PSV), end diastolic velocity (EDV), and time average mean velocity (TAMX) were reduced. This was corroborated by a second study showed a reduction in PSV and EDV in the ipsilateral, and PSV in the contralateral CRA.46
Prognosis
Patients with TON, who sustain indirect trauma, do not lose consciousness, have a good initial VA, have lower grade RAPD, and show signs of visual recovery within 48 hours have a better prognosis.47 Absence of an optic canal fracture has been found to be a good prognostic factor in some studies but not in others.48 49 If picked up early, iatrogenic TON because of impingement of the ON by orbital plates, bears a good prognosis. Adjusting the position of the plate relieves the compression and the TON may resolve.
Management
Treatment of TON is controversial and multiple approaches have been proposed. These include conservative, medical, surgical, and combined management. Where indicated (evidence of ON compression), surgical decompression can be individualized and performed. In other cases, conservative management may be all that is necessary.
Conservative
A visual recovery rate of 40 to 60% has been reported in indirect TON cases managed conservatively, with baseline VA being the most important predictor of the final outcome.48 49 These figures are very much comparable with those achieved in patients treated with steroids, surgery, or a combination of both.50 However, these results may be biased as patients with good initial VA would more likely be treated conservatively.
Medical
Corticosteroids have been used to treat TON since the early 1980s following animal studies that demonstrated their neuroprotective effects after trauma. The positive effects observed included improved microcirculation, energy metabolism, postinjury histology, and functional outcomes.2 8 37 In addition, the ability of steroids to reduce swelling and their antioxidant properties have been postulated to be of benefit.51 Though promising, these initial studies were anecdotal and inadequate as they were nonrandomized, retrospective, and did not have controls making it difficult to draw conclusions.
With various agents, modes of administration, durations, and dosages, medical treatment can be highly variable. Using the example of methylprednisolone, dosages can be low (< 100 mg/d), moderate (100–499 mg/d), high (500–1,999 mg/d), very high (2,000–5,400 mg/d), and megadose (> 5,400 mg/d).
Corticosteroids have showed efficacy in the treatment of acute spinal cord injury and this finding formed the basis for its application in the treatment of TON. National Acute Spinal Cord Injury Study (NASCIS) I, II, and III comprise a series of three randomized controlled clinical trials evaluating the use of high-dose corticosteroids in the treatment of acute spinal cord injury. NASCIS I and II investigated if treatment resulted in better outcomes or not. It concluded that if steroid therapy was implemented within 3 hours of injury, there was no difference in outcomes but if given within 3 to 8 hours of injury, it is associated with improved outcomes.52 NASCIS III investigated the duration of treatment and concluded that when steroids were given within 3 to 8 hours of injury, continuing the treatment for 48 hours was associated with better outcomes than if it was only continued for 24 hours.53
However, both the results and applicability of these studies have been called into question. In NASCIS, the randomization process was possibly imbalanced and may have favored the steroid group. After 1 year of treatment, the mean difference between the steroid and placebo groups indicated a statistically significant benefit only for motor scores but not sensory scores. Even so, this result was obtained from a post hoc analysis of a subgroup of the trial (129 of 487 participants) and may not be representative. The same analysis also suggested use of steroids may be detrimental to treatment as in the group of patients treated with steroids more than 8 hours from injury, as outcomes were poorer when compared with patients in the placebo group. These studies investigate the treatment of injury to the spinal cord, which consists of both gray and white matter whereas the optic nerve only consists solely of white matter. This histological difference may not allow for the results of these studies to be directly applied to the treatment of TON.54 55 In addition, differences in vascular supply (large anastomoses instead of end vessels) and bony cavity in which it sits (wide vertebral canal instead of narrow optic canal) make comparison between the spinal cord and optic nerve difficult.
A randomized, double blind, placebo-controlled clinical trial compared the effect of intravenous high-dose corticosteroid therapy to placebo for the treatment of recent TON.56 Rajiniganth et al concluded there was no difference in improvement in VA between treatment arms. This was corroborated by two other studies,25 30 one of which also concluded that even in patients with poorer initial visual function, outcomes were similar with or without the use of steroids.
In addition to the efficacy of corticosteroids, their adverse effects should be considered as well. In an experimental study comparing the use of high, very high, and megadoses of methylprednisolone and saline for the treatment of optic nerve crush injuries in a rat model,51 saline-treated rats retained the greatest number of axons and there was a dose-dependent decline with increasing dosages of methylprednisolone used.
A large number of patients presenting with TON have concomitant head injury. As such, it is prudent to investigate the implications of steroid treatment for traumatic brain injury (TBI). The corticosteroid randomization after significant head injury (CRASH) was a multicenter, randomized, and placebo-controlled study that investigated the efficacy and safety of corticosteroids (methylprednisolone, 2 g loading dose, 0.4 g/h infusion for 48 hours) in patients with acute TBI. The study was aborted even before recruitment was completed as preliminary results showed an increased risk of death within 14 days after corticosteroid treatment was started.57 58
Other complications include gastrointestinal bleeding, wound infections, pneumonia, pancreatitis, sepsis, and acute psychosis, with the last four being observed with the use of higher doses of methylprednisolone.59 The duration of steroid application between studies is highly variable. Some studies began to taper the dose of steroids after 11 days and others ceased steroids completely after 9 days of treatment.
Surgery
Options for surgical management of TON include partial removal the bony optic canal (in cases of optic canal fracture fragments impinging the ON), ON fenestration and opening up the annulus of Zinn.26 These procedures principally serve to decompress the optic canal and ON as swelling and the resultant vascular compromise can cause secondary damage.60 There are multiple surgical techniques described in the literature that include intracranial, transethmoidal, endonasal and sublabial approaches, and both endoscopic as well as open surgery can be performed.61 62 In addition to choice of treatment, the timing of its implementation is debatable as well and there is no consensus about the gold standard of surgical treatment.
Various case studies have showed benefit from optic canal decompression surgery. However, the data are largely from small retrospective studies and comparison of studies is very difficult as there is a wide range of intracranial and extracranial techniques used. Inconsistent adjuvant steroid regimes provide another confounding factor, as does a recruitment bias toward a subgroup with worse baseline visual acuities or failure of corticosteroid treatment.
The International Optic Nerve Trauma Study (IONTS) was a nonrandomized, multicenter trial that looked at 133 patients with TON who were given either conservative (no treatment), medical (methylprednisolone), or surgical (optic canal decompression) treatment.63 It was a randomized clinical trial that was subsequently converted into a nonrandomized comparative study as patients who did not improve with steroid therapy were then also surgically managed.
The IONTS lacked uniformity regarding corticosteroid dose, timing of treatment, and indications for optic canal decompression. In the study, neither high-dose steroid therapy nor canal decompression surgery conferred any additional benefit compared with close observation. Significantly, a large percentage (57%) of those not treated showed three or more lines of improvement in VA.
The documented improvement after ON decompression with or without steroid therapy varies from 27 to 82%.64 65 The authors believe that these figures have such a large range due to poorly defined indications for decompression surgery. Hence, for surgery to be of benefit to the patient, it should only be performed when there is radiological evidence of optic canal fracture (and impingement of ON by fracture fragment), intraneural edema or an ON sheath hematoma. When incising the ON sheath, one should do so superomedially to avoid the ophthalmic artery that often runs inferolaterally.
The safety of surgical intervention has been examined in numerous studies and reported complications include injury to the ophthalmic artery, injury to the carotid artery, cerebrospinal fluid (CSF) leak, meningitis, and intraorbital infections.66 Complications may also be due to approach itself and a cadaveric study showed that transsphenoidal medial wall decompression of the ON canal with dural sheath opening might induce physical damage to the nerve.67
Endoscopic ON decompression (EOND) is an alternative to open surgery and is often done with an endonasal transsphenoidal approach, accessing the optic canal above the opticocarotid recess.68 69 70 This offers a less morbid approach that heals without a visible scar. With this approach, the roof and medial wall of the optic canal can be removed, annulus of Zinn opened and ON sheath incised. Other approaches include transconjunctival71 and transcranial (with extradural anterior clinoidectomy).72
In a study of 96 patients on whom endonasal transsphenoidal EOND was performed, results were far from satisfactory with only 40.6% of patients demonstrating at least improvement of one line on a Snellen chart.73 Subgroup analysis of patients who presented with light perception versus no light perception showed a large difference in efficacy between the two at 83.3 to 26.4%. This suggests that with a VA of at least light perception, EOND should be efficacious but in patients with no light perception, other approaches may be required. A study of 41 children (43 eyes) similarly concluded that the initial VA and the time difference between injury and surgery are the most important factors affecting prognosis.74 Interestingly, it also concluded that age of the patient does not influence the outcome of EOND.
In patients with epistaxis and TON, there should be a suspicion of carotid cavernous segment pseudoaneurysm and these patients should not undergo EOND.75 Instead, vascular-enhanced CT and digital subtraction angiography should be employed for diagnosis and promptly treated with vascular embolization (either stent or coil) as it can be fatal. In cases with CSF leakage, a transcranial approach through a supraorbital keyhole is more appropriate.76 The optic canal can be decompressed superiorly through the removal of its roof and anterior clinoid process and dural defect repaired with tensor fascia lata grafts anchored using fat or tissue glue.
Combined
Surgery has often been combined with medical therapy and vice versa in the treatment of TON; either synergistically or after one type of treatment fails to yield any improvement in vision. However, a study of 42 patients with TON did not find any significant differences in improvement of vision between the steroid and steroid with surgery arms.77
Timing of Intervention
The contrasting results of two studies from the Massachusetts Eye and Ear Infirmary emphasize the importance of early diagnosis and prompt management. From 1976 to 1987, 33 cases of TON were seen with 6 (18%) having no light perception vision and 9 (27%) having visual improvement.78 From 1987 to 1989, 14 cases of TON were seen with 5 (36%) having no light perception but with prompt diagnosis (within 1 week) and treatment (optic canal surgery, when indicated, was done within 24 hours) 11 (79%) experienced visual improvement.64 Even though the second population had a poorer prognosis, with prompt diagnosis and treatment, outcomes were better.
Neuroprotective and Neuroregenerative Treatments
Following an insult to a neuron from the central nervous system (CNS), it fails to regenerate. In addition, undamaged neurons have only a limited ability to form compensatory circuits, and there is relatively little replacement of lost neurons, placing severe constraints on functional recovery.79 Possible explanations for these include a decline in the intrinsic growth capacity of a neuron, inadequate levels of trophic factors, and the inhibitory environment at the site of injury. Hence, for both the anatomical and functional recovery of the ON to occur following trauma, both neuroprotection (promoting RGC survival by limiting apoptosis) and neuroregeneration need to take place.
Crystallin
The neuroprotective and axon growth promoting effects of lens injury have been attributed to certain purified lens proteins (crystallins). It is a member of the small heat shock protein family and has the potential to act as an anti-inflammatory agent in the neuroprotective process. Alpha-crystallin (α-crystallin) has been shown to promote RGC survival by two mechanisms, namely, retinal microglial cell inhibition and inhibition of tissue necrosis factor α-(TNF-α) and iNOS protein expression.80 Beta/gamma crystallin (β/γ crystallin) promotes infiltration of activated macrophages and activates retinal astrocytes, Müller cells and resident Microglia. In addition, β-crystallin induces astrocytes to produce ciliary neurotrophic factor (CNTF). These effects ultimately culminate in RGC survival and axonal regeneration with greater effects seen in β/γ-crystallin use compared with α-crystallin use.81
Erythropoietin
In a study comparing intravitreal erythropoietin (EPO) and conservative management for patients with indirect TON, patients treated with EPO showed a greater improvement in best corrected visual acuity (BCVA).82 This was supported by studies showing the neuroprotective and neuroregenerative effects of EPO in axotomized rats.83 84 Apart from transient hypotension during the infusion, there were no adverse drug reactions reported with the use of EPO in the study. A multicenter trial, Traumatic Optic Neuropathy Treatment Trial (TONTT), sponsored by the Tehran University of Medical Sciences, compares the use of EPO to methylprednisolone and is currently recruiting patients.
Glutamate Inhibitors
Glutamate is the major excitatory neurotransmitter in the eye that induces RGC apoptosis via binding to N-methyl diacetyl aspartate (NMDA) and kainate receptors. Glutamate inhibitors and NMDA receptor antagonists have been shown to promote RGC survival in rat models of ON injury.85
Lomerizine
The increase in intracellular calcium, via the activation of caspases and other substances and pathways within the neuron, plays an important role in apoptosis in the ON. The administration of lomerizine, a new calcium channel blocker, has been shown to reduce macrophage aggregation, reduce oxidative stress, and protected RGCs from secondary death. However, it did not prevent demyelination of the ON.86 87
Minocycline
Minocycline, a broad spectrum tetracycline antibiotic has been investigated as a potential treatment for primary open angle glaucoma as it has been shown to promote trabecular meshwork cell and ON head astrocyte survival.88 This antiapoptotic effect has also been demonstrated in the RGCs of rats with glaucomatous eyes and following ON transection.89
Neurotrophic Factors
Neurotrophic factors such as brain-derived neurotrophic factor (BDNF), and CNTF are essential to RGC survival and their absence noted in ON damage. Implantation of a segment of peripheral nerve graft into the posterior chamber of the eye of rats was found to be sufficient to stimulate the RGCs to regenerate axons through the ON itself, which suggested this was possibly due to secretion of trophic factors by Schwann cells. Following damage to the nerve, there is loss of retrograde supply of BDNF, which helps support the survival of existing neurons and encourages the growth and differentiation of new ones. Administration of BDNF has shown improved RGC survival.90 91 CNTF on the contrary, promotes neuroregeneration via neurite extension, and loss of CNTF receptors at the injury site has been noted in several studies.
Nonencephalitogenic Myelin Peptides
Modulating the immune response following trauma to the CNS has shown some promise as it can limit the damage and promote healing.92 93 Immunization of rats with nonencephalitogenic myelin peptides or myelin-based protein (MBP) triggered a T-cell–mediated immune response that slowed the degeneration of RGCs following a crush injury.94 Glatiramer acetate (Copolymer 1, Cop-1 or Copaxone) is a MBP that has been approved by the Food and Drug Administration (FDA) for reducing the frequency of relapses of multiple sclerosis. These results may one day translate into treatment for glaucoma and TON.
Oncomodulin
Oncomodulin (Ocm) was shown to be able to cause RGC axonal regeneration in the presence of mannose cyclic adenosine monophosphate (cAMP).95 This was further reinforced as its immunodepletion eliminated the axonal regeneration promoting effects of macrophage-conditioned media. Ocm is a small calcium-binding protein secreted by macrophages and neutrophils during ocular inflammation and has been shown to induce greater outgrowth than other factors such as BDNF, FGF2, CNTF, or GDNF.96 High levels of Ocm messenger ribonucleic acid and protein have been demonstrated within 1 day of injection of zymosan into the eye.97 Ocm crosses the inner limiting membrane and binds heavily to RGCs and their dendrites as evidenced by studying its displacement of a 22-aa blocking peptide, a competitive inhibitor of Ocm. This binding is cAMP-dependent and displacement was not seen when a cAMP antagonist was added.98 However, Ocm does not account for all axonal regeneration observed, as there must be elevated cAMP levels for it to work, suggesting that factors that cause this elevation are required as well. In addition, though immunodepleted macrophage–conditioned media devoid of Ocm does not cause axonal regeneration, it has been shown to promote RGC survival.
Tacrolimus (FK506)
FK506, a potent immunosuppressant that has been traditionally used to promote graft survival in organ transplantation, has shown beneficial effects on sciatic nerve recovery in several animal studies.99 100 FK506 treatment induces fibroblast apoptosis and thereby reduces the formation of scar tissue. This avoids the loss of myelin and, therefore, plays a protective or a sparing role after injury, which is thought to aid in ON protection.101 However, much is not known about its adverse effects with a case report102 even suggesting that it may be neurotoxic, as a patient developed a common peroneal nerve palsy with its use.
Therapeutic Hypothermia
A study in Sprague Dawley rats103 concluded that immediate short-duration hypothermia provides long-term protection in an in vivo model of traumatic axonal injury. After stretch injuries to the ON, immediate hypothermia of 32°C for 3 hours showed statistically reduced axonal degeneration in the core.
Tissue Necrosis Factor-α and Nitric Oxide Synthase Inhibitors
TNF-α and nitric oxide are both proapoptotic molecules and their inhibition could lead to greater RGC survival. Administration of nitric oxide synthase inhibitors enhanced RGC survival and delayed retrograde degeneration of RGC axons after axotomy.104
Ocular Transplantation
Though whole eye (with ON) transplantation may be an avenue to provide a patient with a complete and intact optical system, it is currently unsuccessful in mammals because of the ganglion cell axons inability to withstand transection, the inability to maintain circulation to the axons, and finally immune rejection of the transplant. There is however, evidence of some visual recovery with transplantations in cold-blooded vertebrates.105
Conclusion
In the light of the findings mentioned earlier, the authors have constructed a clinical pathway (Fig. 2) for the investigation and management of TON.
Fig. 2.
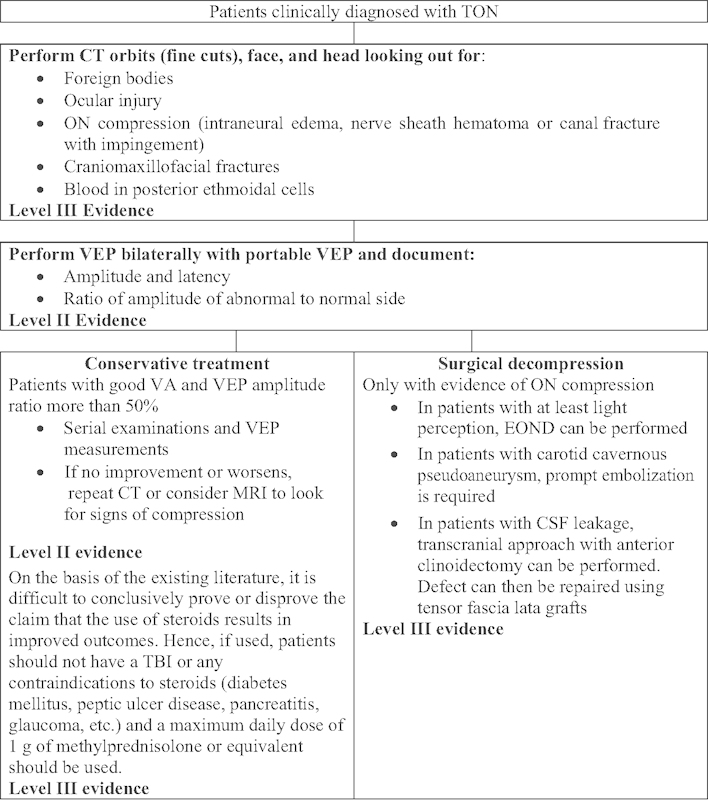
Clinical pathway. CT, computed tomography; EOND, endoscopic optic nerve decompression; MRI, magnetic resonance imaging; ON, optic nerve; TBI, traumatic brain injury; TON, traumatic optic neuropathy; VA, visual acuity; VET, visual-evoked potential.
References
- 1.Steinsapir K D Goldberg R A Traumatic optic neuropathy: an evolving understanding Am J Ophthalmol 20111516928–933., e2 [DOI] [PubMed] [Google Scholar]
- 2.Warner N, Eggenberger E. Traumatic optic neuropathy: a review of the current literature. Curr Opin Ophthalmol. 2010;21(6):459–462. doi: 10.1097/ICU.0b013e32833f00c9. [DOI] [PubMed] [Google Scholar]
- 3.Osborne N N, Chidlow G, Layton C J, Wood J P, Casson R J, Melena J. Optic nerve and neuroprotection strategies. Eye (Lond) 2004;18(11):1075–1084. doi: 10.1038/sj.eye.6701588. [DOI] [PubMed] [Google Scholar]
- 4.Steinsapir K D, Goldberg R A. Traumatic optic neuropathy: a critical update. Compr Ophthalmol Update. 2005;6:11–21. [Google Scholar]
- 5.Cirovic S, Bhola R M, Hose D R. et al. Computer modelling study of the mechanism of optic nerve injury in blunt trauma. Br J Ophthalmol. 2006;90(6):778–783. doi: 10.1136/bjo.2005.086538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh F B. Pathological-clinical correlations. I. Indirect trauma to the optic nerves and chiasm. II. Certain cerebral involvements associated with defective blood supply. Invest Ophthalmol. 1966;5(5):433–449. [PubMed] [Google Scholar]
- 7.Gross C E, DeKock J R, Panje W R, Hershkowitz N, Newman J. Evidence for orbital deformation that may contribute to monocular blindness following minor frontal head trauma. J Neurosurg. 1981;55(6):963–966. doi: 10.3171/jns.1981.55.6.0963. [DOI] [PubMed] [Google Scholar]
- 8.Anderson R L, Panje W R, Gross C E. Optic nerve blindness following blunt forehead trauma. Ophthalmology. 1982;89(5):445–455. doi: 10.1016/s0161-6420(82)34769-7. [DOI] [PubMed] [Google Scholar]
- 9.Crompton M R. Visual lesions in closed head injury. Brain. 1970;93(4):785–792. doi: 10.1093/brain/93.4.785. [DOI] [PubMed] [Google Scholar]
- 10.Pirouzmand F. Epidemiological trends of traumatic optic nerve injuries in the largest Canadian adult trauma center. J Craniofac Surg. 2012;23(2):516–520. doi: 10.1097/SCS.0b013e31824cd4a7. [DOI] [PubMed] [Google Scholar]
- 11.Lee V, Ford R L, Xing W, Bunce C, Foot B. Surveillance of traumatic optic neuropathy in the UK. Eye (Lond) 2010;24(2):240–250. doi: 10.1038/eye.2009.79. [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg-Cohen N, Miller N R, Repka M X. Traumatic optic neuropathy in children and adolescents. J AAPOS. 2004;8(1):20–27. doi: 10.1016/j.jaapos.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Bonavolontà G. Postoperative blindness following orbital surgery. Orbit. 2005;24(3):195–200. doi: 10.1080/01676830500192092. [DOI] [PubMed] [Google Scholar]
- 14.Cruz A A, dos Santos A C. Blindness after Le Fort I osteotomy: a possible complication associated with pterygomaxillary separation. J Craniomaxillofac Surg. 2006;34(4):210–216. doi: 10.1016/j.jcms.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Shibuya T Y, Feinberg S M, Mathog R H. et al. Visual risks of facial fracture repair in the setting of traumatic optic neuropathy. Arch Otolaryngol Head Neck Surg. 2006;132(3):258–264. doi: 10.1001/archotol.132.3.258. [DOI] [PubMed] [Google Scholar]
- 16.Roccia F, Boffano P, Guglielmi V. et al. Role of the maxillofacial surgeon in the management of severe ocular injuries after maxillofacial fractures. J Emerg Trauma Shock. 2011;4(2):188–193. doi: 10.4103/0974-2700.82204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vásquez L M, González-Candial M. Permanent blindness after endoscopic sinus surgery. Orbit. 2011;30(2):108–110. doi: 10.3109/01676830.2010.546554. [DOI] [PubMed] [Google Scholar]
- 18.Kim J Y, Kim H J, Kim C-H, Lee J-G, Yoon J-H. Optic nerve injury secondary to endoscopic sinus surgery: an analysis of three cases. Yonsei Med J. 2005;46(2):300–304. doi: 10.3349/ymj.2005.46.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J W, Chin B R, Park H S, Lee S H, Kwon T G. Cranial nerve injury after Le Fort I osteotomy. Int J Oral Maxillofac Surg. 2011;40(3):327–329. doi: 10.1016/j.ijom.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y L, Lee L A, Lim K E. Surgical consideration to optic nerve protrusion according to sinus computed tomography. Otolaryngol Head Neck Surg. 2006;134(3):499–505. doi: 10.1016/j.otohns.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 21.Urolagin S B, Kotrashetti S M, Kale T P, Balihallimath L J. Traumatic optic neuropathy after maxillofacial trauma: a review of 8 cases. J Oral Maxillofac Surg. 2012;70(5):1123–1130. doi: 10.1016/j.joms.2011.09.045. [DOI] [PubMed] [Google Scholar]
- 22.Robinson D Wilcsek G Sacks R Orbit and orbital apex Otolaryngol Clin North Am 2011444903–922., viii [DOI] [PubMed] [Google Scholar]
- 23.Spoor T C, Hartel W C, Lensink D B, Wilkinson M J. Treatment of traumatic optic neuropathy with corticosteroids. Am J Ophthalmol. 1990;110(6):665–669. doi: 10.1016/s0002-9394(14)77065-5. [DOI] [PubMed] [Google Scholar]
- 24.Razo-Blanco-Hernández D M Lima-Gómez V Asymmetrical bilateral optic neuropathy. Case report [in Spanish] Cir Cir 2009774309–312., 287–290 [PubMed] [Google Scholar]
- 25.Ropposch T, Steger B, Meço C. et al. The effect of steroids in combination with optic nerve decompression surgery in traumatic optic neuropathy. Laryngoscope. 2013;123(5):1082–1086. doi: 10.1002/lary.23845. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal R, Shah M, Mireskandari K, Yong G K. Controversies in ocular trauma classification and management: review. Int Ophthalmol. 2013;33(4):435–445. doi: 10.1007/s10792-012-9698-y. [DOI] [PubMed] [Google Scholar]
- 27.Yu-Wai-Man P, Griffiths P G. Steroids for traumatic optic neuropathy. Cochrane Database Syst Rev. 2013;6:CD006032. doi: 10.1002/14651858.CD006032.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raji M R, Manfredi S J, Gabriele O F, Sprinkle P M, Weinstein G W. Computerized tomography-evaluation in facial trauma associated with blindness. Am J Neuroradiol. 1982;3(1):92. [Google Scholar]
- 29.Seiff S R, Berger M S, Guyon J, Pitts L H. Computed tomographic evaluation of the optic canal in sudden traumatic blindness. Am J Ophthalmol. 1984;98(6):751–755. doi: 10.1016/0002-9394(84)90693-7. [DOI] [PubMed] [Google Scholar]
- 30.Lee K F, Muhd Nor N I, Yaakub A, Wan Hitam W H. Traumatic optic neuropathy: a review of 24 patients. Int J Ophthalmol. 2010;3(2):175–178. doi: 10.3980/j.issn.2222-3959.2010.02.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takehara S, Tanaka T, Uemura K. et al. Optic nerve injury demonstrated by MRI with STIR sequences. Neuroradiology. 1994;36(7):512–514. doi: 10.1007/BF00593510. [DOI] [PubMed] [Google Scholar]
- 32.Parab R, Fung C I, Van Der Merwe G. Blunt facial trauma causing isolated optic nerve hematoma. Case Rep Radiol. 2013;2013:235209. doi: 10.1155/2013/235209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mine S, Yamakami I, Yamaura A. et al. Outcome of traumatic optic neuropathy. Comparison between surgical and nonsurgical treatment. Acta Neurochir (Wien) 1999;141(1):27–30. doi: 10.1007/s007010050262. [DOI] [PubMed] [Google Scholar]
- 34.Kubal W S. Imaging of orbital trauma. Radiographics. 2008;28(6):1729–1739. doi: 10.1148/rg.286085523. [DOI] [PubMed] [Google Scholar]
- 35.Lee H J, Jilani M, Frohman L, Baker S. CT of orbital trauma. Emerg Radiol. 2004;10(4):168–172. doi: 10.1007/s10140-003-0282-7. [DOI] [PubMed] [Google Scholar]
- 36.Yeh S, Foroozan R. Orbital apex syndrome. Curr Opin Ophthalmol. 2004;15(6):490–498. doi: 10.1097/01.icu.0000144387.12739.9c. [DOI] [PubMed] [Google Scholar]
- 37.Holmes M D, Sires B S. Flash visual evoked potentials predict visual outcome in traumatic optic neuropathy. Ophthal Plast Reconstr Surg. 2004;20(5):342–346. doi: 10.1097/01.iop.0000134272.55294.4c. [DOI] [PubMed] [Google Scholar]
- 38.Bodanapally U K, Kathirkamanathan S, Geraymovych E. et al. Diagnosis of traumatic optic neuropathy: application of diffusion tensor magnetic resonance imaging. J Neuroophthalmol. 2013;33(2):128–133. doi: 10.1097/WNO.0b013e3182842553. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Shi W, Li M. et al. Time-dependent diffusion tensor changes of optic nerve in patients with indirect traumatic optic neuropathy. Acta Radiol. 2014;55(7):855–863. doi: 10.1177/0284185113506900. [DOI] [PubMed] [Google Scholar]
- 40.Yang Q T, Fan Y P, Zou Y. et al. Evaluation of traumatic optic neuropathy in patients with optic canal fracture using diffusion tensor magnetic resonance imaging: a preliminary report. ORL J Otorhinolaryngol Relat Spec. 2011;73(6):301–307. doi: 10.1159/000330723. [DOI] [PubMed] [Google Scholar]
- 41.Thuen M, Olsen O, Berry M. et al. Combination of Mn(2+)-enhanced and diffusion tensor MR imaging gives complementary information about injury and regeneration in the adult rat optic nerve. J Magn Reson Imaging. 2009;29(1):39–51. doi: 10.1002/jmri.21606. [DOI] [PubMed] [Google Scholar]
- 42.Cunha L P, Costa-Cunha L V, Malta R F, Monteiro M L. Comparison between retinal nerve fiber layer and macular thickness measured with OCT detecting progressive axonal loss following traumatic optic neuropathy. Arq Bras Oftalmol. 2009;72(5):622–625. doi: 10.1590/s0004-27492009000500004. [DOI] [PubMed] [Google Scholar]
- 43.Mohan K, Kecova H, Hernandez-Merino E, Kardon R H, Harper M M. Retinal ganglion cell damage in an experimental rodent model of blast-mediated traumatic brain injury. Invest Ophthalmol Vis Sci. 2013;54(5):3440–3450. doi: 10.1167/iovs.12-11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chauhan B C, Stevens K T, Levesque J M. et al. Longitudinal in vivo imaging of retinal ganglion cells and retinal thickness changes following optic nerve injury in mice. PLoS ONE. 2012;7(6):e40352. doi: 10.1371/journal.pone.0040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi W, Wang H Z, Song W X, Yang W L, Li W Y, Wang N L. Axonal loss and blood flow disturbances in the natural course of indirect traumatic optic neuropathy. Chin Med J (Engl) 2013;126(7):1292–1297. [PubMed] [Google Scholar]
- 46.Ustymowicz A, Mariak Z, Obuchowska I, Mariak Z, Kochanowicz J. Blood flow disturbances in the central retinal artery in patients with traumatic optic neuropathy. Med Sci Monit. 2009;15(7):CR366–CR371. [PubMed] [Google Scholar]
- 47.Tabatabaei S A, Soleimani M, Alizadeh M. et al. Predictive value of visual evoked potentials, relative afferent pupillary defect, and orbital fractures in patients with traumatic optic neuropathy. Clin Ophthalmol. 2011;5:1021–1026. doi: 10.2147/OPTH.S21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carta A, Ferrigno L, Salvo M, Bianchi-Marzoli S, Boschi A, Carta F. Visual prognosis after indirect traumatic optic neuropathy. J Neurol Neurosurg Psychiatry. 2003;74(2):246–248. doi: 10.1136/jnnp.74.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chou P I, Sadun A A, Chen Y C. et al. Clinical experiences in the management of traumatic optic neuropathy. Neuroophthalmology. 1996;16:325–336. [Google Scholar]
- 50.Ishikawa A, Okabe H, Nakagawa Y, Kiyosawa M. Treatment and following-up of traumatic optic neuropathy. Neuro-Ophthalmology Japan. 1996;13(2):175–183. [Google Scholar]
- 51.Steinsapir K D, Goldberg R A, Sinha S, Hovda D A. Methylprednisolone exacerbates axonal loss following optic nerve trauma in rats. Restor Neurol Neurosci. 2000;17(4):157–163. [PubMed] [Google Scholar]
- 52.Bracken M B, Shepard M J, Collins W F. et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322(20):1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 53.Bracken M B, Shepard M J, Collins W F Jr. et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J Neurosurg. 1992;76(1):23–31. doi: 10.3171/jns.1992.76.1.0023. [DOI] [PubMed] [Google Scholar]
- 54.Nesathurai S. Steroids and spinal cord injury: revisiting the NASCIS 2 and NASCIS 3 trials. J Trauma. 1998;45(6):1088–1093. doi: 10.1097/00005373-199812000-00021. [DOI] [PubMed] [Google Scholar]
- 55.Coleman W P, Benzel D, Cahill D W. et al. A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of methylprednisolone in acute spinal cord injury. J Spinal Disord. 2000;13(3):185–199. doi: 10.1097/00002517-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Rajiniganth M G, Gupta A K, Gupta A, Bapuraj J R. Traumatic optic neuropathy: visual outcome following combined therapy protocol. Arch Otolaryngol Head Neck Surg. 2003;129(11):1203–1206. doi: 10.1001/archotol.129.11.1203. [DOI] [PubMed] [Google Scholar]
- 57.Edwards P, Arango M, Balica L. et al. CRASH trial collaborators. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet. 2005;365(9475):1957–1959. doi: 10.1016/S0140-6736(05)66552-X. [DOI] [PubMed] [Google Scholar]
- 58.Roberts I, Yates D, Sandercock P. et al. CRASH trial collaborators. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 2004;364(9442):1321–1328. doi: 10.1016/S0140-6736(04)17188-2. [DOI] [PubMed] [Google Scholar]
- 59.Braughler J M, Hall E D, Means E D, Waters T R, Anderson D K. Evaluation of an intensive methylprednisolone sodium succinate dosing regimen in experimental spinal cord injury. J Neurosurg. 1987;67(1):102–105. doi: 10.3171/jns.1987.67.1.0102. [DOI] [PubMed] [Google Scholar]
- 60.Samardzic K, Samardzic J, Janjetovic Z, Samardzic I, Sekelj S, Latic-Hodzic L. Traumatic optic neuropathy—to treat or to observe? Acta Inform Med. 2012;20(2):131–132. doi: 10.5455/aim.2012.20.131-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fukado Y. Results in 400 cases of surgical decompression of the optic nerve. Mod Probl Ophthalmol. 1975;14:474–481. [PubMed] [Google Scholar]
- 62.Fujitani T, Inoue K, Takahashi T, Ikushima K, Asai T. Indirect traumatic optic neuropathy—visual outcome of operative and nonoperative cases. Jpn J Ophthalmol. 1986;30(1):125–134. [PubMed] [Google Scholar]
- 63.Levin L A, Beck R W, Joseph M P, Seiff S, Kraker R. The treatment of traumatic optic neuropathy: the International Optic Nerve Trauma Study. Ophthalmology. 1999;106(7):1268–1277. doi: 10.1016/s0161-6420(99)00707-1. [DOI] [PubMed] [Google Scholar]
- 64.Joseph M P, Lessell S, Rizzo J, Momose K J. Extracranial optic nerve decompression for traumatic optic neuropathy. Arch Ophthalmol. 1990;108(8):1091–1093. doi: 10.1001/archopht.1990.01070100047032. [DOI] [PubMed] [Google Scholar]
- 65.Miller N R. The management of traumatic optic neuropathy. Arch Ophthalmol. 1990;108(8):1086–1087. doi: 10.1001/archopht.1990.01070100042030. [DOI] [PubMed] [Google Scholar]
- 66.Li H, Zhou B, Shi J, Cheng L, Wen W, Xu G. Treatment of traumatic optic neuropathy: our experience of endoscopic optic nerve decompression. J Laryngol Otol. 2008;122(12):1325–1329. doi: 10.1017/S0022215108002296. [DOI] [PubMed] [Google Scholar]
- 67.Onofrey C B, Tse D T, Johnson T E. et al. Optic canal decompression: a cadaveric study of the effects of surgery. Ophthal Plast Reconstr Surg. 2007;23(4):261–266. doi: 10.1097/IOP.0b013e3180cac220. [DOI] [PubMed] [Google Scholar]
- 68.Horiguchi K, Murai H, Hasegawa Y, Mine S, Yamakami I, Saeki N. Endoscopic endonasal trans-sphenoidal optic nerve decompression for traumatic optic neuropathy—technical note. Neurol Med Chir (Tokyo) 2010;50(6):518–522. doi: 10.2176/nmc.50.518. [DOI] [PubMed] [Google Scholar]
- 69.Kong D S, Shin H J, Kim H Y. et al. Endoscopic optic canal decompression for compressive optic neuropathy. J Clin Neurosci. 2011;18(11):1541–1545. doi: 10.1016/j.jocn.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 70.Wang D H, Zheng C Q, Qian J, Barr J J, Anderson A G Jr. Endoscopic optic nerve decompression for the treatment of traumatic optic nerve neuropathy. ORL J Otorhinolaryngol Relat Spec. 2008;70(2):130–133. doi: 10.1159/000114537. [DOI] [PubMed] [Google Scholar]
- 71.Chen C T Huang F Tsay P K et al. Endoscopically assisted transconjunctival decompression of traumatic optic neuropathy J Craniofac Surg 200718119–26., discussion 27–28 [DOI] [PubMed] [Google Scholar]
- 72.Yang Y Wang H Shao Y Wei Z Zhu S Wang J Extradural anterior clinoidectomy as an alternative approach for optic nerve decompression: anatomic study and clinical experience Neurosurgery 200659402ONS253–ONS262., discussion ONS262 [DOI] [PubMed] [Google Scholar]
- 73.Yang Q T, Zhang G H, Liu X, Ye J, Li Y. The therapeutic efficacy of endoscopic optic nerve decompression and its effects on the prognoses of 96 cases of traumatic optic neuropathy. J Trauma Acute Care Surg. 2012;72(5):1350–1355. doi: 10.1097/TA.0b013e3182493c70. [DOI] [PubMed] [Google Scholar]
- 74.Peng A, Li Y, Hu P, Wang Q. Endoscopic optic nerve decompression for traumatic optic neuropathy in children. Int J Pediatr Otorhinolaryngol. 2011;75(8):992–998. doi: 10.1016/j.ijporl.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 75.Kang Z, Li J, Zou Y, Yang Q. Diagnosis and treatment of traumatic optic neuropathy with carotid artery cavernous segment pseudoaneurysm. Laryngoscope. 2013;123(11):2591–2597. doi: 10.1002/lary.24013. [DOI] [PubMed] [Google Scholar]
- 76.Chen Y H, Lin S Z, Chiang Y H, Ju D T, Liu M Y, Chen G J. Supraorbital keyhole surgery for optic nerve decompression and dura repair. J Neurotrauma. 2004;21(7):976–981. doi: 10.1089/0897715041526140. [DOI] [PubMed] [Google Scholar]
- 77.Yang W G, Chen C T, Tsay P K, de Villa G H, Tsai Y J, Chen Y R. Outcome for traumatic optic neuropathy—surgical versus nonsurgical treatment. Ann Plast Surg. 2004;52(1):36–42. doi: 10.1097/01.sap.0000096442.82059.6d. [DOI] [PubMed] [Google Scholar]
- 78.Lessell S. Indirect optic nerve trauma. Arch Ophthalmol. 1989;107(3):382–386. doi: 10.1001/archopht.1989.01070010392031. [DOI] [PubMed] [Google Scholar]
- 79.Chen D F, Jhaveri S, Schneider G E. Intrinsic changes in developing retinal neurons result in regenerative failure of their axons. Proc Natl Acad Sci U S A. 1995;92(16):7287–7291. doi: 10.1073/pnas.92.16.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fischer D, Hauk T G, Müller A, Thanos S. Crystallins of the beta/gamma-superfamily mimic the effects of lens injury and promote axon regeneration. Mol Cell Neurosci. 2008;37(3):471–479. doi: 10.1016/j.mcn.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 81.Wu N, Yin Z Q, Wang Y. Traumatic optic neuropathy therapy: an update of clinical and experimental studies. J Int Med Res. 2008;36(5):883–889. doi: 10.1177/147323000803600503. [DOI] [PubMed] [Google Scholar]
- 82.Kashkouli M B, Pakdel F, Sanjari M S. et al. Erythropoietin: a novel treatment for traumatic optic neuropathy-a pilot study. Graefes Arch Clin Exp Ophthalmol. 2011;249(5):731–736. doi: 10.1007/s00417-010-1534-3. [DOI] [PubMed] [Google Scholar]
- 83.Weishaupt J H, Rohde G, Pölking E, Siren A L, Ehrenreich H, Bähr M. Effect of erythropoietin axotomy-induced apoptosis in rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45(5):1514–1522. doi: 10.1167/iovs.03-1039. [DOI] [PubMed] [Google Scholar]
- 84.King C E, Rodger J, Bartlett C, Esmaili T, Dunlop S A, Beazley L D. Erythropoietin is both neuroprotective and neuroregenerative following optic nerve transection. Exp Neurol. 2007;205(1):48–55. doi: 10.1016/j.expneurol.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 85.Dkhissi O, Chanut E, Wasowicz M. et al. Retinal TUNEL-positive cells and high glutamate levels in vitreous humor of mutant quail with a glaucoma-like disorder. Invest Ophthalmol Vis Sci. 1999;40(5):990–995. [PubMed] [Google Scholar]
- 86.Fitzgerald M, Bartlett C A, Evill L, Rodger J, Harvey A R, Dunlop S A. Secondary degeneration of the optic nerve following partial transection: the benefits of lomerizine. Exp Neurol. 2009;216(1):219–230. doi: 10.1016/j.expneurol.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 87.Fitzgerald M, Payne S C, Bartlett C A, Evill L, Harvey A R, Dunlop S A. Secondary retinal ganglion cell death and the neuroprotective effects of the calcium channel blocker lomerizine. Invest Ophthalmol Vis Sci. 2009;50(11):5456–5462. doi: 10.1167/iovs.09-3717. [DOI] [PubMed] [Google Scholar]
- 88.Kernt M, Neubauer A S, Eibl K H. et al. Minocycline is cytoprotective in human trabecular meshwork cells and optic nerve head astrocytes by increasing expression of XIAP, survivin, and Bcl-2. Clin Ophthalmol. 2010;4:591–604. doi: 10.2147/opth.s11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levkovitch-Verbin H, Kalev-Landoy M, Habot-Wilner Z, Melamed S. Minocycline delays death of retinal ganglion cells in experimental glaucoma and after optic nerve transection. Arch Ophthalmol. 2006;124(4):520–526. doi: 10.1001/archopht.124.4.520. [DOI] [PubMed] [Google Scholar]
- 90.Chen H, Weber A J. BDNF enhances retinal ganglion cell survival in cats with optic nerve damage. Invest Ophthalmol Vis Sci. 2001;42(5):966–974. [PubMed] [Google Scholar]
- 91.Di Polo A, Aigner L J, Dunn R J, Bray G M, Aguayo A J. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Müller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A. 1998;95(7):3978–3983. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schwartz M, Moalem G. Beneficial immune activity after CNS injury: prospects for vaccination. J Neuroimmunol. 2001;113(2):185–192. doi: 10.1016/s0165-5728(00)00447-1. [DOI] [PubMed] [Google Scholar]
- 93.Schwartz M. Protective autoimmunity as a T-cell response to central nervous system trauma: prospects for therapeutic vaccines. Prog Neurobiol. 2001;65(5):489–496. doi: 10.1016/s0301-0082(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 94.Kipnis J, Yoles E, Schori H, Hauben E, Shaked I, Schwartz M. Neuronal survival after CNS insult is determined by a genetically encoded autoimmune response. J Neurosci. 2001;21(13):4564–4571. doi: 10.1523/JNEUROSCI.21-13-04564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yin Y, Cui Q, Li Y. et al. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23(6):2284–2293. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin Y, Henzl M T, Lorber B. et al. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci. 2006;9(6):843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- 97.Kurimoto T, Yin Y, Omura K. et al. Long-distance axon regeneration in the mature optic nerve: contributions of oncomodulin, cAMP, and pten gene deletion. J Neurosci. 2010;30(46):15654–15663. doi: 10.1523/JNEUROSCI.4340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yin Y, Cui Q, Gilbert H Y. et al. Oncomodulin links inflammation to optic nerve regeneration. Proc Natl Acad Sci U S A. 2009;106(46):19587–19592. doi: 10.1073/pnas.0907085106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hizay A Demirel B M Gokhan G Sarikcioglu L Demir N Does FK506 reduce the size of the watershed area after vascular injury of the sciatic nerve? Rom J Morphol Embryol 201152(3, Suppl):1077–1080. [PubMed] [Google Scholar]
- 100.Que J, Cao Q, Sui T, Du S, Kong D, Cao X. Effect of FK506 in reducing scar formation by inducing fibroblast apoptosis after sciatic nerve injury in rats. Cell Death Dis. 2013;4:e526. doi: 10.1038/cddis.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sarikcioglu L, Demir N, Akar Y, Demirtop A. Effect of intrathecal FK506 administration on intraorbital optic nerve crush: an ultrastructural study. Can J Ophthalmol. 2009;44(4):427–430. doi: 10.3129/i09-071. [DOI] [PubMed] [Google Scholar]
- 102.Jain A, Mathew P J, Modi M, Mangal K. Unilateral common peroneal nerve palsy following renal transplantation: a case report of tacrolimus neurotoxicity. J Postgrad Med. 2011;57(2):126–128. doi: 10.4103/0022-3859.81871. [DOI] [PubMed] [Google Scholar]
- 103.Ma M, Matthews B T, Lampe J W, Meaney D F, Shofer F S, Neumar R W. Immediate short-duration hypothermia provides long-term protection in an in vivo model of traumatic axonal injury. Exp Neurol. 2009;215(1):119–127. doi: 10.1016/j.expneurol.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tezel G, Yang X, Yang J, Wax M B. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res. 2004;996(2):202–212. doi: 10.1016/j.brainres.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 105.Ellenberg D, Shi J, Jain S. et al. Impediments to eye transplantation: ocular viability following optic-nerve transection or enucleation. Br J Ophthalmol. 2009;93(9):1134–1140. doi: 10.1136/bjo.2008.155267. [DOI] [PMC free article] [PubMed] [Google Scholar]


