Abstract
Background
The neuropeptide RFamide-related peptide-3 (RFRP-3; mammalian ortholog to GnIH) can inhibit LH release and increases feeding, but the regulation and development of RFRP-3 neurons remains poorly characterized, especially in mice.
Methods and Results
We first confirmed that peripheral injections of murine RFRP-3 peptide could markedly suppress luteinizing hormone secretion in adult mice, as in other species. Second, given RFRP-3′s reported orexigenic properties, we performed double-label in situ hybridization for metabolic genes in Rfrp neurons of mice. While Rfrp neurons did not readily co-express NPY, TRH, or MC4R, a small subset of Rfrp neurons did express leptin receptor in both sexes. Surprisingly, we identified no changes in Rfrp expression or neuronal activation in adult mice after acute fasting. However, we determined that Rfrp mRNA levels in the DMN were significantly reduced in adult Obese (Ob) mice of both sexes. Given the lower Rfrp levels observed in adult Ob mice, we asked whether leptin might also regulate RFRP-3 neuron development. Rfrp gene expression changed markedly over juvenile development, correlating with the timing of the juvenile “leptin surge” known to govern hypothalamic feeding circuit development. However, the dramatic developmental changes in juvenile Rfrp expression did not appear to be leptin-driven, as the pattern and timing of Rfrp neuron development were unaltered in Ob juveniles.
Conclusion
Leptin status modulates RFRP-3 expression in adulthood, but is not required for normal development of the RFRP-3 system. Leptin's regulation of adult RFRP-3 neurons likely occurs via primarily indirect signaling, and may be secondary to obesity, as only a small subset of RFRP-3 neurons express LepRb.
Keywords: RFRP-3, GnIH, leptin, reproduction, development
Introduction
Neuronal networks of the hypothalamus integrate peripheral endocrine and metabolic signals to regulate gonadotropin-releasing hormone (GnRH) neurons, which in turn stimulate the reproductive axis. In most cases, hormonal and metabolic information are transmitted to GnRH neurons indirectly via upstream reproductive neural circuitry. One possible intermediary neuropeptide is RFamide-related peptide 3 (RFRP-3), the mammalian ortholog of avian gonadotropin-inhibiting hormone (GnIH) (1,2). In rodents, RFRP-3 treatment effectively inhibits both GnRH neuronal activity and luteinizing hormone (LH) secretion through central GnRH-dependent mechanisms (3-6). In mice and rats, some GnRH neurons are apposed by RFRP-3 axonal fibers (2,7,8), and a subset of GnRH neurons express Gpr147 (8,9), a high affinity receptor for RFRP-3 (10,11). In rodents, RFRP-3-producing neurons reside in a scattered pattern solely within and just ventral to the dorsal-medial nucleus of the hypothalamus (collectively abbreviated as DMN) (2,12). Likewise, the rodent Rfrp gene, encoding RFRP-3, is expressed in a scattered pattern exclusively in the DMN and, to a much lesser extent, ventrally in the neighboring area just dorsal of the VMN (2,9,10). The DMN is known to regulate aspects of energy balance, feeding behavior, and thermoregulation (13-15). However, the precise role(s) of RFRP-3 neurons in the DMN is unclear, in part due to a lack of knowledge of both the neuropeptides and receptors co-expressed in RFRP-3 neurons and the identity of regulatory factors that govern RFRP-3 synthesis and secretion.
Leptin, a hormone secreted from adipocytes, has strong effects on hypothalamic regulation of satiety, energy expenditure, and body weight, not to mention a stimulatory (permissive) role in reproductive function. Obese mice (Ob) have a non-functional leptin gene and are morbidly overweight, hyperphagic, have low LH levels, and are infertile (16,17), illustrating the importance of leptin in maintenance of both energy homeostasis and reproduction. Leptin does not directly regulate GnRH neurons, as the long form of the leptin receptor (LepRb), which is responsible for signal transduction, is not expressed in GnRH neurons (18,19). Rather, leptin acts on GnRH neurons indirectly, through upstream intermediates which have yet to be fully indentified. RFRP-3 neurons may be one potential relay system through which leptin signals are mediated, as the DMN (where RFRP-3 neurons reside) is a highly leptin-responsive brain region (20-22). Central injections of RFRP-3 not only inhibit LH secretion, but also stimulate feeding behavior (23,24). Moreover, RFRP-3 neurons are activated by chronic mild food restriction in hamsters (25), and RFRP-3′s receptor, Gpr147, is required in mice to suppress LH secretion after acute food deprivation (26), suggesting that RFRP-3 plays a role in both energy balance and reproduction, as does leptin (27). Thus, it is possible that leptin might inhibit the production and/or secretion of RFRP-3 in order to facilitate reproductive function and/or suppress feeding behavior.
In addition to its roles in adulthood, leptin has important developmental effects on the hypothalamus. During the second week of postnatal life, serum leptin levels increase drastically and transiently in a postnatal leptin “surge” (28). This temporary increase in juvenile leptin levels regulates the development of axonal projections from the arcuate nucleus to the DMN, as well as other brain regions (29,30). We previously demonstrated that DMN Rfrp expression, as measured by cell number and Rfrp mRNA levels per cell, is dramatically higher in juveniles on postnatal day 10 than at birth (9), but whether this developmental difference is caused, fully or in part, by the juvenile leptin surge is unknown.
In this study, we addressed whether neural Rfrp expression is regulated by metabolic manipulations, such as leptin-deficiency seen in Ob animals or short-term food deprivation. We also ascertained if the developmental maturation of the neural Rfrp system is leptin-dependent, owing to the developmental surge in leptin secretion during the juvenile period. Specifically, we determined whether 1) the mouse RFRP-3 peptide suppresses LH secretion, as reported for the rat RFRP-3 peptide variant in other species, 2) Rfrp neurons co-express important metabolic genes, including LepRb, that are known to be expressed in the DMN, 3) Rfrp expression and/or neuronal activation is enhanced during food deprivation or altered in adult Ob mice, and 4) the developmental pattern of Rfrp expression in postnatal mice parallels developmental leptin secretion (the juvenile leptin surge), and if so, whether normal Rfrp development is dependent on leptin signaling.
Materials and Methods
Animals, Gonadectomies, and Tissue Collection
Experiments utilized either C57BL6 mice or mice from the Ob strain (WT and Ob). Mice from the Ob line were purchased from Harlan Laboratories and maintained on a C57BL6 background in the lab. Both strains were studied in adulthood and in development. All animals were housed on a 12:12 light-dark cycle, with food and water available ad libitum, except where indicated in experiment 4. All experiments were conducted in accordance with the NIH Animal Care and Use Guidelines and with approval of the Animal Care and Use Committee of the University of California, San Diego.
For adult C57BL6 mice, 7-9 week old female (diestrous) or male mice were anesthetized with isoflurane, weighed, blood was collected via retro-orbital bleeding, and then rapidly decapitated. Prior to RFRP-3 injections and for the food deprivation experiment all C57BL6 mice were bilaterally gonadectomized (GDX), as previously described (9). Gonadectomy was implemented to promote high levels of circulating LH for RFRP-3 or food restriction to inhibit, and to simultaneously control for differences in sex steroids between individuals since estradiol and testosterone can both mildly suppress Rfrp levels in mice of both sexes (9,31). For adult OB mice, 7-8 week old female or male WT or Ob mice were GDX (again, to control for sex steroids between genotypes and allow for maximal Rfrp expression levels in the absence of steroid inhibition) and then sacrificed 7 days later for blood and tissue collection.
For developmental experiments, infantile and juvenile pups were generated by C57BL6 breeder pairs or heterozygous Ob breeder pairs (producing WT, Ob and heterozygous littermates). The date of birth was designated as postnatal day (PND) 1. Newborn and juvenile pups of various ages were anesthetized with isoflurane, weighed, and rapidly decapitated. Trunk blood was collected from each animal to measure serum leptin levels. For mice of the OB strain, tail samples were taken postmortem to determine the Ob genotype. Note that the obesity phenotype in Ob mice is not present until 4 weeks of age or later (16,17).
Brains from all animals were collected at sacrifice, frozen on dry ice, stored at -80°C, and then sectioned on a cryostat into five coronal series of 20 μm brain sections which were thaw-mounted onto Superfrost-plus slides. Slides were stored at -80°C until use for in-situ hybridization.
Hormone Assays
Blood from adult animals was collected by retro-orbital bleed. Blood from juvenile animals was collected via trunk blood. Serum luteinizing hormone (LH) was measured by the UVA Ligand Core (range 0.04 - 37.4 ng/mL). Serum leptin was measured using Quantikine Mouse Leptin ELISA kit (R&D Systems) following the manufacturer's protocol (range 1.25 ng/mL - 80 ng/mL).
Single-label and Double-label In Situ Hybridization (ISH)
The Rfrp, c-fos, Trh, and LepRb cRNA ISH riboprobes have been described and validated previously (9,32-34). Neuropeptide Y (Npy) and melanocortin receptor 4 (Mc4r) were cloned from mouse hypothalamic RNA into pBluescript SK- plasmid and transcribed using T7 polymerase. The probes correspond to the following bases of their respective murine mRNAs; Npy: bases 7-468 (NM_023456); Trh: bases 308-572 (NM_009426); LepRb: bases 3379-3912 (NM_146146); Mc4r: bases 589-1442 (NM_016977).
Single-label ISH was performed as previously described (9,35). Briefly, for each assay, slide-mounted brain sections encompassing the entire DMN from one of the 5 sets of serial brain sections were fixed in 4% paraformaldehyde, pretreated with acetic anhydride, rinsed in 2× SSC (sodium citrate, sodium chloride), delipidated in chloroform, dehydrated in ethanol, and air-dried. Radiolabeled (33P) Rfrp antisense riboprobe (0.04 pmol/ml) was combined with tRNA, heat-denatured, added to hybridization buffer, and applied to each slide (100 μl/slide). Slides were cover-slipped and placed in a 55°C humidity chamber overnight. The slides were then washed in 4× SSC and placed into RNAse A treatment for 30 min at 37°C, then in RNAse buffer without RNase at 37°C for 30 min. After washing in 2× SSC at room temperature, slides were washed in 0.1× SSC at 62°C for 1 hour, dehydrated in ethanols, and air-dried. Slides were then dipped in Kodak NTB emulsion, air-dried, and stored at 4°C for 3-4 days (depending on the assay) before being developed and cover-slipped.
Double-label ISH was performed as previously described (35,36). Briefly, one set of slide-mounted brain sections encompassing the entire DMN were fixed in 4% paraformaldehyde, pretreated with acetic anhydride, rinsed in 2× SSC (sodium citrate, sodium chloride), delipidated in chloroform, dehydrated in ethanols, and air-dried. Radio-labeled (33P) antisense Npy, Trh, LepRb, or Mc4r (0.05 pmol/ml) were combined with Digoxigenin (DIG)-labeled Rfrp riboprobes (Roche Digoxigenin labeling kit, 1:500) along with tRNA, denatured by boiling, and dissolved together in hybridization buffer. The probe mix was then applied to slides and hybridized at 55°C overnight. The slides were then washed in 4× SSC and placed into RNAse A treatment for 30 min at 37°C, then in RNAse buffer without RNase at 37°C for 30 min. After washing in 2× SSC at room temperature, slides were washed in 0.1× SSC at 62°C for 1 hour. Slides were then incubated in 2× SSC with 0.05% Triton X-100 and 3% sheep serum (NSS) for 75 min at room temperature and then incubated overnight at room temperature with anti-DIG antibody conjugated to alkaline phosphatase [(Roche) diluted 1:500 in Buffer 1 containing 1% NSS and 0.3% Triton X-100]. The next day, slides were washed with Buffer 1 and then incubated with Vector Red alkaline phosphatase substrate (Vector Labs) for 1 h at room temperature. The slides were then air-dried, dipped in Kodak NTB emulsion, stored at 4°C, and developed and cover-slipped 3-9 days later, depending on the assay.
ISH slides were analyzed with an automated image processing system (Dr. Don Clifton, University of Washington) by a person blinded to the treatment group (37). For single-label Rfrp experiments, the software counted the number of ISH silver grain clusters representing Rfrp cells as well as the number of silver grains over each cell (which provides a semi-quantitative index of Rfrp mRNA expressed per cell) (38-40). Cells were considered Rfrp positive when the number of silver grains in a cluster exceeded that of background by 3-fold. We have previously described two obvious Rfrp cell populations interspersed within the DMN that dramatically differ in silver grain (i.e., mRNA) expression. Therefore, as in previous studies, we divided Rfrp cells into two categories based on their Rfrp mRNA expression level; “high expressing” (HE) cells have a silver grain cluster diameter greater than > 20 μm whereas “low expressing” (LE) cell have a silver grain cluster less than < 20 μm. Previous studies have determined that silver grain number and silver grain cell area are highly dependent, representative of mRNA differences, and not due to morphological cell size differences (9). All Rfrp cells from one set of serial sections were quantified in each animal. For double-label ISH assays, all red fluorescent DIG-containing cells (Rfrp cells) from one set of serial brain sections were identified under fluorescence microscopy and the grain-counting software was used to quantify silver grains (representing either LepRb, Npy, Trh, or Mc4r mRNA) overlying each DIG cell. Signal-to-background ratios for individual cells were calculated, and a cell was considered double-labeled if its ratio was > 3 (35,36,41,42).
Statistical Analysis
All data are expressed as the mean ± SEM for each group. In all experiments, differences were analyzed by student's t-test, ANOVA or 2-way ANOVA (for age and genotype), followed by post-hoc comparisons for individual age/genotype groups via Fisher's (Protected) LSD. Statistical significance was set at p < 0.05. All analyses were performed in Statview 5.0.1 (SAS Institute, Cary, NC).
Experiment 1: Does RFRP-3 inhibit LH secretion in mice?
The conserved role of RFRP-3 in the inhibition of LH secretion is assumed to be true in mice but, surprisingly, data demonstrating this has not yet been reported. In order to test if the mouse RFRP-3 peptide is functionally able to suppress LH secretion in mice, as occurs with other RFRP-3 variants in other species, adult ovariectomized mice were subjected to either 100 ng or 500 ng of the murine RFRP-3 peptide (VNMEAGTRSHFPSLPQRF-NH2, Genscript USA Inc.) dissolved in 100 μL saline, or saline vehicle. 20 minutes after intraperitoneal injection, blood was collected by retro-orbital bleed and the serum was assayed for LH (n = 7-8 per treatment group).
Experiment 2: What metabolic neuropeptides and receptors are expressed in Rfrp neurons?
The phenotypic identity of Rfrp neurons is virtually unknown. Besides estrogen and glucocorticoid receptors, other receptors or secreted co-factors, such as neurotransmitters or neuropeptides, have not been identified in Rfrp neuron. Any number of metabolic genes expressed in the DMN region may co-localize with Rfrp, and if so, would give valuable insight to the regulation and potential metabolic functions of RFRP-3 neurons. We therefore examined whether transcripts of important metabolic genes already known to be expressed in the DMN specifically co-localize with Rfrp. Adult C57BL6 mice of both sexes (females in diestrous) were sacrificed and their brains collected for double label ISH analyses. Using alternate series of coronal brain sections (encompassing the entire rostral to caudal span of the DMN) from each mouse, we examined in 4 separate assays the co-expression of neuropeptide Y (Npy) (43-45), thyrotropin-releasing hormone (Thr) (34,46), leptin receptor long form (LepRb) (47), and melanocortin receptor 4 (Mc4r) (48) in Rfrp neurons (n = 4-6 per sex).
Experiment 3: Is Rfrp expression or neuron activity responsive to short-term metabolic challenge?
Given that RFRP-3 treatment increases food intake (7,49) and that a subset of Rfrp neurons express the leptin receptor (Experiment 2), it is possible that transiently diminished serum leptin levels achieved via short-term food deprivation may alter Rfrp expression and/or Rfrp neuronal activation. Furthermore, a recent report demonstrated that LH secretion is not suppressed after 12 h of food deprivation (FD) in Gpr147 knockout mice, suggesting RFRP-3 is essential during the first 12 h of FD to suppress LH (26). To further examine this, adult C57BL6 female mice were first GDX (to equate sex steroids between groups, since E2 can inhibit Rfrp levels) and were then later subjected to short-term (12-hour) FD or kept on ad libitum (ad lib) feeding (controls) (n = 7 per group). All food was removed from FD animals at lights off (1800 h) and all animals were sacrificed 12 h later at lights on (0600 h) the following morning. Cages of ad lib control mice were opened, but food was not removed. Brains and blood were collected immediately at the end of the 12 h FD or ad lib feeding period. Rfrp expression in the DMN was analyzed by single-label ISH and, in an alternate set of brain sections from the same animals, double-label ISH was performed for Rfrp and c-fos, a marker of neuronal activation. Blood serum collected at the time of sacrifice was assayed for circulating LH and leptin.
Experiment 4: Is Rfrp expression altered in adult Ob animals?
Since Experiment 2 found that a subset of Rfrp neurons express LepRb, we hypothesized that Rfrp expression may be leptin regulated. To determine if Rfrp levels are altered with chronic leptin deprivation, we measured Rfrp neuron numbers and cellular Rfrp expression levels using single label ISH in adult male and female GDX Ob mice (leptin deficient) and their WT GDX littermates (n = 5-7 animals per genotype per sex).
Experiment 5: Does Rfrp expression during postnatal development coincide with serum leptin levels?
Experiment 4 demonstrated that adult Ob mice display altered Rfrp expression, suggesting that leptin may regulate RFRP-3 neurons. In development, there is a large transient rise in serum leptin that occurs during the second week of life in mice. Around this same age, Rfrp expression is significantly higher than on the day of birth (9). In order to see if the juvenile leptin surge coincides with developmental changes in Rfrp neurons, C57BL6 breeder pairs were allowed to deliver pups naturally and the day of birth (postnatal day 1; PND1) was noted for each litter (only litters of 4-8 pups were used). Pups were sacrificed by rapid decapitation on PND 1, 3, 6, 8, 10, 12, 14, and 16 (n = 5-9 animals per age, per sex). Trunk blood was collected for serum hormone analysis, and brains were collected and frozen on dry ice. For each sex, brain slices containing the entire DMN were assayed and analyzed for Rfrp expression by single-label ISH, using the HE and LE Rfrp cell criteria described previously (see Single-label In Situ Hybridization section above) (36); each sex was assayed independently. Serum leptin was measured by ELISA. Along with the postnatal mice, cohorts of control adult diestrous female or male mice were simultaneously assayed for Rfrp expression and serum leptin levels.
In a complementary experiment, to determine if the long form leptin receptor is expressed in juvenile Rfrp neurons at different developmental stages, an alternate set of brain slides from female PND 1, 10 and 12 mice (along with adult diestrous female controls) was assayed for LepRb co-expression in Rfrp neurons using double label ISH (n = 6-7 animals per age group).
Experiment 6: Are the juvenile changes in Rfrp expression during development dependent on leptin?
Given the synchronous developmental changes in juvenile Rfrp expression and postnatal leptin secretion observed in Experiment 5, we hypothesized that the specifically-timed developmental changes in Rfrp neurons may be leptin dependent. To test this, we repeated experiment 5 using heterozygous Ob breeding pairs to produce WT, Ob, and heterozygous pups. Brains, trunk blood, and tail snips for genotyping were collected on PND1, 6, 10, 12, and 16. Only litters of 4-8 pups were used. Brains from WT and Ob males of these various postnatal ages were analyzed for Rfrp mRNA expression levels via single-label ISH (n = 5-9 animals per age per genotype). Serum leptin for each animal was measured via ELISA to confirm the presence or absence of circulating leptin.
Results
Experiment 1: The murine RFRP-3 peptide inhibits LH secretion in adult female mice
To determine if peripherally-administered murine RFRP-3 peptide can suppress LH secretion in mice, as occurs with other RFRP-3 variants in other species, 100 ng or 500 ng of RFRP-3 or saline was injected i.p. in adult GDX female mice. Both the 100 ng and 500 ng doses of murine RFRP-3 were able to significantly suppress LH secretion, to nearly 70% of saline-treated control levels (Figure 1, p < 0.05). There was no difference in the efficacy of the two doses of murine RFRP-3 on LH suppression.
Figure 1.
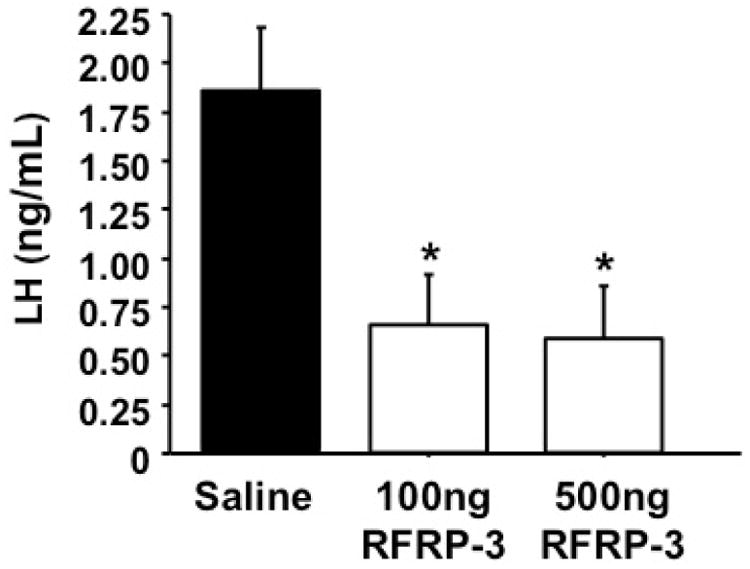
Effect of peripheral RFRP-3 injection on LH secretion in mice. Adult gonadectomized female mice were injected i.p. with 100 ng or 500 ng of RFRP-3 dissolved in 100 μL of saline, or 100 μL of saline alone, and bled 20 minutes later. RFRP-3 significantly suppressed LH secretion in gonadectomized mice at both doses (p < 0.05).
Experiment 2: A subset of Rfrp neurons express LepRb mRNA
Double label ISH was performed for energy balance-related genes known to be expressed in the DMN (34,43-48) to determine if they are co-expressed in Rfrp neurons of adult male or female mice. We found that virtually no Rfrp neurons co-expressed Npy or Trh, despite notable Npy and Trh expression in the same DMN region, often near Rfrp neurons (Figure 2; Table 1). Next, to assess the possibility that metabolic hormones/neuropeptides might directly regulate the RFRP-3 system, we looked for co-expression of several metabolic signaling factor receptors in Rfrp neurons. Long form leptin receptor (LepRb) mRNA, which is expressed in the rodent DMN, was found to be notably co-expressed in a subset of Rfrp neurons, suggesting that a proportion of adult Rfrp neurons may be leptin responsive. The degree of LepRb-Rfrp co-expression was similar in both sexes (∼15%). An even smaller subset of Rfrp neurons (∼8%) in both sexes were found to co-express the primary melanocortin receptor, Mc4r. A summary of the co-expression levels for each gene with Rfrp is in Table 1.
Figure 2.
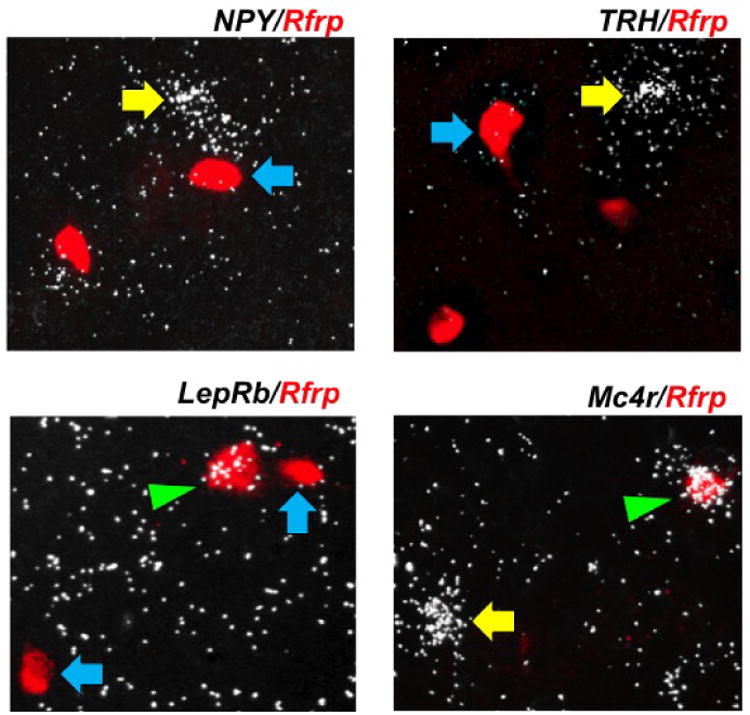
Expression of hypothalamic energy balance genes in the dorsal-medial hypothalamus. Representative photomicrographs of double label in-situ hybridization of neuropeptide Y (NPY), thyrotropin-releasing hormone (TRH), long form leptin receptor (LepRb) or melanocortin receptor 4 (Mc4r) (silver grains) in Rfrp neurons (red fluorescence). Single labeled Rfrp neurons are identified with blue arrows and single labeled NPY, TRH, LepRb or Mc4r neurons are marked with yellow arrows. Double labeled LepRb/Rfrp and double labeled Mc4r/Rfrp neurons are marked with a green arrowheads.
Table 1.
Summary of four double label in-situ hybridization assays for metabolic-related genes in the DMN co-expressed with Rfrp neurons. Adult diestrous (DE) females and intact male mice were assayed for neuropeptide Y (NPY), thyrotropin-releasing hormone (TRH), long form leptin receptor (LepRb) or melanocortin receptor 4 (Mc4r) mRNA in Rfrp cells in the DMN. Values are average percent co-expression with ± SEM. n = 4-6 animals per sex.
| Intact Males | DE Females | |
|---|---|---|
| NPY/Rfrp | 1.5 ± 0.7% | 3.0 ± 1.5% |
| TRH/Rfrp | 1.1 ± 0.5% | 1.6 ± 0.8% |
| LepRb/Rfrp | 16.9 ± 1.6% | 13.0 ± 1.6% |
| Mc4r/Rfrp | 6.9 ± 1.0% | 8.7 ± 0.7% |
Experiment 3: Rfrp neurons in mice appear unresponsive to 12-hour food deprivation
To test if energetic challenge induced by short-term food deprivation modifies Rfrp mRNA expression levels or the activity of RFRP-3 neurons, adult female mice were subjected to short-term (12 h) food deprivation (12h FD). Demonstrating the effectiveness of the energetic challenge, 12h FD mice had a significant decrease in body weight (5.3%; p<0.05) and significant decreases in serum LH and leptin relative to ad lib control females (Figure 3A-C, p < 0.05). However, despite this negative impact of 12h FD on body weight and endocrine physiology, there were no significant differences in either the total number of Rfrp neurons or the total Rfrp mRNA between ad lib and FD animals (Figure 3D-F). Moreover, when Rfrp neurons were categorized as either HE or LE, there were no statistical differences between ad lib and FD animals (data not shown).
Figure 3.
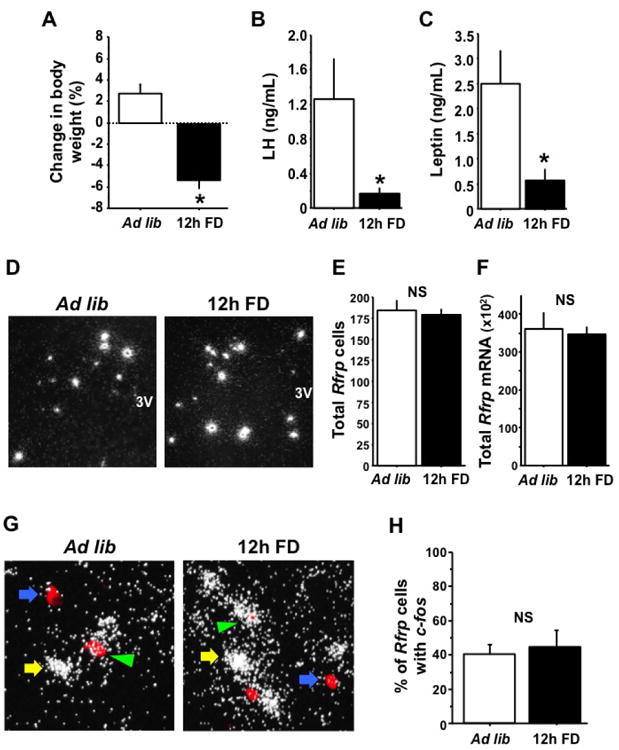
Effects of food deprivation on Rfrp expression and Rfrp neuron activation in gonadectomized (GDX) female mice. [A] The percent change in body weight after 12 hours of food deprivation (12h FD) or ad-libitum access to food (Ad lib). Food deprivation had a significant effect on the change in body weight (p < 0.05) with deprived animals losing almost 6% of body weight on average. [B] Serum luteinizing hormone (LH) is significantly less in 12h FD GDX animals than Ad lib animals (p < 0.05). [C] Serum leptin is significantly less in 12h FD GDX animals than Ad lib animals (p < 0.05). [D] Representative photomicrographs if single label in-situ hybridization (ISH) for Rfrp mRNA in Ad lib and 12h FD female GDX mice. 3V = third ventricle. [E] Quantification of single label ISH for Rfrp expressing cells; the total number of Rfrp neurons is not significantly different between Ad lib and 12h FD animals. [F] Quantification of total Rfrp mRNA from single label ISH; the total number of Rfrp neurons is not significantly different between Ad lib and 12h FD animals. [G] Representative photomicrographs if double label ISH for c-fos and Rfrp mRNAs in Ad lib and 12h FD female GDX mice. Double labeled c-fos and Rfrp (green arrowhead) and single labeled c-fos cells (yellow arrow) and single labeled Rfrp neurons (blue arrow) are shown. [H] Quantification of percent c-fos and Rfrp co-expression between Ad lib and 12h FD animals. There was no significant difference between the two groups.
In addition to mRNA levels, we also used double-label ISH to examine alterations in neuronal activation of RFRP-3 neurons in 12h FD mice. As with the single label assay, the total number of Rfrp cells in this double-label assay did not differ between 12h FD and ad-lib mice (117 ± 8 cells versus 121 ± 8 cells, respectively). Moreover, there was no group difference in the percent of Rfrp neurons demonstrating recent neuronal activation, as measured by c-fos mRNA co-expression in Rfrp neurons (Figure 3G-H).
Experiment 4: Rfrp expression is decreased in adult Ob mice of both sexes
This experiment assessed whether there are notable differences in Rfrp expression in adult Ob (leptin-deficient) and WT mice that were all GDX beforehand to remove any masking effects of circulating E2 (which moderately decreases Rfrp expression). As expected, Ob animals of both sexes were significantly heavier, weighing almost twice as much, on average, than their WT counterparts (p < 0.05, data not shown). In the brain, the total number of detectable Rfrp neurons was not significantly different between Ob and WT mice in either sex (Figure 4A-B). However, using the HE and LE Rfrp cell criteria, there was a significant decrease in the number of HE Rfrp neurons in female Ob mice compared to female WTs (Figure 4A, p < 0.05), with no significant differences in the number of LE Rfrp cells between genotypes. Likewise, a similar reduction in the number of HE Rfrp neurons was detected in male Ob mice compared to WT male littermates (Figure 4B, p < 0.05). In addition to these changes in cell number, the total level of Rfrp mRNA in the DMN was significantly lower in both male and female Ob mice compared to their WT littermate controls (Figure 4A-B, p < 0.05).
Figure 4.
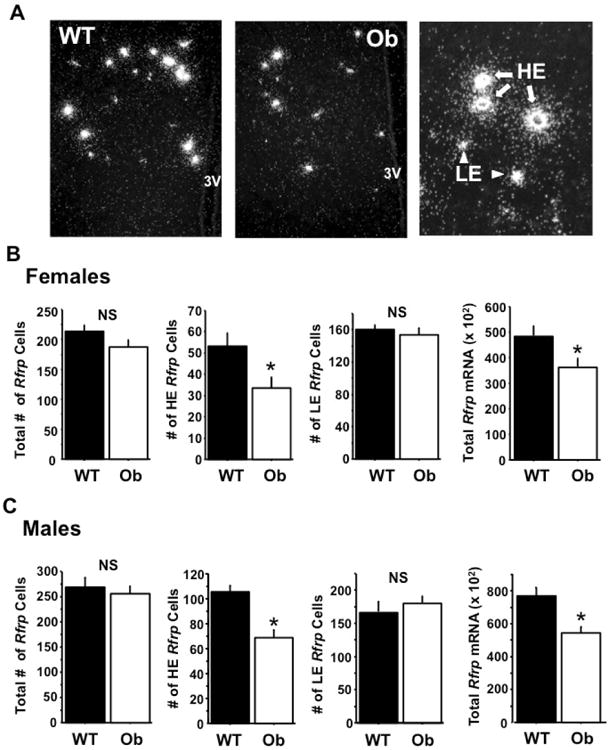
Rfrp expression in obese (Ob) male and female gonadectomized (GDX) mice. [A] Representative photomicrographs of single label in-situ hybridization for Rfrp mRNA in wildtype (WT) and Ob female GDX mice. 3V = third ventricle [B] Quantification of total Rfrp neurons, high expressing (HE) Rfrp neurons, low expressing (LE) Rfrp neurons, and total Rfrp mRNA from single label ISH in female GDX mice. The number of HE cells and the total Rfrp mRNA was significantly less in Ob females than WT littermates (p < 0.05). There were no significant differences between the total number of Rfrp neurons or number of LE cells. [C] Quantification of total Rfrp neurons, HE Rfrp neurons, LE Rfrp neurons, and total Rfrp mRNA from single label ISH in male GDX mice. The number of HE cells and the total Rfrp mRNA was significantly less in Ob males than WT littermates (p < 0.05). There were no significant differences between the total number of Rfrp neurons or number of LE cells.
Experiment 5: Changes in Rfrp expression over postnatal development correlate with the leptin surge
We previously reported that Rfrp levels in the brain are dramatically higher in juvenile mice (on PND 10) compared to birth. Since Experiment 4 demonstrated that Rfrp expression is significantly altered in adult Ob animals of both sexes, we hypothesized that the juvenile leptin surge (28) might govern the developmental changes in Rfrp expression. We first determined if there was a correlation between juvenile leptin levels and postnatal developmental changes in Rfrp expression. C57BL6 mice of both sexes were sacrificed on PND1, 3, 6, 8, 10, 12, 14, 16 and their brains assayed for Rfrp expression (Figure 5) and compared to serum leptin levels at each age. The total number of detectable Rfrp neurons was highest at birth and was significantly lower at later postnatal ages, with the lowest number of Rfrp neurons in adulthood (diestrous stage for females) (Figure 6A). However, the amount of Rfrp being expressed per cell was notably lowest at birth and increased substantially with age. Indeed, the total amount of Rfrp mRNA in the DMN increased with postnatal development, beginning to rise around PND 6 and peaking at PND 12 before dropping again at subsequent older ages (Figure 6B, p < 0.05). This robust increase in total Rfrp mRNA during the 2nd week of postnatal life appears to reflect a large increase in the number of HE Rfrp cells, which are virtually absent at birth but substantially increase in abundance beginning around PND 6, peaking at maximal levels at PND 12 before dropping slightly at subsequent older ages (Figures 6C, p < 0.05). Unlike HE Rfrp cells, the number of LE cells decreases slowly and steadily throughout these postnatal ages, with the lowest number of LE cells in adulthood (Figure 6D, p < 0.05). Virtually identical results were found in similarly-aged male mice (data not shown).
Figure 5.
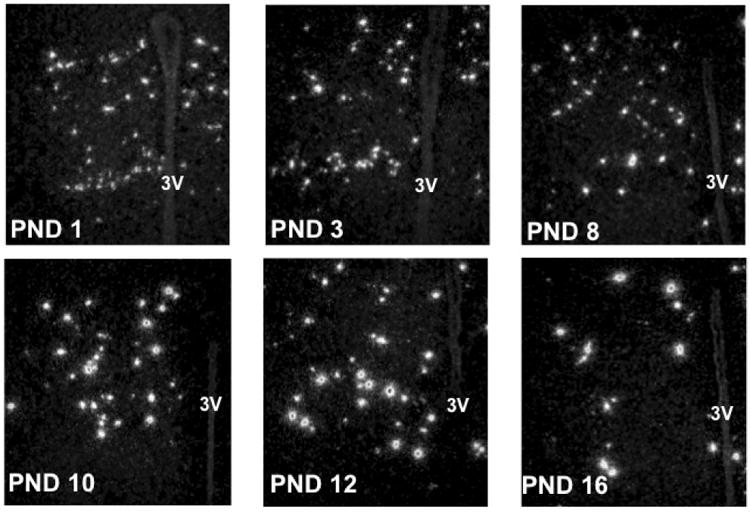
Rfrp expression over the first 16 days of postnatal life. Representative photomicrographs of single label in-situ hybridization for Rfrp mRNA on postnatal days (PND) 1, 3, 6, 8, 10, 12, 14, 16 in female mice. Day of birth is designated PND1. 3V = third ventricle.
Figure 6.
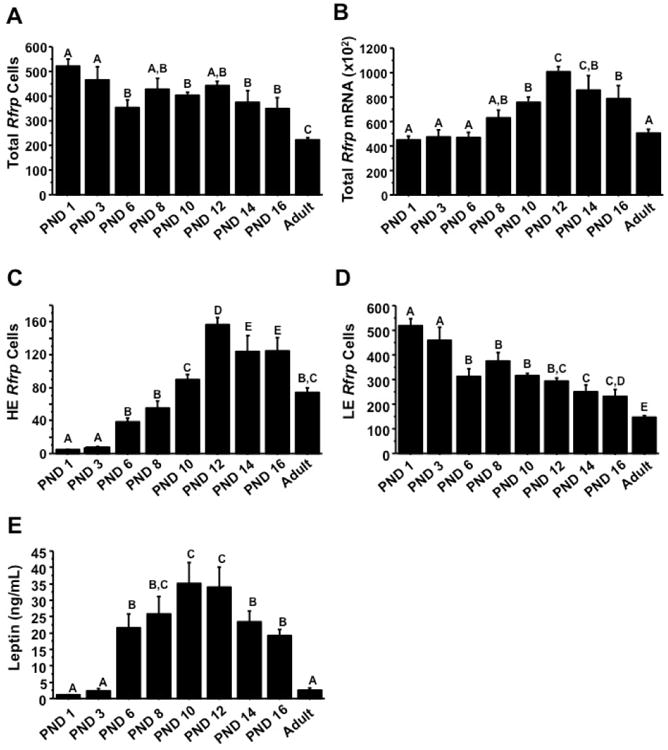
Summary of changes in Rfrp expression over the first 16 days of postnatal life in female mice from single label in-situ hybridization. Rfrp mRNA was quantified on postnatal days (PND) 1, 3, 6, 8, 10, 12, 14, 16 and diestrous female adult mice. [A] Quantification of total number of Rfrp neurons during the first two weeks of life. Different letters indicate significantly different groups (p < 0.05). [B] Quantification of total Rfrp mRNA during the first two weeks of life. Different letters indicate significantly different groups (p < 0.05). [C] Quantification of the number of high expressing (HE) Rfrp neurons during the first two weeks of life. Different letters indicate significantly different groups (p < 0.05). [D] Quantification of the number of low expressing (LE) Rfrp neurons during the first two weeks of life. Different letters indicate significantly different groups (p < 0.05). [E] Average serum leptin levels of mice sacrificed across development on PND 1, 3, 6, 8, 10, 12, 14, 16 and a cohort of diestrous female adult mice. Different letters indicate significantly different groups (p < 0.05).
Serum leptin levels were measured in each animal to see if they correlated with Rfrp levels. For both sexes, mean serum leptin levels were essentially undetectable at birth (PND 1) and on PND 3, and were first readily detectable at PND 6. Leptin levels increased robustly during the second week of life, peaking at PND 10 and 12 (the leptin surge), and subsequently began to fall on PNDs 14 and 16, being low again in adult animals (Figure 6E, p < 0.05). Leptin levels were significantly higher than adult female (diestrous) levels at all postnatal ages examined, except for PND 1 and 3 (p < 0.05). Interestingly, these postnatal changes in leptin levels, reflective of the leptin surge, appeared to correlate well with the observed increases in the number of HE Rfrp cells and total Rfrp mRNA, with both starting to increase around PND 6 and both reaching maximal levels around PND 10 and PND 12 (Figure 6).
Experiment 6: Juvenile Rfrp expression is not dependent on leptin during development
The dramatic developmental increase in HE Rfrp neurons and total Rfrp mRNA observed around PND 10 and 12 correlates with the zenith of the juvenile leptin surge (Experiment 5), suggesting that the two events may be related. To test this possibility, we determined both whether Rfrp neurons co-express LepRb during specific postnatal ages and whether the normal developmental pattern and levels of Rfrp expression are altered in juvenile mice lacking leptin. In postnatal brains examined for Rfrp/LepRb co-expression, a subset of Rfrp cells clearly co-expressed notable LepRb mRNA (Figure 7A); the degree of co-expression did not vary with age, being 15-20% from birth through adulthood (Figure 7B).
Figure 7.
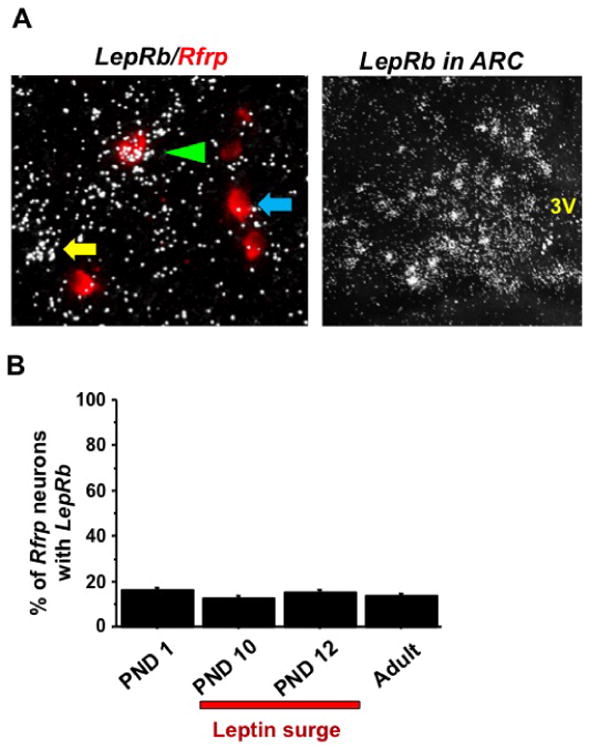
Long-form leptin receptor (LepRb) expression in some Rfrp neurons during postnatal life. [A] [Left] Representative photomicrographs of double label in-situ hybridization LepRb (silver grains) in Rfrp neurons (red fluorescence) in the DMN region. A single labeled Rfrp neurons is identified with a blue arrows and a single labeled LepRb neuron is marked with a yellow arrow. Double labeled LepRb/Rfrp is marked with a green arrowhead. [Right] Strong LepR mRNA staining in the ARC nucleus (left side of nucleus shown) in the same double label LepRb/Rfrp assay. 3V, third ventricle. [B] Quantification of the percent co-expression of LepRb and Rfrp during postnatal development. Animals sacrificed on postnatal days (PND) 1, 10, 12 and a cohort of adult diestrous females were assayed. No significant differences were seen between ages. The leptin surge peaks around PND10 and 12, as indicated by the red bar.
To determine if leptin signaling is required, directly or indirectly, for the normal pattern of Rfrp neuron development in juveniles, female Ob and WT brains were analyzed for Rfrp expression on PND1, 6, 10, 12 and 16 (Figure 8, 9). Despite the strong correlation between developmental Rfrp and leptin changes observed in normal mice, there were no significant differences in the number of Rfrp neurons, total amount of Rfrp mRNA, or the number of HE and LE Rfrp cells between Ob and WT mice at any developmental age (Figure 9A-D). The same developmental pattern in Rfrp expression seen in Experiment 5 was observed in both genotypes, with identical magnitude increases detected in total Rfrp mRNA and the number of HE cells. Moreover, all Rfrp expression increases occurred at the same specific postnatal ages, regardless of genotype. Confirming the genotypes and leptin milieu, serum leptin was significantly elevated between PND 6 and 12 (representing the leptin surge), and lower on PND 16 than 12 in WT animals (p < 0.05) but remained undetectable in Ob animals at all ages (Figure 9E).
Figure 8.
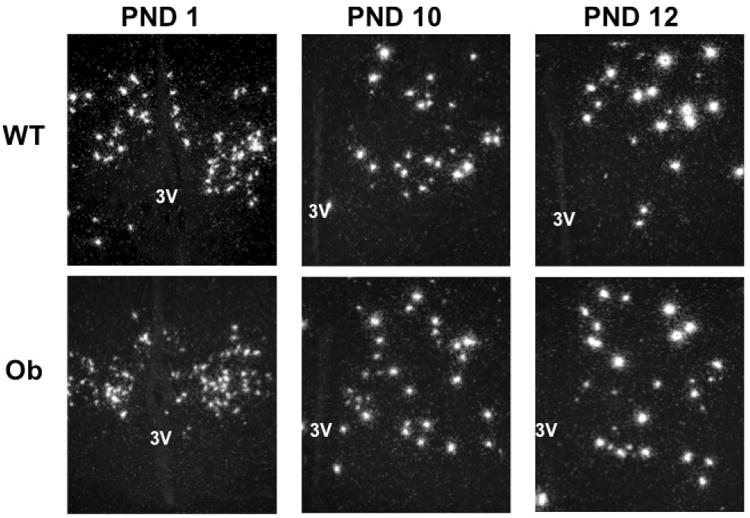
Rfrp expression over postnatal life in female Obese (Ob) and wildtype (WT) littermates. Representative photomicrographs of single label in-situ hybridization for Rfrp mRNA on postnatal days (PND) 1, 10, and 12 are shown. 3V = third ventricle.
Figure 9.
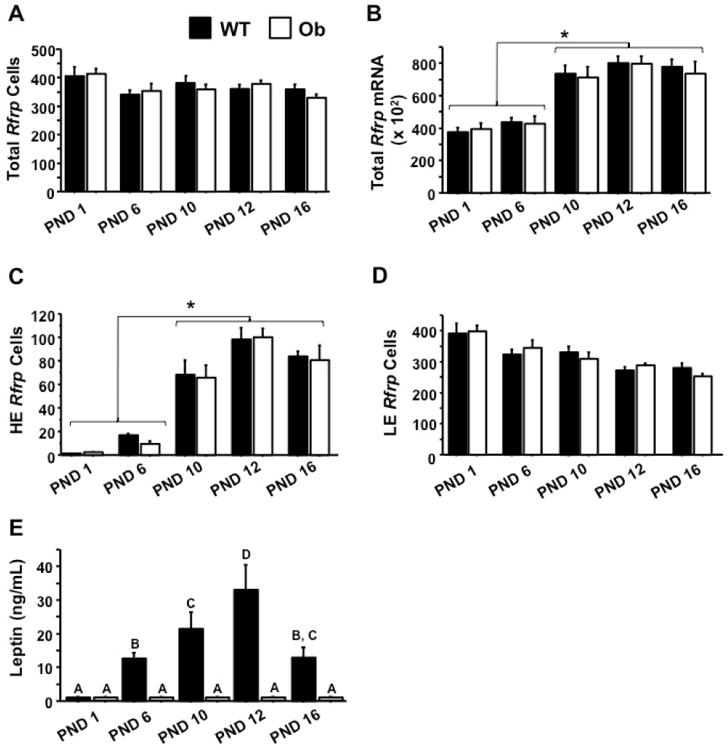
Summary of changes in Rfrp expression on postnatal days (PND) 1, 6, 10, 12, and 16 in female Obese (Ob) and wildtype (WT) animals. [A] Quantification of total number of Rfrp neurons during the leptin surge. There were no significant changes between genotypes. [B] Quantification of total Rfrp mRNA during the leptin surge. Rfrp expression is significantly higher on postnatal (PND) 10, 12, and 16 than on PND 1 or PND 6 (p < 0.05). [C] Quantification of the number of high expressing (HE) Rfrp neurons during the leptin surge. The number of Rfrp neurons is significantly higher on PND10, 12, and 16 than on PND 1 or PND 6 (p < 0.05). [D] Quantification of the number of low expressing (LE) Rfrp neurons during the leptin surge. Different letters indicate significantly different groups (p < 0.05). [E] Serum leptin concentration in Ob and WT female mice as measured by ELISA. Leptin is highest on postnatal days 10 and 12, dropping on 16, demonstrating the postnatal leptin surge. Leptin concentration in WT mice were significantly elevated between PND 6 and PND 16, peaking around PND 12 (p < 0.05). Leptin was not detectable on the day of birth in WT mice or in Ob animals at any age.
Discussion
The physiological roles RFRP-3 plays and how the hypothalamic RFRP-3 system develops and is regulated are still poorly understood. In addition to providing the first demonstration of peripherally administered RFRP-3′s ability to potently inhibit LH secretion in vivo in mice, our current findings also show that a subset of RFRP-3 neurons express the long-form of the leptin receptor and that Rfrp mRNA expression is diminished in Ob (leptin deficient) animals of both sexes. We also demonstrate that Rfrp expression changes in a dramatic and unusual manner in postnatal development, mirroring age-specific juvenile changes in serum leptin. However, examination of the developmental maturation of Rfrp neurons in Ob pups revealed that neither the timing nor magnitude of these developmental changes in Rfrp expression are not dependent on leptin.
Despite the reported effect of the rat variant of RFRP-3 to inhibit LH secretion in many species, including rodents (rats and hamsters), the effect of the murine variant of RFRP-3 on LH has not been reported in any species, including mice themselves. As the RFRP-3 field moves towards transgenic and knockout animals (26,50), mouse models will become increasingly important and affirming our understanding of murine RFRP-3 pharmacology and physiology is essential. Our present finding of markedly suppressed LH secretion 20 min after peripheral injection of 2 doses of murine RFRP-3 in adult female mice matches the results found in rat, hamster, and sheep using the rat RFRP-3 peptide (2,51-54). Until just recently, RFPR-3 of any variant had surprisingly never been administered to mice. León et al. reported that a truncated rat RFRP-3 peptide given via intracerebroventricular injection modestly decreased serum LH (by ∼ 25%) in WT GDX female mice (26). That reduction in LH was considerably less than the suppressive effects of peripheral murine RFRP-3 observed in the present study, in which we found ∼ 70% decrease in serum LH after peripheral murine RFRP-3 injections at 2 different doses. Besides potential species differences in murine versus rat RFRP-3 peptide efficacy, the difference in magnitude of effect might be due to dosing or the route of administration. Interestingly, the 100 ng and 500 ng doses were equally effective in suppressing LH secretion, suggesting that the suppressive effect of RFRP-3 may already be maximal near the 100 ng dose. As our RFRP-3 was administered peripherally, we cannot discern at what level(s) of the HPG axis the lowered LH secretion is attributable to. However, most data in rodents suggests that RFRP-3′s effects on LH are induced in the brain, via diminished GnRH signaling, rather than at the pituitary (8). Yet, one study recently described in vitro effects of RFRP-3 on the pituitary in murine gonadotrope LβT2 cells (55).
Several studies have demonstrated an orexigenic effect of RFRP-3 (7,24,49), suggesting that RFRP-3 may be involved in receiving and/or transmitting energy balance signals. Both NPY and TRH are neuropeptide cell populations that reside, and are even expressed in a similar pattern as Rfrp, in the DMN (34,45). However Rfrp did not co-localize with either of these neuropeptides, demonstrating that it is a unique neuropeptide population within the DMN. We also examined two metabolic hormone receptors, the long form leptin receptor, LepRb, and a melanocortin receptor, Mc4r, which are known to also be expressed in the DMN. A small subset of Rfrp neurons expressed each of these receptors, with the leptin receptor being the more prominently expressed, suggesting that some Rfrp neurons may be a direct target of leptin signaling. However, most Rfrp neurons did not express LepR, suggesting that any major effects of leptin signaling— or its absence—on Rfrp neurons may in fact be indirect.
Ob mice are infertile due to a lack of gonadotropin secretion (27), and since RFRP-3 is known to inhibit LH secretion, and some Rfrp neurons express the leptin receptor, we hypothesized that Rfrp expression would be higher in Ob mice. However, using GDX adult Ob males and females, we found the opposite outcome: Rfrp expression is lower in adult Ob mice than their wildtype littermates. This was true for both sexes. During the writing of this manuscript, Rizwan et al. (56) published data suggesting that RFRP-3 neurons are not regulated by leptin. That study's immunohistochemical (IHC) data showed no difference between the number of RFRP-3-immunoreactive neurons in GDX, E2-replaced Ob mice and WT littermates, and minimal phosphorylation of signal transducer and activator of transcription 3 (STAT3) in RFRP-3 neurons after leptin injections. Those results suggest that RFRP-3 neurons are not directly regulated by leptin and that RFRP-3-immunoreactive levels are unaffected by leptin deficiency, differing from our present results in which Rfrp levels were significantly diminished in Ob mice. It is possible that the exogenous E2 replacement in the Rizwan study may have masked any difference between WT and Ob animals, as E2 moderately represses Rfrp (9,31), and may have done so similarly in both genotypes. In contrast, our present data quantifying gene expression (rather than protein) levels demonstrates that total Rfrp mRNA levels are significantly repressed in GDX Ob animals (no E2 given, so Rfrp expression is not being inhibited by sex steroids). Additionally, in our Ob mice, we identified a primary deficit in Rfrp expression specifically in the HE Rfrp cell population, which is a cellular distinction that has not yet been analyzed using IHC. The functional significance of the lower Rfrp levels in Ob mice is not yet known; we note that RFRP-3 has proposed roles in several other physiological/behavioral systems beyond reproduction, such as feeding, stress, and anxiety, and the observed decreases in Rfrp levels in Ob mice may reflect RFRP-3′s involvement in these processes rather than the reproductive impairments of these animals.
Although we show a notable decrease in overall Rfrp levels in adult Ob mice of both sexes, it remains unclear if this is due to changes in direct or indirect leptin signaling to RFRP-3 cells. Rizwan et al. showed minimal phosphorylation of STAT3 in RFRP-3 neurons after leptin treatment, ranging from ∼3% in mice to 7-13% in rats, suggesting that any effects of leptin would likely be indirect. In the present study, using double-label techniques, we found direct anatomical evidence for a slightly higher degree of LepRb expression in Rfrp neurons, 15-20% in female and male mice, suggesting that some effects of leptin could in fact be direct on a subset of Rfrp neurons. Yet, based on the overall low-to moderate percent of total Rfrp neurons expressing LepRb, we concur with Rizwan et al., that the majority of RFRP-3 neurons in rodents are likely not directly responsive to leptin signaling. If so, the diminished Rfrp levels observed in Ob mice may reflect any of the following: 1) direct leptin signaling in only a small subset (∼15-20%) of Rfrp cells, causing only those cells to change gene expression, 2) indirect leptin signaling on upstream leptin-responsive circuits that themselves directly regulate RFRP-3 neurons, 3) influence of non-leptin metabolic signals that are secondarily altered in the obese state of Ob mice. This last possibility seems likely, since Rfrp expression was normal in young juvenile Ob mice, in which leptin signaling is absent but obesity has yet to emerge. Of note, despite the observed low-to-moderate levels of LepR-Rfrp coexpression, very strong LepR expression was observed in the ARC and other areas of the same brain sections, demonstrating the robustness of the LepR probe staining in these assays.
Central injections of RFRP-3 reportedly stimulate food intake (23,24), and RFRP-3 neurons are activated by chronic mild (20%) food restriction in hamsters (25). Moreover, a recent report demonstrated that LH secretion is not suppressed after 12 h of FD in Gpr147 knockout mice (lacking the RFRP-3 receptor), suggesting that RFRP-3 is involved in the metabolic regulation of LH (26). We therefore examined whether Rfrp expression levels or Rfrp neuronal activation is altered in adult female mice subjected to 12 h FD. This short-term FD had no effect on Rfrp expression or Rfrp neuronal activation. These results are surprising, as body weight and LH levels were significantly lower after FD, reflecting effective metabolic challenge and inhibition of the reproductive system. Thus, the lower LH levels observed in our metabolically challenged females, which we hypothesized to be a direct or indirect effect of enhanced RFRP-3, appear to be decreased by some other mechanism. The previously observed increase in Rfrp neuronal activation in hamsters that were food restricted to 80% of normal diet for much longer periods of time (8 days or more (25)) may suggest that metabolic alteration of Rfrp neurons may either be a part of chronic or mild nutritional stress, rather than acute, severe changes in energy availability, as in our 12 h FD paradigm.
Over the course of postnatal development, there are drastic age-specific changes in neural Rfrp expression, especially in the late infantile and juvenile phases. Previously, we demonstrated that both the number of HE Rfrp cells and the total amount of Rfrp mRNA per cell are considerably lower at birth than on PND 10, at which time the number of LE Rfrp cells is lower than at birth (9). Here, we determined a more detailed time-line during the first two weeks of life. In normal C57BL6 mice, the developmental increase in HE Rfrp cells and total Rfrp mRNA appears around PND 6-8, with a peak in these measures around PND 12. This was in striking synchronous alignment with the occurrence of the postnatal leptin surge. However, using Ob mice, we found that neither the timing nor magnitude of this developmental pattern of the Rfrp population is leptin dependent. The developmental changes observed in Rfrp expression may be due to other metabolic hormones, such as ghrelin or insulin (57-59), or reproductive hormones from maturing gonads (60-63), all of which have been shown to modify gene expression in the developing hypothalamus. Identifying the cause of the dramatic Rfrp changes during development, and just as importantly, their functional significance, will be an important avenue of future inquiry.
In conclusion, the data presented here provide further insight into the regulation and development of RFRP-3 neurons in mice. We show for the first time that peripherally administered murine RFRP-3 peptide can strongly inhibit LH secretion in adult female mice, consistent with reports for other RFRP-3 variants in other species. We also show that although Rfrp neurons appears to be a distinct population of cells from NPY or TRH cells in the DMN, there does appear to be a small subpopulation of Rfrp neurons that co-express LepRb, allowing for the possibility of direct leptin signaling in these cells. This finding is extended by the novel observation that adult Ob animals have less Rfrp expression than their WT littermates, though whether this decrease in Rfrp expression is due to direct or indirect effect of leptin signaling, or other metabolic cues associated with obesity, remains to be determined. Additionally, we demonstrate that Rfrp expression in female mice is resistant to short-term food deprivation, despite previous reports demonstrating effects of long-term mild food restriction on the activation of RFRP-3 neurons in other species. Thus, the observed decrease in LH under short-term food deprivation in mice is unlikely to be due to changes in RFRP-3 neurons. Lastly, we show for the first time the detailed developmental pattern of Rfrp expression in the rodent brain during infantile and juvenile life, and though this pattern correlates with leptin levels, we find it is independent on leptin signaling. The mechanism and physiological significance of these dramatic Rfrp changes during juvenile development remains to be determined.
Acknowledgments
This research was supported by NIH grant R01 HD065856. Additional support was provided by the Eunice Kennedy Shriver NICHD/NIH (SCCPIR) grant U54-HD012303 (U.C. San Diego) and U54 HD-28934 (University of Virginia Ligand Assay and Analysis Core). The authors thank Kristen Tolson, Joshua Kim, and Elena Luo for technical assistance and comments on this manuscript.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275(2):661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- 2.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A. 2006;103(7):2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson GM, Relf HL, Rizwan MZ, Evans JJ. Central and peripheral effects of RFamide-related peptide-3 on luteinizing hormone and prolactin secretion in rats. Endocrinology. 2009;150(4):1834–1840. doi: 10.1210/en.2008-1359. [DOI] [PubMed] [Google Scholar]
- 4.Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology. 2009;150(6):2799–2804. doi: 10.1210/en.2008-1623. [DOI] [PubMed] [Google Scholar]
- 5.Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol. 2009;587(Pt 7):1401–1411. doi: 10.1113/jphysiol.2008.166447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson GM, Augustine RA, Rizwan MZ, Cornes PA. Neuroscience 2011. Washington D.C: 2011. RFamide-related peptide-3 (RFRP-3) regulates LH secretion exclusively via a GnRH-dependent mechanism in the rat. [Google Scholar]
- 7.Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav. 2007;51(1):171–180. doi: 10.1016/j.yhbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizwan MZ, Poling MC, Corr M, Cornes PA, Augustine RA, Quennell JH, Kauffman AS, Anderson GM. RFamide-Related Peptide-3 Receptor Gene Expression in GnRH and Kisspeptin Neurons and GnRH-Dependent Mechanism of Action. Endocrinology. 2012;153(8):3770–3779. doi: 10.1210/en.2012-1133. [DOI] [PubMed] [Google Scholar]
- 9.Poling MC, Kim J, Dhamija S, Kauffman AS. Development, Sex Steroid Regulation, and Phenotypic Characterization of RFamide-Related Peptide (Rfrp) Gene Expression and RFamide Receptors in the Mouse Hypothalamus. Endocrinology. 2012;153(4):1827–1840. doi: 10.1210/en.2011-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol. 2000;2(10):703–708. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- 11.Liu Q, Guan XM, Martin WJ, McDonald TP, Clements MK, Jiang Q, Zeng Z, Jacobson M, Williams DL, Jr, Yu H, Bomford D, Figueroa D, Mallee J, Wang R, Evans J, Gould R, Austin CP. Identification and characterization of novel mammalian neuropeptide FF-like peptides that attenuate morphine-induced antinociception. J Biol Chem. 2001;276(40):36961–36969. doi: 10.1074/jbc.M105308200. [DOI] [PubMed] [Google Scholar]
- 12.Yano T, Iijima N, Kakihara K, Hinuma S, Tanaka M, Ibata Y. Localization and neuronal response of RFamide related peptides in the rat central nervous system. Brain Res. 2003;982(2):156–167. doi: 10.1016/s0006-8993(03)02877-4. [DOI] [PubMed] [Google Scholar]
- 13.Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav. 2002;76(3):431–442. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- 14.Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc Natl Acad Sci U S A. 2006;103(32):12150–12155. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bi S. Role of dorsomedial hypothalamic neuropeptide Y in energy homeostasis. Peptides. 2007;28(2):352–356. doi: 10.1016/j.peptides.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41(12):317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 18.Quennell JH, Mulligan AC, Tups A, Liu X, Phipps SJ, Kemp CJ, Herbison AE, Grattan DR, Anderson GM. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology. 2009;150(6):2805–2812. doi: 10.1210/en.2008-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louis GW, Greenwald-Yarnell M, Phillips R, Coolen LM, Lehman MN, Myers MG., Jr Molecular mapping of the neural pathways linking leptin to the neuroendocrine reproductive axis. Endocrinology. 2011;152(6):2302–2310. doi: 10.1210/en.2011-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology. 1997;138(2):839–842. doi: 10.1210/endo.138.2.5033. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira VX, Jr, Fazio MA, Miranda MT, da Silva JM, Bittencourt JC, Elias CF, Miranda A. Leptin fragments induce Fos immunoreactivity in rat hypothalamus. Regul Pept. 2005;127(1-3):123–132. doi: 10.1016/j.regpep.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9(2):117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson MA, Fraley GS. Rat RFRP-3 alters hypothalamic GHRH expression and growth hormone secretion but does not affect KiSS-1 gene expression or the onset of puberty in male rats. Neuroendocrinology. 2008;88(4):305–315. doi: 10.1159/000145718. [DOI] [PubMed] [Google Scholar]
- 24.Clarke IJ, Smith JT, Henry BA, Oldfield BJ, Stefanidis A, Millar RP, Sari IP, Chng K, Fabre-Nys C, Caraty A, Ang BT, Chan L, Fraley GS. Gonadotropin-inhibitory hormone is a hypothalamic peptide that provides a molecular switch between reproduction and feeding. Neuroendocrinology. 2012;95(4):305–316. doi: 10.1159/000332822. [DOI] [PubMed] [Google Scholar]
- 25.Klingerman CM, Williams WP, 3rd, Simberlund J, Brahme N, Prasad A, Schneider JE, Kriegsfeld LJ. Food Restriction-Induced Changes in Gonadotropin-Inhibiting Hormone Cells are Associated with Changes in Sexual Motivation and Food Hoarding, but not Sexual Performance and Food Intake. Front Endocrinol (Lausanne) 2012;2:101. doi: 10.3389/fendo.2011.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leon S, Garcia-Galiano D, Ruiz-Pino F, Barroso A, Manfredi-Lozano M, Romero-Ruiz A, Roa J, Vazquez MJ, Gaytan F, Blomenrohr M, van Duin M, Pinilla L, Tena-Sempere M. Physiological Roles of Gonadotropin-Inhibitory Hormone Signaling in the Control of Mammalian Reproductive Axis: Studies in the NPFF1 Receptor Null Mouse. Endocrinology. 2014:en20141030. doi: 10.1210/en.2014-1030. [DOI] [PubMed] [Google Scholar]
- 27.Swerdloff RS, Batt RA, Bray GA. Reproductive hormonal function in the genetically obese (ob/ob) mouse. Endocrinology. 1976;98(6):1359–1364. doi: 10.1210/endo-98-6-1359. [DOI] [PubMed] [Google Scholar]
- 28.Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101(5):1020–1027. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304(5667):108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 30.Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci. 2004;24(11):2797–2805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molnar CS, Kallo I, Liposits Z, Hrabovszky E. Estradiol down-regulates RF-amide-related peptide (RFRP) expression in the mouse hypothalamus. Endocrinology. 2011;152(4):1684–1690. doi: 10.1210/en.2010-1418. [DOI] [PubMed] [Google Scholar]
- 32.Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996;387(2-3):113–116. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- 33.Finn PD, Steiner RA, Clifton DK. Temporal patterns of gonadotropin-releasing hormone (GnRH), c-fos, and galanin gene expression in GnRH neurons relative to the luteinizing hormone surge in the rat. J Neurosci. 1998;18(2):713–719. doi: 10.1523/JNEUROSCI.18-02-00713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugrue ML, Vella KR, Morales C, Lopez ME, Hollenberg AN. The thyrotropin-releasing hormone gene is regulated by thyroid hormone at the level of transcription in vivo. Endocrinology. 2010;151(2):793–801. doi: 10.1210/en.2009-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dror T, Franks J, Kauffman AS. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol Reprod. 2013;88(6):146. doi: 10.1095/biolreprod.113.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poling MC, Quennell JH, Anderson GM, Kauffman AS. Kisspeptin neurones do not directly signal to RFRP-3 neurones but RFRP-3 may directly modulate a subset of hypothalamic kisspeptin cells in mice. J Neuroendocrinol. 2013;25(10):876–886. doi: 10.1111/jne.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chowen JA, Clifton DK. Semiquantitative analysis of cellular somatostatin mRNA levels by in situ hybridization histochemistry. Method Neurosci. 1991;5:137–158. [Google Scholar]
- 38.Kauffman AS, Navarro VM, Kim J, Clifton D, Steiner RA. Sex Differences in the Regulation of Kiss1/NKB Neurons in Juvenile Mice: Implications for the Timing of Puberty. Am J Physiol Endocrinol Metab. 2009;(00461):02009. doi: 10.1152/ajpendo.00461.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology. 2010;151(12):5807–5817. doi: 10.1210/en.2010-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology. 2011;152(5):2020–2030. doi: 10.1210/en.2010-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kauffman A, Sun Y, Kim J, Khan AR, Shu J, Neal-Perry G. Vasoactive Intestinal Peptide Modulation of the Steroid-Induced LH Surge Involves Kisspeptin Signaling in Young but Not in Middle-Aged Female Rats. Endocrinology. 2014:en20131793. doi: 10.1210/en.2013-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Giorgio NP, Semaan SJ, Kim J, Lopez PV, Bettler B, Libertun C, Lux-Lantos VA, Kauffman AS. Impaired GABAB receptor signaling dramatically up-regulates Kiss1 expression selectively in nonhypothalamic brain regions of adult but not prepubertal mice. Endocrinology. 2014;155(3):1033–1044. doi: 10.1210/en.2013-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sahu A, Kalra PS, Kalra SP. Food deprivation and ingestion induce reciprocal changes in neuropeptide Y concentrations in the paraventricular nucleus. Peptides. 1988;9(1):83–86. doi: 10.1016/0196-9781(88)90013-7. [DOI] [PubMed] [Google Scholar]
- 44.Chan YY, Steiner RA, Clifton DK. Regulation of hypothalamic neuropeptide-Y neurons by growth hormone in the rat. Endocrinology. 1996;137(4):1319–1325. doi: 10.1210/endo.137.4.8625906. [DOI] [PubMed] [Google Scholar]
- 45.Li C, Chen P, Smith MS. The acute suckling stimulus induces expression of neuropeptide Y (NPY) in cells in the dorsomedial hypothalamus and increases NPY expression in the arcuate nucleus. Endocrinology. 1998;139(4):1645–1652. doi: 10.1210/endo.139.4.5905. [DOI] [PubMed] [Google Scholar]
- 46.Lechan RM, Jackson IM. Immunohistochemical localization of thyrotropin-releasing hormone in the rat hypothalamus and pituitary. Endocrinology. 1982;111(1):55–65. doi: 10.1210/endo-111-1-55. [DOI] [PubMed] [Google Scholar]
- 47.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395(4):535–547. [PubMed] [Google Scholar]
- 48.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457(3):213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 49.Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol. 2008;199(1):105–112. doi: 10.1677/JOE-08-0197. [DOI] [PubMed] [Google Scholar]
- 50.Soga T, Kitahashi T, Clarke IJ, Parhar IS. Gonadotropin-inhibitory hormone promoter-driven enhanced green fluorescent protein expression decreases during aging in female rats. Endocrinology. 2014;155(5):1944–1955. doi: 10.1210/en.2013-1786. [DOI] [PubMed] [Google Scholar]
- 51.Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, Iqbal J, Li Q, Tilbrook A, Morgan K, Pawson AJ, Tsutsui K, Millar RP, Bentley GE. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology. 2008;149(11):5811–5821. doi: 10.1210/en.2008-0575. [DOI] [PubMed] [Google Scholar]
- 52.Rizwan MZ, Porteous R, Herbison AE, Anderson GM. Cells expressing RFamide-related peptide-1/3, the mammalian gonadotropin-inhibitory hormone orthologs, are not hypophysiotropic neuroendocrine neurons in the rat. Endocrinology. 2009;150(3):1413–1420. doi: 10.1210/en.2008-1287. [DOI] [PubMed] [Google Scholar]
- 53.Pineda R, Garcia-Galiano D, Sanchez-Garrido MA, Romero M, Ruiz-Pino F, Aguilar E, Dijcks FA, Blomenrohr M, Pinilla L, van Noort PI, Tena-Sempere M. Characterization of the inhibitory roles of RFRP3, the mammalian ortholog of GnIH, in the control of gonadotropin secretion in the rat: in vivo and in vitro studies. Am J Physiol Endocrinol Metab. 2010;299(1):E39–46. doi: 10.1152/ajpendo.00108.2010. [DOI] [PubMed] [Google Scholar]
- 54.Ancel C, Bentsen AH, Sebert ME, Tena-Sempere M, Mikkelsen JD, Simonneaux V. Stimulatory effect of RFRP-3 on the gonadotrophic axis in the male Syrian hamster: the exception proves the rule. Endocrinology. 2012;153(3):1352–1363. doi: 10.1210/en.2011-1622. [DOI] [PubMed] [Google Scholar]
- 55.Son YL, Ubuka T, Millar RP, Kanasaki H, Tsutsui K. Gonadotropin-inhibitory hormone inhibits GnRH-induced gonadotropin subunit gene transcriptions by inhibiting AC/cAMP/PKA-dependent ERK pathway in LbetaT2 cells. Endocrinology. 2012;153(5):2332–2343. doi: 10.1210/en.2011-1904. [DOI] [PubMed] [Google Scholar]
- 56.Rizwan MZ, Harbid AA, Inglis MA, Quennell JH, Anderson GM. Evidence That Hypothalamic RFamide Related Peptide-3 Neurones are not Leptin-Responsive in Mice and Rats. J Neuroendocrinol. 2014;26(4):247–257. doi: 10.1111/jne.12140. [DOI] [PubMed] [Google Scholar]
- 57.Bouret SG. Organizational actions of metabolic hormones. Front Neuroendocrinol. 2013;34(1):18–26. doi: 10.1016/j.yfrne.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hayashida T, Nakahara K, Mondal MS, Date Y, Nakazato M, Kojima M, Kangawa K, Murakami N. Ghrelin in neonatal rats: distribution in stomach and its possible role. J Endocrinol. 2002;173(2):239–245. doi: 10.1677/joe.0.1730239. [DOI] [PubMed] [Google Scholar]
- 59.Steculorum SM, Bouret SG. Maternal diabetes compromises the organization of hypothalamic feeding circuits and impairs leptin sensitivity in offspring. Endocrinology. 2011;152(11):4171–4179. doi: 10.1210/en.2011-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mannan MA, O'Shaughnessy PJ. Ovarian steroid metabolism during post-natal development in the normal mouse and in the adult hypogonadal (hpg) mouse. J Reprod Fertil. 1988;82(2):727–734. doi: 10.1530/jrf.0.0820727. [DOI] [PubMed] [Google Scholar]
- 61.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 62.Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev. 2009;30(7):883–925. doi: 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- 63.Poling MC, Kauffman AS. Organizational and activational effects of sex steroids on kisspeptin neuron development. Front Neuroendocrinol. 2013;34(1):3–17. doi: 10.1016/j.yfrne.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


