Abstract
Cyclophosphamide in combination with busulfan (Bu) or total body irradiation (TBI) are the most commonly used myeloablative conditioning regimens in patients with Chronic Myeloid Leukemia (CML). We used data from the Center for International Bone Marrow Transplantation Research to compare outcomes in adults who underwent hematopoietic cell transplantation for CML in first chronic phase following myeloablative conditioning with cyclophosphamide (Cy) in combination with TBI, oral Bu or intravenous (IV) Bu. Four hundred thirty-eight adults received human leukocyte antigen (HLA)-matched sibling grafts and 235 received well-matched grafts from unrelated donors from 2000 through 2006. Important differences existed between the groups in distribution of donor relation, exposure to tyrosine kinase inhibitors and year of transplantation. In multivariate analysis, relapse occurred less frequently among patients receiving IV Bu compared to TBI (RR=0.36; P=0.022) or oral Bu (RR=0.39; P=0.028), but non-relapse mortality and survival were similar. A significant interaction was detected between donor relation and the main effect in leukemia-free survival (LFS). Among recipients of HLA-identical sibling grafts, but not URD grafts, LFS was better in patients receiving IV (RR=0.53; P=0.025) or oral Bu (RR=0.64; P=0.017) compared to TBI. In CML in first chronic phase, Cy in combination with IV Bu was associated with less relapse than TBI or oral Bu. LFS was better following IV or oral Bu compared to TBI.
Introduction
Tyrosine kinase inhibitors (TKIs) have replaced allogeneic hematopoietic cell transplantation (HCT) as initial therapy of patients with chronic myeloid leukemia (CML). Nevertheless, many patients with CML eventually receive an allotransplant. Determining the best pretransplant conditioning regimen is important.
Cyclophosphamide combined with total body irradiation (Cy/TBI) has historically been the standard pretransplant conditioning regimen. 1-4 The combination of Cy with a fixed dose of oral busulfan (BuCy) has also proven effective in CML.5 A randomized comparison of Cy/TBI to BuCy in patients with CML undergoing human leukocyte antigen (HLA)-identical sibling transplantation reported comparable relapse, leukemia-free survival (LFS) and overall survival (OS). BuCy was better tolerated, however, with shorter hospitalization and less acute graft-versus-host disease (GvHD).6 A second randomized study reported similar outcomes but with fewer relapses in the BuCy cohort. 7
The development of an assay for plasma Bu was initially reported in 1983, 8 but an assay was not commercially available until 1996. 9 Studies of Bu kinetics revealed that oral Bu is erratically absorbed and that oral administration of a fixed-dose results in wide variations in plasma Bu levels.10,11,12,13 Low plasma levels are associated with increased risks of graft-failure and relapse and high levels with increased toxicity. 10,11,12 Dose adjustment of oral Bu, based on plasma levels following the initial dose, decreases the variability and may improve outcomes.14 An intravenous (IV) formulation of Bu was developed and its use in patients was first reported in 2002. 15,16 It provides complete bioavailability, much more consistent plasma levels and less acute toxicity and 100-day mortality than an oral fixed-dose.15,16 Although a retrospective study in Acute Myeloid Leukemia (AML) from the European Group for Blood and Marrow Transplantation failed to show significant differences in outcome, 17 a recent large retrospective study in patients with AML in first remission from the Center for International Bone Marrow Transplant Research (CIBMTR) reported significantly less non-relapse mortality (NRM) and late relapse, and better LFS and OS with Cy in combination with IV, but not oral, Bu compared with TBI. 18 A recent prospective cohort analysis in persons with MDS, AML and CML reported better survival following IV Bu than with TBI.19
No prospective or retrospective study has compared Cy in combination with IV Bu, oral Bu or TBI in patients with CML in chronic phase. We used data from the CIBMTR to compare outcomes following these regimens.
Patients and methods
Data sources
The CIBMTR is a working group of more than 500 transplant centers worldwide that voluntarily contribute data on allogeneic and autologous transplants. Detailed demographic, disease, and transplant characteristics and outcome data are collected on a sample of registered patients including all unrelated donor (URD) transplants facilitated by the National Marrow Donor Program in the United States. Observational studies conducted by the CIBMTR are carried out with a waiver of informed consent and in compliance with HIPAA regulations as determined by the Institutional Review Board and the Privacy Officer of the Medical College of Wisconsin.
Patients
The study population consisted of all patients ≥ 18 years of age reported to the CIBMTR who received a first HCT with an HLA-identical sibling or well-matched URD20 from 2000-2006 for CML in first chronic phase after pretransplant conditioning with Cy/TBI (single-dose ≥5.5 Gy, fractionated ≥9 Gy) or Bu (≥9mg/kg) combined with Cy and no other anti-cancer drugs. The data set was derived from CIBMTR comprehensive report forms. Patients with a genetically-identical twin or cord blood donor, an ex vivo T cell depleted graft, a less than well-matched URD, or receiving Cy post-transplant were excluded. Data regarding Bu pharmacokinetics (PK) were not collected.
Study end points and definitions
The primary outcome studied was overall survival. Patients were considered to have an event at the time of death from any cause; survivors were censored at last contact. NRM was defined as death without evidence of leukemia recurrence; relapse, defined by hematologic, cytogenetic, or molecular criteria, was considered a competing event. LFS was defined as time to treatment failure (death or relapse). For relapse, NRM and LFS, patients alive in continuous complete remission were censored at last follow-up. Times to neutrophil and platelet recovery were calculated as the time from transplantation to achieving the first of three consecutive days with neutrophils >0.5 × 109/L and platelets > 20×109/L, 7 days from the last platelet transfusion. Acute GvHD was graded according to consensus criteria based on the pattern of severity of abnormalities in skin, gastrointestinal tract and liver. 21 Chronic GvHD was diagnosed by standard criteria. 22 For hematopoietic recovery and GvHD, death without the event was considered a competing event.
Statistical Methods
In univariate analysis, probabilities of LFS and OS were calculated using the Kaplan-Meier method, with the variance estimated by Greenwood’s formula. Hematopoietic recovery, GvHD, NRM, and relapse were estimated using the cumulative incidence method to account for competing risks.
In multivariate analysis, a forward stepwise selection procedure was performed using the proportional hazards Cox model for OS, LFS, NRM, GvHD, and relapse to adjust for the following variables considered for inclusion in each model - subject: age, gender, and Karnofsky performance score at transplant; disease: interval from diagnosis to transplant and TKI use prior to HCT, and transplant-related: donor-recipient gender and Cytomegalovirus (CMV) serological status, donor relation and graft source, year of transplant, ATG or alemtuzumab use, GvHD prophylaxis, and planned use of growth factors post-transplant. P<0.05 was used to select variables to enter and to retain as covariates in the model. The proportional hazards assumption was assessed for each variable by testing its time dependency. Interactions were checked between each selected variable and the main effect.
Adjusted LFS and survival probabilities were estimated through the direct adjusted survival curves estimation method.23 SAS software, version 9.3 (SAS Institute, Cary, NC) was used in all analyses.
Results
Demographics and univariate analysis
Six hundred seventy-three adults received a first HCT from an HLA-matched sibling (N=438) or well-matched unrelated donor (URD; N=235) from January 1, 2000 through December 31, 2006 for CML in first chronic phase following myeloablative preparation with Cy combined with TBI, oral Bu or IV Bu. The median follow up of surviving patients is five years. Characteristics of patients categorized according to pretransplant conditioning regimen are described in Table 1. Patients who received IV Bu were older (median age 39 years, 42% > 40 years) than those receiving TBI (median age 35 years, 31% > 40 years) or oral Bu (median age 34 years, 29% > 40 years). Eighty-three percent of patients receiving oral Bu and 67% receiving IV Bu received a transplant from an HLA-identical sibling compared to 36% of those receiving TBI. Sixty-seven percent of IV Bu patients, compared to 27% of oral Bu and 36% of TBI patients, received at least one TKI before transplant. Nine percent of oral Bu and 13% of IV Bu patients received anti-thymocyte globulin (ATG) or alemtuzumab compared to 18% of those receiving TBI. Sixty-eight percent of IV Bu patients, compared to 37% oral Bu and 23% of TBI patients, underwent HCT from 2004-2006, the last 3 years of study. Median and interquartile range (IQR) of radiation dose was 12 Gy (IQR: 12-13.2 Gy) and Cy dose was 119.5 mg/kg (IQR: 98 – 120 mg/kg) for patients receiving TBI. Median Cy doses were 119 mg/kg (IQR: 105 – 120 mg/kg) and 109 mg/kg (IQR: 98 – 120 mg/kg) for those receiving oral and IV Bu. Median Cy does were identical (119mg/kg) for patients receiving BuCy regardless of whether the donor was an HLA-identical sibling or unrelated. The median and IQR Bu dose was 15.7 mg/kg (IQR: 14 – 16 mg/kg) for patients receiving oral Bu and 12 mg/kg (IQR: 10 – 13 mg/kg) for those receiving IV Bu.
Table 1.
Characteristics of patients
| Characteristics of patients | Cy TBI | Oral BuCy | IV BuCy | Overall p- value |
|---|---|---|---|---|
| Number of patients | 222 | 354 | 97 | |
| Number of centers | 75 | 68 | 47 | |
| Patient-Related | ||||
| Age, median (range), years | 35 (18- 59) |
34 (18- 59) |
39 (18- 61) |
0.018 |
| 18-30 | 73 (33) | 136 (38) | 28 (29) | 0.167 |
| 31-40 | 80 (36) | 116 (33) | 28 (29) | |
| 41-50 | 47 (21) | 75 (21) | 27 (28) | |
| >50 | 22 (10) | 27 ( 8) | 14 (14) | |
| Sex | 0.293 | |||
| Male | 145 (65) | 211 (60) | 56 (58) | |
| Karnofsky performance score at transplant | 0.142 | |||
| <90% | 19 ( 9) | 21 ( 6) | 11 (11) | |
| >=90% | 193 (87) | 326 (92) | 83 (86) | |
| Missing | 10 ( 5) | 7 (2) | 3 (3) | |
| Disease-Related | ||||
| Time from diagnosis to transplant, median (range), months | 10 (3 - 79) | 9 (2 - 96) | 10 (2 - 149) |
0.813 |
| TKI use pre transplant | <0.001 | |||
| No | 143 (64) | 258 (73) | 32 (33) | |
| Yes | 79 (36) | 96 (27) | 65 (67) | |
| Transplant-Related | ||||
| Donor-recipient sex match | 0.503 | |||
| M-M | 102 (46) | 139 (39) | 37 (38) | |
| F-M | 43 (19) | 72 (20) | 19 (20) | |
| M-F | 44 (20) | 70 (20) | 24 (25) | |
| F-F | 33 (15) | 73 (21) | 17 (18) | |
| Donor Relation | <0.001 | |||
| HLA-identical sibling | 80 (36) | 293 (83) | 65 (67) | |
| Well-matched URD | 142 (64) | 61 (17) | 32 (33) | |
| Donor-recipient CMV serological status | <0.001 | |||
| +/+ | 75 (34) | 74 (21) | 26 (27) | |
| +/− | 56 (25) | 197 (56) | 35 (36) | |
| −/+ | 36 (16) | 26 ( 7) | 13 (13) | |
| −/− | 49 (22) | 45 (13) | 17 (18) | |
| Missing | 6 (3) | 12 ( 3) | 6 (6) | |
| HLA-iden sibling donor age, median (range), years | 37 (13 - 70) |
34 (3 - 65) | 40 (20 - 61) |
<0.001 |
| Unrelated donor age, median (range), years | 35 (19 - 61) |
33 (20 - 46) |
38 (21 - 51) |
0.063 |
| Graft type | <0.001 | |||
| Bone Marrow | 146 (66) | 168 (47) | 49 (51) | |
| Peripheral blood | 76 (34) | 186 (53) | 48 (49) | |
| Conditioning regimen | ||||
| Bu dose, median (range), mg/kg | -- | 16 (10 - 25) |
12 (9-17) | -- |
| Cy/TBI (nonfrac 550-750) | 9 ( 4) | 0 | 0 | |
| Cy/TBI (nonfrac 800-1200) | 4 ( 2) | 0 | 0 | |
| Cy/TBI (frac 900-1170) | 13 ( 6) | 0 | 0 | |
| Cy/TBI (frac 1200-1300) | 123 (55) | 0 | 0 | |
| Cy/TBI (frac 1320-1395) | 40 (18) | 0 | 0 | |
| Cy/TBI (frac 1400-1500) | 33 (15) | 0 | 0 | |
| ATG or alemtuzumab use | 0.004 | |||
| Yes | 41 (18) | 32 (9) | 13 (13) | |
| No | 181 (82) | 322 (91) | 84 (87) | |
| GVHD prophylaxis * | <0.001 | |||
| TAC + MMF +− others | 6 (3) | 1 (<1) | 4 (4) | |
| TAC + MTX +− others (except MMF) | 49 (22) | 23 (6) | 44 (45) | |
| TAC + others (except MTX, MMF) | 6 (3) | 2 (<1) | 0 | |
| TAC alone | 2 (<1) | 0 | 1 (1) | |
| CSA + MMF +− others (except TAC) | 6 (3) | 4 ( 1) | 2 (2) | |
| CSA + MTX +− others (except TAC, MMF) | 140 (63) | 307 (87) | 40 (41) | |
| CSA + others (except TAC, MTX, MMF) | 5 (2) | 3 (<1) | 1 (1) | |
| CSA alone | 6 (3) | 4 (1) | 1 (1) | |
| Other | 0 | 2 (<1) | 3 (3) | |
| Missing | 2 (<1) | 8 (2) | 1 (1) | |
| Growth factors given post transplant | 0.299 | |||
| No | 172 (77) | 253 (71) | 72 (74) | |
| Yes | 49 (22) | 101 (29) | 25 (26) | |
| Missing | 1 (<1) | 0 | 0 | |
| Year of transplant | <0.001 | |||
| 2000 | 81 (36) | 93 (26) | 9 (9) | |
| 2001 | 45 (20) | 44 (12) | 8 (8) | |
| 2002 | 28 (13) | 59 (17) | 5 (5) | |
| 2003 | 15 (7) | 26 (7) | 10 (10) | |
| 2004 | 14 ( 6) | 43 (12) | 28 (29) | |
| 2005 | 21 (9) | 54 (15) | 19 (20) | |
| 2006 | 18 (8) | 35 (10) | 18 (19) | |
| Median follow-up of survivors, range, months | 72 (2-127) | 56 (2- 129) |
59 (3- 119) |
Cy indicates Cyclophosphamide; TBI, total body irradiation; Bu, busulfan; ATG, antithymocyte globulin; GvHD, graft versus host disease; TAG, tacrolimus; MMF, mycophenolate mofetil; MTX, methotrexate; GSA, cyclosporine, TKI, tyrosine kinase inhibitor
Neutrophil recovery at 28 days was similar among the groups, but platelet recovery at 28 days occurred in a higher proportion of oral (75%, 95% CI: 71-80%) or IV Bu (77%, 95% CI: 68-85%) patients than those receiving TBI (64%, 95% CI: 58-70%, P=0.009; Table 2). The incidences of hepatic veno-occlusive disease and interstitial pneumonia at 100 days, and NRM, LFS and OS at 5 years did not differ significantly among the three groups. (Table 2)
Table 2.
Univariate analysis
| Outcomes | Cy TBI Probability (95% CI) |
Oral BuCy Probability (95% CI) |
IV BuCy Probability (95% CI) |
Overall p- values |
|---|---|---|---|---|
| Neutrophil recovery | ||||
| NEval | 222 | 354 | 97 | |
| @ 28 days | 92 (88-95) | 91 (88-94) | 95 (90-98) | 0.391 |
| Platelet recovery | ||||
| NEval | 221 | 346 | 96 | |
| @ 28 days | 64 (58-70) | 75 (71-80) | 77 (68-85) | 0.009 |
| @ 100 days | 90 (85-93) | 93 (90-95) | 96 (91-99) | 0.103 |
| Acute GVHD (II-IV) | ||||
| NEval | 222 | 352 | 97 | |
| @ 100 days | 56 (50-63) | 43 (39-49) | 46 (37-56) | 0.014 |
| Acute GVHD (III-IV) | ||||
| NEval | 222 | 354 | 97 | |
| @ 100 days | 24 (19-30) | 20 (16-24) | 26 (18-35) | 0.290 |
| Hepatic Veno-occlusive Disease | ||||
| NEval | 222 | 354 | 97 | |
| @ 100 days | 5 (2-9) | 9 (7-13) | 6 (2-12) | 0.17 |
| Interstitial Pneumonia | ||||
| NEval | 222 | 354 | 97 | |
| @ 100 days | 9 (5-13) | 5 (3-8) | 4 (1-9) | 0.22 |
| Chronic GVHD | ||||
| NEval | 216 | 348 | 95 | |
| @ 5 years | 55 (48-61) | 62 (57-68) | 67 (57-76) | 0.082 |
| Non-relapse mortality | ||||
| NEval | 216 | 350 | 95 | |
| @ 1 year | 25 (20-31) | 20 (16-24) | 16 (9-24) | 0.142 |
| @ 3 years | 31 (25-38) | 24 (20-29) | 22 (14-31) | 0.139 |
| @ 5 years | 31 (25-38) | 25 (21-30) | 36 (25-48) | 0.119 |
| Relapse | ||||
| NEval | 216 | 350 | 95 | |
| @ 5 years | 17 (12-23) | 17 (12-22) | 7 (2-14) | 0.014 |
| Leukemia free survival | ||||
| NEval | 216 | 350 | 95 | 0.102 |
| @ 1 year | 67 (60-73) | 74 (69-78) | 80 (72-88) | 0.031 |
| @ 3 years | 55 (48-62) | 62 (57-68) | 74 (64-82) | 0.006 |
| @ 5 years | 52 (45-59) | 58 (52-64) | 57 (45-69) | 0.384 |
| Overall survival | ||||
| NEval | 222 | 354 | 97 | 0.196 |
| @ 1 year | 74 (68-80) | 80 (75-84) | 84 (76-91) | 0.118 |
| @ 3 years | 67 (60-73) | 74 (69-78) | 77 (68-85) | 0.097 |
| @ 5 years | 66 (59-72) | 72 (67-77) | 61 (50-73) | 0.152 |
N Eval indicates number evaluable; Cy, cyclophosphamide; TBI, total body irradiation; IV, intravenous; Bu, busulfan, GvHD, graft versus host disease
The incidence of relapse (hematologic, cytogenetic or molecular) at 5 years was 17% (95% CI: 12-23%) for TBI, 17% (95% CI: 12-22%) for oral Bu and 7% (95% CI: 2-14%) for IV Bu (P=0.014). Univariate analyses of specific clinical outcomes and covariates are summarized in supplemental Table S1. Thirty eight patients (4 for no hematopoietic recovery, 5 for graft failure, 7 for relapse, and 22 for whom the indication was missing) underwent either a second HCT (n=11) or donor lymphocyte infusion (n=27). Of these 38 patients, 26 are still alive.
Multivariate Analysis
Relapse occurred significantly less frequently among patients receiving IV Bu compared to TBI (RR=0.36, 95% CI: 0.15 – 0.86; P=0.022) or oral Bu (RR=0.39, 0.17 – 0.90; P=0.028). NRM and OS were similar among the groups (Table 3).
Table 3.
Relative risks and 95% confidence intervals of a multivariate analysis
| Oral BuCy vs. TBI RR (95% CI) |
IV BuCy vs. TBI RR (95% CI) |
Oral Bucy vs. IV BuCy RR (95% CI) |
Overall p | |
|---|---|---|---|---|
| aGVHD II-IVa | 0.87 (0.66, 1.13) | 0.81 (0.57, 1.14) | 1.07 (0.77, 1.49) | 0.405 |
| aGVHD III-IV for HLA sibsb |
0.71 (0.42, 1.18) | 0.89 (0.44, 1.81) | 0.79 (0.43, 1.47) | 0.37 |
| aGVHD III-IV for URDb |
1.76 (1.02, 3.04) | 2.62 (1.34, 5.12) | 0.67 (0.33, 1.36) | 0.01 |
| cGVHD for HLA sibsc |
1.22 (0.85, 1.74) | 1.31 (0.83, 2.05) | 0.93 (0.65, 1.33) | 0.47 |
| cGVHD for URDc |
2.73 (1.82, 4.10) | 1.52 (0.94, 2.47) | 1.79 (1.04, 3.08) | <.0001 |
| LFS for HLA sibsd |
0.64 (0.44, 0.92) | 0.53 (0.31, 0.92) | 1.19 (0.72, 1.98) | 0.025 |
| LFS for URDd | 1.69 (1.11, 2.56) | 1.32 (0.75, 2.32) | 1.28 (0.71, 2.32) | 0.046 |
| Relapsee | 0.94 (0.61, 1.45) | 0.36 (0.15, 0.86) | 2.58 (1.11, 6.03) | 0.067 |
| NRMf | 1.17 (0.81, 1.68) | 1.26 (0.78, 2.03) | 0.93 (0.60, 1.45) | 0.576 |
| OSg | 1.15 (0.81, 1.62) | 1.19 (0.75, 1.88) | 0.97 (0.63, 1.48) | 0.679 |
* Bolded indicates p-value<0.05; aGVHD, acute GVHD; cGVHD, chronic GVHD; NRM, non-relapse mortality; LFS, leukemia-free survival; OS, overall survival
Other Significant factors in the multivariate model include:
Graft type, Donor relation
Donor relation, year of transplant
Donor relation, Sex match, graft type, ATG or alemtuzumab use
Donor relation, recipient age, TKI
None
Donor relation, recipient age, graft type, year of transplant
Year of transplant, recipient age, donor relation
The interaction term between donor relation (HLA-identical sibling, URD) and the main effect variable (oral Bu vs. IV Bu vs. TBI) was significant for acute GVHD ≥ Grade 3, chronic GVHD and LFS. The results for each donor relation are presented separately based on the multivariate models that included donor relation as a covariate as well as the significant interaction term. Among patients receiving grafts from HLA-identical siblings, the incidences of acute GvHD ≥ Grade 3 and chronic GvHD were similar for all three groups. For patients with URD, however, compared to TBI the incidence of acute GvHD ≥ Grade 3 was higher in those receiving oral (RR=1.76, 95% CI: 1.02 – 3.04; P=0.043) or IV Bu (RR=2.62, 95% CI: 1.34-5.12; P=0.005). The incidence of chronic GvHD among patients with unrelated donors was higher for those receiving oral (RR=2.73, 95% CI: 1.82-4.10; P<0.0001), but not IV, Bu compared to TBI.
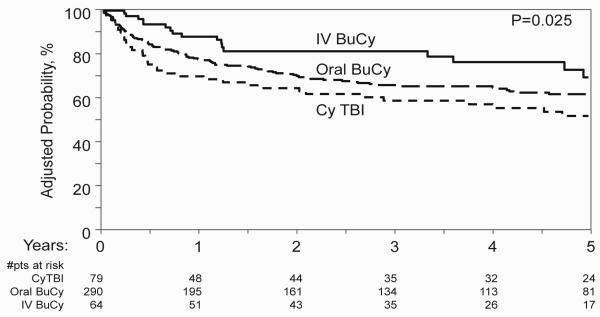
LFS was significantly better among recipients of HLA-identical sibling (Figure 1), but not URD, grafts receiving oral (RR=0.64, 95% CI: 0.44-0.92; P=0.017) or IV Bu (RR=0.53, 95% CI: 0.31-0.92; P=0.025) compared to TBI. In order to determine whether administration of higher radiation doses might contribute to inferior outcomes with TBI, the TBI cohort was divided into those receiving standard (<12.5 Gy) and high dose TBI in a separate multivariate analysis (Table 4). IV Bu remained associated with lower relapse (RR=0.38, 95% CI: 0.16-0.94; P=0.037) and, among recipients of related grafts, better LFS (RR=0.55, 95% CI: 0.31-0.98; P=0.044) than standard dose TBI.
Figure 1.
Adjusted probabilities of LFS according to preparative regimen for recipients of grafts from HLA-identical sibling (adjusted covariates: TKI use before HCT, recipient age).
Table 4.
Relative risks and 95% confidence intervals of a multivariate analysis (TBI dose divided into standard and high dose)
| Oral BuCy vs. TBI (≤ 1250) RR (95% CI) |
IV BuCy vs. TBI (≤ 1250) RR (95% CI) |
TBI (>1250) vs. TBI(<1250) RR (95% CI) |
Oral Bucy vs. IV BuCy RR (95% CI) |
Oral BuCy vs. TBI (> 1250) RR (95% CI) |
IV BuCy vs. TBI (> 1250) RR (95% CI) |
Overall p | |
|---|---|---|---|---|---|---|---|
| aGVHD II-IVa | 0.82(0.61, 1.09) | 0.76 (0.53, 1.1) | 0.84 (0.58, 1.22) |
1.07 (0.77, 1.5) | 0.98 (0.67, 1.42) | 0.91 (0.59, 1.41) | 0.434 |
| aGVHD III-IV for HLA sibsb |
0.87 (0.47, 1.6) | 1.10 (0.5, 2.41) | 1.96 (0.81, 4.72) |
0.79 (0.43, 1.46) |
0.44 (0.21, 0.94) | 0.56 (0.23, 1.37) | 0.193 |
| aGVHD III-IV for URDb |
2.04 (1.09, 3.8) |
3.05 (1.46,
6.35) |
1.44 (0.73, 2.83) |
0.67 (0.33, 1.36) |
1.42 (0.73, 2.75) | 2.12 (0.98, 4.57) | 0.018 |
| cGVHD for HLA sibsc |
1.17 (0.78, 1.76) | 1.26 (0.77, 2.06) |
0.87 (0.41, 1.84) |
0.93 (0.65, 1.32) |
1.36 (0.69, 2.66) | 1.46 (0.71, 3.02) | 0.643 |
| cGVHD for URDc |
2.07 (1.35, 3.18) | 1.14 (0.69, 1.89) |
0.46 (0.28,
0.76) |
1.81 (1.06,
3.12) |
4.52 (2.6, 7.83) | 2.49 (1.36, 4.55) | <.0001 |
| LFS for HLA sibsd |
0.66 (0.43, 0.99) |
0.55 (0.31,
0.98) |
1.1 (0.56, 2.16) | 1.19 (0.72, 1.97) |
0.6 (0.32, 1.09) | 0.5 (0.24, 1.03) | 0.059 |
| LFS for URDd | 1.96 (1.23, 3.13) | 1.53 (0.84, 2.8) | 1.48 (0.89, 2.45) |
1.28 (0.71, 2.31) |
1.33 (0.8, 2.21) | 1.04 (0.55, 1.96) | 0.046 |
| Relapsee | 0.99 (0.6, 1.63) |
0.38 (0.16,
0.94) |
1.17 (0.58, 2.36) |
2.58 (1.11,
6.03) |
0.84 (0.45, 1.58) | 0.33 (0.12, 0.87) | 0.131 |
| NRMf | 1.27 (0.84, 1.9) | 1.36 (0.82, 2.26) |
1.26 (0.77, 2.06) |
0.93 (0.6, 1.45) | 1 (0.62, 1.62) | 1.08 (0.61, 1.91) | 0.595 |
| OSg | 1.26 (0.86, 1.85) | 1.3 (0.8, 2.12) | 1.31 (0.83, 2.09) |
0.97 (0.63, 1.48) |
0.96 (0.61, 1.51) | 0.99 (0.57, 1.71) | 0.568 |
*Bolded indicates p-value<0.05; aGVHD, acute GVHD; cGVHD, chronic GVHD; NRM, non-relapse mortality; LFS, leukemia-free survival; OS, overall survival
Other Significant factors in the multivariate model include:
Graft type, Donor relation
Donor relation, year of transplant
Donor relation, Sex match, graft type, ATG or alemtuzumab use
Donor relation, recipient age, TKI
None
Donor relation, recipient age, graft type, year of transplant
Year of transplant, recipient age, donor relation
LFS was worse for recipients of URD grafts who received oral, but not IV, Bu compared to TBI (RR=1.69, 95% CI: 1.11-2.56; P=0.014).The use of a TKI prior to HCT was not adversely associated with any of the reported outcomes and was associated with better LFS. (RR=0.69, 95% CI: 0.53-0.92; P=0.01)
Discussion
Although transplantation is no longer first-line treatment for CML in first chronic phase many patients who are resistant to, or intolerant of, tyrosine kinase inhibitors continue to undergo the procedure.24,25,26 CIBMTR registration retrieval for the United States alone identified 120 allogeneic transplants, of whom 113 recipients were ≥18 years of age, for CML in first chronic phase in 2012 and 2013 (Wael Saber, personal communication). Our retrospective analysis shows that, in patients with chronic phase CML, relapse occurred significantly less often among those who received IV Bu compared to oral Bu or TBI, regardless of whether patients received standard or high doses of TBI. LFS was better among those receiving HLA-identical sibling grafts who received IV or oral Bu compared to TBI including when the analysis was limited to those patients who received standard TBI doses.
OS, however, did not differ among the groups. The effectiveness of TKIs and donor lymphocyte infusions in extending survival following relapse in CML27, and the limited (5 years) follow up of the present study probably account for similar OS despite the differences in relapse and LFS. Nevertheless, cure is the ultimate goal of HCT in CML. The lower incidence of relapse and better LFS with IV Bu (compared to TBI) support its use in patients receiving HLA-identical sibling grafts. LFS following HLA-identical sibling grafts was also superior to TBI in patients receiving oral Bu. It is likely that PK dosing was widely utilized among these patients. 18 The oral formulation has been largely displaced by the IV formulation,28 but PK-based oral dosing might yield similar results. This was not specifically addressed in the present study. Important differences in LFS between IV Bu and TBI were not identified for recipients of URD grafts with the available sample size in this study.
In AML, studies of Cy combined with fixed dose oral Bu have reported some disadvantages, including higher relapse rates, compared to TBI. 29,30 In contrast, compared to TBI, IV Bu was associated with lower relapse rates beyond 1 year, less NRM and better LFS and survival in patients with AML in CR.18 It would seem that the advantage of IV Bu might be magnified in CML where oral fixed-dose Bu showed advantages over TBI, including less toxicity6 and relapse7, and favorable results were reported with dose-adjusted oral Bu. 14 Notably, NRM was comparatively low in the present study in the IV Bu group relative to the TBI cohort at 1 and 3 years, but similar at 5 years. A higher proportion of patients receiving IV Bu who were alive at 3 years subsequently died from NRM compared to those receiving TBI, but no clear pattern in the cause of death emerged. In particular, only one death attributed to chronic GvHD occurred beyond three years in the IV Bu cohort (data not shown). We also have no precise explanation for the higher incidence of acute GvHD ≥ Grade III with Bu compared with TBI in recipients of URD grafts, however, TBI patients were more likely to have received marrow and ATG or alemtuzumab. These data contrast with reports of less GvHD with Bu in CML 6 and AML, 17 although those studies were performed with HLA identical sibling donors. Importantly, the use of a TKI before HCT did not adversely influence the outcomes reported and was associated with better LFS. These results support a previous report that imatinib use before HCT did not adversely influence transplant outcomes.31 The significantly lower incidence of relapse with IV Bu and better LFS with IV (or oral) Bu compared to TBI in recipients of HLA-identical related grafts were present regardless of whether TKI use was considered in the multivariate model.
There are, of course, limitations to this retrospective analysis. First, it is not known why individual patients received specific preparative regimens. Second, there were important differences between the groups, especially in the distribution of related and unrelated donors, TKI exposure, and year when transplantation was performed. Multivariate analyses were performed to account for these differences, but the relatively few patients in the IV Bu arm, particularly those receiving grafts from URD, limits the effectiveness of that approach. In addition, over the course of the study, the use of molecular detection of relapse became more widespread. The application of more sensitive techniques to detect relapse, however, would likely result in earlier detection in patients undergoing transplantation in the later years of study, potentially leading to an underestimation of the difference in relapse with IV Bu. Also, data were not collected for PK studies and dose adjustment, which is reported to affect outcomes with IV 32 as well as oral Bu. 33 We were therefore unable to analyze the potential benefit of PK-directed dosing in patients receiving oral or IV Bu.
Absent results of a randomized trial, the association of IV Bu with lower relapses rates in first chronic phase CML patients, and better LFS compared to TBI among recipients of HLA-identical sibling grafts favors its use in that setting.
Supplementary Material
Highlights.
IV busulfan is associated with lower relapse rates than oral busulfan or TBI following myeloablative HCT for CML.
IV and oral busulfan are associated with better LFS than TBI in patients receiving grafts from HLA-identical siblings.
Use of a TKI prior to transplant was associated with better LFS.
Acknowledgements
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; Swedish Orphan Biovitrum; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement: The authors declare no competing financial interests.
References
- 1.Clift RA, Buckner CD, Thomas ED, et al. Treatment of chronic granulocytic leukemia in chronic phase by allogeneic marrow transplantation. Lancet. 1982 Sep;2(8299):621–623. doi: 10.1016/s0140-6736(82)92735-0. [DOI] [PubMed] [Google Scholar]
- 2.Goldman JM, Baughan AS, McCarthy DM, et al. Marrow transplantation for patients in the chronic phase of chronic granulocytic leukemia. Lancet. 1982 Sep;2(8299):623–625. doi: 10.1016/s0140-6736(82)92736-2. [DOI] [PubMed] [Google Scholar]
- 3.Goldman JM, Apperley JF, Jones L, et al. Bone marrow transplantation for patients with chronic myeloid leukemia. N Engl J Med. 1986 Jan;314(4):202–207. doi: 10.1056/NEJM198601233140403. [DOI] [PubMed] [Google Scholar]
- 4.Thomas ED, Clift RA, Fefer A, et al. Marrow transplantation for the treatment of chronic myelogenous leukemia. Ann Intern Med. 1986 Feb;104(2):155–163. doi: 10.7326/0003-4819-104-2-155. [DOI] [PubMed] [Google Scholar]
- 5.Biggs JC, Szer J, Crilley P, et al. Treatment of chronic myeloid leukemia with allogeneic bone marrow transplantation after preparation with BuCy 2. Blood. 1992 Sep;80(5):1352–1357. [PubMed] [Google Scholar]
- 6.Clift RA, Buckner CD, Thomas ED, et al. Marrow transplantation for chronic myeloid leukemia: a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide. Blood. 1994 Sep;84(6):2036–2043. [PubMed] [Google Scholar]
- 7.Devergie A, Blaise D, Attal M, et al. Allogeneic bone marrow transplantation for chronic myeloid leukemia in first chronic phase: a randomized trial of busulfan-cytoxan versus cytoxan-total body irradiation as preparative regimen: a report from the French Society of Bone Marrow Graft (SFGM) Blood. 1995 Apr;85(8):2263–2268. [PubMed] [Google Scholar]
- 8.Ehrsson H, Hassan M, Ehrnebo M, et al. Busulfan kinetics. Clin Pharmacol Ther. 1983 Jul;34(1):86–9. doi: 10.1038/clpt.1983.134. [DOI] [PubMed] [Google Scholar]
- 9.McCune JS, Baker KS, Blough DK, et al. Variation in prescribing patterns and therapeutic drug monitoring of intravenous busulfan in pediatric hematopoietic cell transplant recipients. J Clin Pharmacol. 2013 Mar;53(3):264–75. doi: 10.1177/0091270012447196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grochow LB, Jones RJ, Brundrett RB, et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol. 1989;25(1):55–61. doi: 10.1007/BF00694339. [DOI] [PubMed] [Google Scholar]
- 11.Slattery JT, Sanders JE, Buckner CD, et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant. 1995 Jul;16(1):31–42. [PubMed] [Google Scholar]
- 12.Slattery JT, Clift RA, Buckner CD, et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood. 1997 Apr;89(8):3055–3060. [PubMed] [Google Scholar]
- 13.Buffery PJ, Allen KM, Chin PK, et al. Thirteen years' experience of pharmacokinetic monitoring and dosing of busulfan: can the strategy be improved? Ther Drug Monit. 2014 Feb;36(1):86–92. doi: 10.1097/FTD.0b013e31829dc940. [DOI] [PubMed] [Google Scholar]
- 14.Radich JP, Gooley T, Bensinger W, et al. HLA-matched related hematopoietic cell transplantation for chronic-phase CML using a targeted busulfan and cyclophosphamide preparative regimen. Blood. 2003 Jul;102(1):31–35. doi: 10.1182/blood-2002-08-2619. [DOI] [PubMed] [Google Scholar]
- 15.Andersson BS, Kashyap A, Gian V, et al. Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: a phase II study. Biol Blood Marrow Transplant. 2002;8(3):145–154. doi: 10.1053/bbmt.2002.v8.pm11939604. [DOI] [PubMed] [Google Scholar]
- 16.Kashyap A, Wingard J, Cagnoni P, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002;8(9):493–500. doi: 10.1053/bbmt.2002.v8.pm12374454. [DOI] [PubMed] [Google Scholar]
- 17.Nagler A, Rocha V, Labopin M, et al. Allogeneic hematopoietic stem-cell transplantation for acute myeloid leukemia in remission: comparison of intravenous busulfan plus cyclophosphamide (Cy) versus total-body irradiation plus Cy as conditioning regimen--a report from the acute leukemia working party of the European group for blood and marrow transplantation. J Clin Oncol. 2013 Oct;31(28):3549–3556. doi: 10.1200/JCO.2013.48.8114. [DOI] [PubMed] [Google Scholar]
- 18.Copelan EA, Hamilton BK, Avalos B, et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared to TBI. Blood. 2013 Dec;122(24):3863–3870. doi: 10.1182/blood-2013-07-514448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bredeson C, Lerademacher J, Kato K, et al. Prospective cohort study comparing intravenous busulfan to total body irradiation in hematopoietic cell transplantation. Blood. 2013 Dec;122(24):3871–3878. doi: 10.1182/blood-2013-08-519009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008 Jul;14(7):748–58. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995 Jun;15(6):825–828. [PubMed] [Google Scholar]
- 22.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980 Aug;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Loberiza FR, Klein JP, et al. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007 Nov;88(2):95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Passweg JR, Baldomero H, Peters C, et al. Hematopoietic SCT in Europe: data and trends in 2012 with special consideration of pediatric transplantation. Bone Marrow Transplant. 2014 Jun;49(6):744–50. doi: 10.1038/bmt.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gratwohl A, Baldomero H, Gratwohl M, et al. Quantitative and qualitative differences in use and trends of hematopoietic stem cell transplantation: a Global Observational Study. Haematologica. 2013 Aug;98(8):1282–90. doi: 10.3324/haematol.2012.076349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013 Aug;122(6):872–84. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martínez C, Gomez V, Tomás JF, et al. Relapse of chronic myeloid leukemia after allogeneic stem cell transplantation: outcome and prognostic factors: the Chronic Myeloid Leukemia Subcommittee of the GETH (Grupo Español de Trasplante Hemopoyético) Bone Marrow Transplant. 2005 Aug;36(4):301–306. doi: 10.1038/sj.bmt.1705063. [DOI] [PubMed] [Google Scholar]
- 28.Champlin RE. Busulfan or TBI: answer to an age-old question. Blood. 2013 Dec;122(24):3856–3857. doi: 10.1182/blood-2013-10-530006. [DOI] [PubMed] [Google Scholar]
- 29.Blaise D, Maraninchi D, Archimbaud E, et al. Allogeneic bone marrow transplantation for acute myeloid leukemia in first remission: a randomized trial of a busulfan-Cytoxan versus Cytoxan-total body irradiation as preparative regimen: a report from the Group d'Etudes de la Greffe de Moelle Osseuse. Blood. 1992 May;79(10):2578–2582. [PubMed] [Google Scholar]
- 30.Gupta V, Lazarus HM, Keating A. Myeloablative conditioning regimens for AML allografts: 30 years later. Bone Marrow Transplant. 2003 Nov;32(10):969–978. doi: 10.1038/sj.bmt.1704285. [DOI] [PubMed] [Google Scholar]
- 31.Lee SJ, Kukreja M, Wang T, et al. Impact of prior imatinib mesylate on the outcome of hematopoietic cell transplantation for chronic myeloid leukemia. Blood. 2008 Oct;112(8):3500–3507. doi: 10.1182/blood-2008-02-141689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pidala J, Kim J, Anasetti C, et al. Pharmacokinetic targeting of intravenous busulfan reduces conditioning regimen related toxicity following allogeneic hematopoietic cell transplantation for acute myelogenous leukemia. J Hematol Oncol. doi: 10.1186/1756-8722-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCune JS, Gibbs JP, Slattery JT. Plasma concentration monitoring of busulfan: does it improve clinical outcome? Clin Pharmacokinet. 2000 Aug;39(2):155–165. doi: 10.2165/00003088-200039020-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.