Abstract
Background
Previous studies have described increased innate immune activation in HIV-1 exposed, sero-negative intra-venous drug users (HESN-IDU), but have not addressed the independent role of injected drugs and/or repeated injections in driving immune activation.
Methods
Here, we investigated innate (NK cells and dendritic cells) and adaptive (HIV-specific antibody and CD8+ T cell) immune parameters among a high-risk cohort of needle-sharing HESN-IDU subjects and compared them to low-risk non-sharing IDU subjects (NS-IDU) and non drug-user controls.
Results
We observed that HIV-specific antibody and CD8+ T cell responses were not detected in HESN-IDU subjects, yet innate immune cell activation was found to be significantly increased on NK cells (CD69 and CD107a upregulation) and MDCs (CD40 and CD83 upregulation) when compared to NS-IDU subjects or non drug-user controls (p<0.01, and p<0.05, respectively). HESN-IDU subjects maintained strong NK cell CD107a degranulation and cytokine (IFN-gamma, TNF-alpha and MIP-1 beta) production following target cell-incubation suggesting that constitutive innate activation does not induce functional exhaustion of innate cells in HESN-IDU subjects. NK activation in HESN-IDU subjects was independent of drug use patterns but was durable over time and correlated with plasma levels of IP-10 by Luminex analysis (rho=0.5073, p=0.0059, n=28).
Conclusions
Our results indicate that heightened innate immune cell activation in HESN-IDU subjects is not the result of the IV-drugs and repeated injection practice itself, but to repeated exposure to factors intrinsic to sharing needles (i.e., exposure to pathogens or heterologous cells among donor blood).
Keywords: HESN, Intravenous Drug-users (IDU), NK Cells, Dendritic Cells, HIV/AIDS
Introduction
The description of HIV-1 exposed individuals that remain sero-negative (HESN) despite repeated high-risk exposure has heightened interest in identifying potential immune-mediated mechanisms of protection from HIV-1. HIV-specific humoral and T cell mediated responses were originally identified in a subset of HESN subjects [1–6], although the magnitude was significantly lower than comparable responses observed in HIV-1 infected individuals [7, 8] and apparently not protective in persistently exposed HESN subjects that later sero-convert [9–11]. Decreased CD4+ T cell activation has been suggested as another correlate of protection from infection [12–14] as have soluble cytokines and anti-HIV peptides [15–19]. In support of the innate cell response as another potential barrier to HIV-1 infection, increased Natural Killer (NK) cell activation has been identified in several high-risk cohorts of HESN subjects exposed through IV-drug use [20–22]. Genotypic data has revealed an enrichment of protective NK receptor alleles in HESN subjects [23, 24] while functional data on NK cells suggests that increased cytokine secretion capacity is another hallmark of high-risk groups that remain uninfected [21, 25, 26]. Together, these results suggest that multiple mechanisms may contribute to the barrier to HIV-1 acquisition, and innate immune cells such as NK cells may further bolster the threshold to HIV-1 infection.
NK cells represent a critical component of the host innate immune response against acute viral infection and serve as a front-line defense against a diverse array of pathogens. Unlike antigen specific T cells, NK cells use the coordinated interaction of both inhibitory and activating receptors to recognize target cells that exhibit signs of stress and display absent or mis-matched MHC Class I (MHC-I) proteins. NK activity is also regulated by accessory cells such as myeloid and plasmacytoid dendritic cells that secrete NK-stimulatory cytokines such as IFN-alpha, IL-12 and IL-15 [27]. This accessory function of dendritic cells is critical for NK cytotoxicity against HIV-1 infected targets [28], and dendritic cell cross-talk with NK cells has been postulated to be important for protection in some cohorts of HESN subjects [29, 30]. Recently, we confirmed previous reports of increased NK activation in HESN-IDU subjects and showed for the first time that DC maturation is also associated with high-risk needle-sharing in IV-drug users from Philadelphia [22]. Nevertheless, it remains unknown if the heightened innate immune activation observed in HESN-IDU subjects is related to the injected drugs and repeated injections or added exposure to innate activating factors directly associated with high-risk needle-sharing activity. Here, we measured phenotypic and functional innate cell parameters that correlated with protection from HIV-1 by comparing high-risk needle-sharing HESN-IDU subjects with low-risk needle exchange IDU program participants.
Materials and Methods
Subject Criteria and Clinical Assessment
30 HIV-1 exposed, sero-negative injection drug users (HESN-IDU) were enrolled from the city of Philadelphia via community-based street outreach in specific neighborhoods previously identified as “risk pockets” [31]. Risk pockets are defined as locations within neighborhoods with a high HIV-1 prevalence where injectable drugs are sold, used, and at times exchanged for sex (Figure 1). In agreement with previous reports [22, 32], we estimate the HIV-1 and HCV sero-prevalence among high-risk needle-sharing HESN-IDU subjects in risk pockets within Philadelphia to be approximately 20% and 70%, respectively. We utilized the “Prognostic Model for Sero-conversion Among Injection Drug Users” [33] to identify high-risk HESN-IDU subjects for our study based upon their frequency of injection and needle sharing behavior. Briefly, subjects from known risk pockets were identified as HESN-IDU if they remained HIV-1 IgG sero-negative despite a history of greater than 2 years of daily injection and frequent (weekly or greater) needle sharing with partners of unknown HIV status.
Figure 1. “Risk pocket” approach to HESN-IDU subject recruitment in the city of Philadelphia.
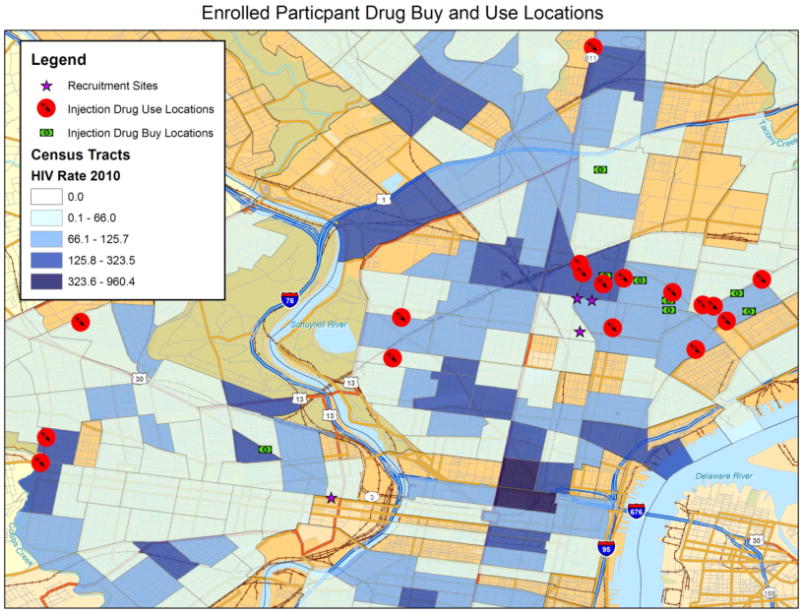
High-risk HIV-1 exposed, sero-negative injection drug users (HESN-IDU) were enrolled from the city of Philadelphia via community-based street outreach in specific neighborhoods previously identified as “risk pockets.” Risk pockets are defined as specific neighborhoods with a high HIV-1 incidence where intra-venous drugs are sold, used, and exchanged for sex. Subjects from known risk pockets were identified as HESN-IDU if they reported a history of greater than 2 years of daily injection and frequent (weekly or greater) needle sharing with partners of unknown HIV status. Low-risk non-sharing IDU (NS-IDU) drug-matched subjects were recruited from local needle exchange centers while no-risk unexposed, non drug-users were recruited from the greater Philadelphia area.
To control for the independent role of injected drugs and/or repeated injections in driving innate immune activation, 15 low-risk, non-sharing injection drug user subjects (NS-IDU) were recruited from local needle exchange centers based upon their high rate of heroin use, which was similar in magnitude to high-risk HESN-IDU subjects (Table 1). 20 no-risk non-drug user controls from the greater Philadelphia area were also recruited as an additional control group. Samples from HESN-IDU subjects, NS-IDU subjects and non-drug user controls were all run contemporaneously throughout the study to reduce the risk of batch-effect. Blood was drawn from all subjects according to Institutional Review Board approval following written informed consent. All HESN-IDU subjects, NS-IDU subjects and HIV-1 infected (sero-positive) subjects screened for the study were referred to drug cessation programs, counseled to enter the local needle-exchange programs to reduce their risk of exposure to blood borne pathogens and offered additional health services as needed.
Table 1.
Demographic characteristics and risk factor behavior for high-risk HESN-IDU subjects and low-risk NS-IDU controls in the cohort.
| Cohort Group | Age | Gender | Ethnicity | HCV Infected | HBV Infected | Frequency of Needle Sharing (Last 6 mos) |
Frequency of Heroin Use (Last 6 mos) |
Frequency of Cocaine/Crack (Last 6 mos) |
Frequency of Unprotected Sex (Last 6 mos) |
|---|---|---|---|---|---|---|---|---|---|
| HESN-008 | 47 | F | C | Yes | Yes | 6–24 | 168–200 | 0 | 0 |
| HESN-016 | 26 | M | C | Yes | No | 6–24 | 145–168 | 145–168 | 90 |
| HESN-019 | 41 | M | AA | Yes | No | 6–24 | 168–200 | 7–24 | 0 |
| HESN-027 | 31 | M | C | Yes | No | 73–144 | 168–200 | 1–6 | 99 |
| HESN-031 | 43 | M | AA | No | No | 6–24 | 168–200 | 168–200 | 10 |
| HESN-037 | 35 | M | C | Yes | Yes | 1–6 | 25–72 | 25–72 | 18 |
| HESN-040 | 29 | M | C | Yes | No | 1–6 | 168–200 | 72–144 | 10 |
| HESN-041 | 42 | M | C | Yes | No | 1–6 | 168–200 | 7–24 | 0 |
| HESN-042 | 43 | F | C | Yes | Yes | 73–144 | 168–200 | 0 | 36 |
| HESN-043 | 40 | M | C | Yes | No | 1–6 | 25–72 | 0 | 0 |
| HESN-044 | 46 | T | C | Yes | Yes | 73–144 | 168–200 | 1–6 | 34 |
| HESN-050 | 58 | M | AA | Yes | No | 1–6 | 168–200 | 0 | 15 |
| HESN-051 | 58 | M | AA | Yes | No | 1–6 | 168–200 | 0 | 46 |
| HESN-052 | 47 | M | AA | Yes | Yes | 7–24 | 1–6 | 168–200 | 2 |
| HESN-053 | 42 | M | H | Yes | No | 1–6 | 168–200 | 0 | 27 |
| HESN-055 | 61 | M | AA | Yes | No | 1–6 | 168–200 | 0 | 2 |
| HESN-056 | 61 | M | AA | No | No | 1–6 | 73–144 | 7–24 | 0 |
| HESN-058 | 54 | M | AA + H | Yes | No | 7–24 | 168–200 | 168–200 | 115 |
| HESN-060 | 32 | M | C | No | No | 1–6 | 7–24 | 1–6 | 15 |
| HESN-063 | 42 | M | C | Yes | No | 7–24 | 73–144 | 0 | 3 |
| HESN-064 | 37 | M | AA | Yes | No | 1–6 | 168–200 | 1–6 | 4 |
| HESN-075 | 51 | M | AA | No | No | 7–24 | 7–24 | 7–24 | 60 |
| HESN-080 | 64 | M | C | Yes | No | 1–6 | 168–200 | 0 | 0 |
| HESN-081 | 56 | F | AA | Yes | Yes | 1–6 | 168–200 | 0 | 3 |
| HESN-088 | 31 | M | H | Yes | No | 25–72 | 168–200 | 168–200 | 168–200 |
| HESN-089 | 32 | M | C | Yes | Yes | 1–6 | 25–72 | 7–24 | 9 |
| HESN-092 | 41 | F | C | Yes | No | 73–144 | 168–200 | 1–6 | 168–200 |
| NS-004 | 29 | F | C | Yes | Yes | 0 | 168–200 | 0 | 168–200 |
| NS-005 | 44 | M | H | No | No | 0 | 168–200 | 0 | 100 |
| NS-020 | 47 | M | AA | Yes | No | 0 | 73–144 | 72–144 | 10 |
| NS-021 | 54 | M | AA | Yes | No | 0 | 168–200 | 0 | 5 |
| NS-024 | 47 | M | C | Yes | No | 0 | 168–200 | 0 | 72 |
| NS-025 | 43 | M | C | Yes | No | 1–6 | 168–200 | 0 | 46 |
| NS-036 | 40 | M | O | No | Yes | 0 | 168–200 | 0 | 0 |
| NS-045 | 42 | F | AA | No | No | 0 | 25–72 | 0 | 0 |
| NS-048 | 54 | F | AA | No | No | 0 | 168–200 | 1–6 | 0 |
| NS-049 | 49 | M | AA | Yes | Yes | 1–6 | 145–168 | 25–72 | 6 |
| NS-057 | 36 | M | AA | Yes | No | 0 | 73–144 | 25–72 | 45 |
| NS-076 | 45 | M | AA | Yes | No | 0 | 168–200 | 1–6 | 48 |
| NS-078 | 54 | F | C | Yes | No | 0 | 168–200 | 1–6 | 30 |
F=Female, M=Male, T=Transgender, AA= African American, C=Caucasian, H=Hispanic, O=Other.
Flow Cytometry
All cell surface antibodies and isotype controls used at the recommended dilution of 0.25 μg antibody per million cells. Peripheral Blood Mono-nuclear Cells (PBMC) were stained with antibodies to phenotypic and functional markers for 15 minutes at room temperature in the dark, washed twice and fixed with Cytofix Buffer (BD Cytometry Systems, San Jose, CA). The following surface antibodies (with clones shown in parentheses) and their appropriate isotype controls were obtained from BD Biosciences or Miletyni Biotech: CD69 FITC (FN50), CD107a PE (H4A3), CD56 PERCP Cy5.5 (B159), CD3 APC or v450 (UCHT1), CD16 APC-H7 (3G8), CD4 FITC (RPA-T4), CD8 PE (RPA-T8), CD38 APC (HIT2), CD11c v450 (B-ly6), Lineage FITC (BD), CD83 PE (HB15e), HLA-DR PERCP Cy5.5 (L243), BDCA-4 APC (REA380), and CD40 APC-H7 (5C3). Intra-cellular staining for IFN-gamma FITC (B27), TNF-alpha v450 (MAB11) and MIP-1 beta APC-H7 (D21–1351) was carried out in 1× Perm/Wash Buffer (BD) as described by the manufacturer. A minimum of one hundred thousand events were collected on a BD LSR-II Flow Cytometer and samples were subsequently analyzed with FlowJo software (Tree Star Incorporated, Ashland OR).
Target Cell-induced NK Degranulation and Cytokine Production Assay
Following an overnight incubation, 5×106 PBMC per condition were washed and incubated in the presence or absence of K562 cells at a 10:1 effector/target ratio in the presence of 10 μl anti-CD107a monoclonal antibody, 0.133 μl of a 3mM stock of Golgi-stop (BD) and 5 μg/mL Brefeldin A (BD) in a 200 μl volume for four hours. PBMC were stained with antibodies to NK cell phenotypic markers and intra-cellularly stained for cytokine expression with antibodies to IFN-gamma, TNF-alpha and MIP-1 beta. The percentage of CD56+/CD3− gated NK cells staining positive for CD107a degranulation and/or cytokine production following incubation with K562 cells was determined after subtraction of background levels of staining in the absence of target cells (No Target Control).
HIV-specific CD8+ T Cell Peptide Stimulation
0.5×106 PBMC were co-cultured with a 1 μg/mL mixture of overlapping 15-mer peptides spanning the HIV-1 Consensus Clade B Gag or Pol proteins (AIDS Research and Reference Reagent Repository, NIH) in the presence of 2.5 μl CD28/CD49d co-stimulation (BD) and 5 μg/mL Brefeldin A for 18 hours in a 200 μl volume. Alternatively, PBMC were stimulated with a 1 μg/mL mixture of a CEF peptide pool comprising 23 peptides consisting of sequences derived from the human Cytomegalovirus, Epstein-Barr and Influenza Viruses (AIDS Research and Reference Reagent Repository, NIH) as a positive control while unstimulated PBMC were used as a negative control. PBMC were washed, stained with antibodies to T Cell phenotypic markers and intra-cellular staining for IFN-gamma was carried out as described above. CD8+ T cells were gated by CD8+/CD3+ staining and the percentage of cells staining positive for CD107a and/or IFN-gamma was determined after subtraction of background levels of staining in unstimulated control cells. Reference PBMC from HIV-1 infected subjects with detectable viremia in the absence of anti-retroviral therapy were used as positive controls for the HIV-specific peptide assay.
HIV-specific Binding Antibody Multiplex Assay (BAMA)
Plasma samples from 28 high-risk HESN-IDU subjects and 14 low-risk non-sharing IDU control subjects were analyzed for HIV-1 specific IgG and IgA responses utilizing a custom HIV-1 binding antibody multiplex assay as previously described [34–37]. Carboxylated fluorescent beads (Luminex Corp, Austin, TX) were covalently coupled to a panel of eight HIV-1 specific antigens: Clade B gp41 Immunodominant epitope (gp41 ID) provided by Dr. M.A. Moody, Duke University), gp41 MN (ImmunoDX, Woburn, MA), A1.con.env03 gp140 CF (consensus clade A envelope gp140 with deletions in the gp41 cleavage site and fusion domain [CF]), B.con.env03 gp140 (Consensus Clade B Env gp140), C.con.env03 gp140 CF (Consensus Clade C Env gp140), Con 6 gp120/B Consensus gp120 Env) and Con S gp140 (Group M Consensus gp140 Env) [38–40]. Samples were incubated with beads conjugated to HIV-1 specific antigens, and HIV-specific IgG and IgA responses were detected with goat anti-human IgA-PE (Jackson Immunoresearch, West Grove, PA) or mouse anti-human IgG-PE (Southern Biotech, Birmingham, AL). Beads were then washed and acquired on a Bio-Plex 200 instrument (Bio-Rad, Hercules, CA) and binding was measured as Mean Fluorescence Intensity (MFI). All experiments were carried out in accordance with Good Clinical Laboratory Practice (GCLP) guidelines. Positive controls included titrated HIV-IG and the following HIV-specific monoclonal antibodies: 7B2 mAb (provided by Drs. James Robinson, Tulane and Barton Haynes, Duke), 4e10 mAb (Polymun Scientific), 2F5 mAb (Polymun Scientific) and b12 IgA mAb (kindly provided by Dr. Dennis Burton). Sample binding to the controls was tracked via Levey-Jennings plots. Each sample was analyzed in two independent BAMA assays. HESN-IDU samples were defined as reactive for a specific antigen if the sample MFI was greater than the average MFI plus 5 standard deviations of a panel of at least 30 seronegative plasma samples. Samples were considered positive for HIV-1 specific antibody if they met the above preset criteria for positivity and had positive binding antibody responses to ≥ 2 HIV antigens.
Cytokine Measurements
Plasma samples we analyzed by the University of Pennsylvania Immunology Core Facility utilizing the Millipore 29-Plex Human Cytokie/Chemokine Magentic Bead Panel according to the manufacture’s recommendations.
Statistical Analysis
All graphic presentations were performed with Prism software (GraphPad Software, La Jolla, CA) and displayed as median with interquartile range. Statistical analysis of two groups was carried out using a Wilcoxon-Mann-Whitney test for two independent groups or a Wilcoxon signed-rank test for paired data. Comparisons of three or more groups was carried out using a Kruskal-Wallis test with a post-hoc Dunn test. Correlations between two variables were carried out using Spearman Correlation of untransformed data with a 95% confidence interval. No data was purposefully excluded from the analysis. All missing data from any subjects is due to technical issues with the assay. In all cases, significant results have two-sided p values of p<0.05, p<0.01, p<0.001 denoted with a single, double or triple asterisk in graphs, respectively. Due to limited sample size, reported p-values are unadjusted for multiple testing.
Results
Increased innate immune NK and MDC activation is associated with high-risk needle-sharing activity among HESN-IDU subjects
We have previously shown that NK activation is increased in HIV-1 exposed, sero-negative injection drug users (HESN-IDU) that share needles in areas of high HIV-1 prevalence compared to control donors that were not injection drug-users [22] (see Figure 1 for risk pocket approach). Here, we addressed the independent role of injected drugs and/or repeated injections in driving innate cell activation by comparing HESN-IDU subjects with non-sharing IDU donors (NS-IDU) who inject opioids at a similar frequency but participate in local needle exchange programs (see Table 1 for subject characteristics and drug use history). We observed that NK cells from high-risk needle-sharing HESN-IDU subjects exhibited increased constitutive CD69 activation and CD107a degranulation compared to both control donors that were not injection drug-users and low-risk NS-IDU subjects that do not share needles (see representative staining in Figure 2A). CD69 activation and CD107a degranulation were both independently increased in HESN-IDU subjects (Supplementary Figure 1B and C) and together were significantly increased in HESN-IDU subjects compared to both non drug-user controls (p<0.001) and low-risk NS-IDU subjects (p<0.01) (Figure 2B). When NK activation was re-tested in a subset of HESN-IDU subjects upon a second blood draw at least three months later, we observed that the heightened NK activation phenotype was durable over time (Figure 2C). Next we measured CD40 and CD83 upregulation on myeloid dendritic cells (MDC) and plasmacytoid dendritic cells (PDC) as a marker for activation and maturation, respectively. We observed that MDC (Figure 2D), but not PDC (Figure 2E), activation/maturation was significantly increased in HESN-IDU subjects compared to both non drug-user controls (p<0.05) and low-risk NS-IDU subjects (p<0.05). We correlated NK activation directly with MDC activation and observed that these two parameters were not associated within the groups in our cohort (Figure 2F–G). However, when comparing innate immune activation between the groups, we observed that the parameters of NK activation (x-axis) and/or MDC activation (y-axis) could be used to identify the majority of HESN-IDU subjects (69%), whereas only a small minority of low-risk NS-IDU subjects (20%) or non drug-user controls (15%) (data not shown) exhibited similar innate immune activation (Figure 2F–G). Together, these results indicate that heightened innate immune cell activation in HESN-IDU subjects is not the result of the IV-drugs and repeated injection practice itself, but to repeated exposure to factors intrinsic to sharing needles (i.e., exposure to donor blood pathogens or heterologous cells).
Figure 2. NK and MDC activation is increased in HESN-IDU subjects and associated with high-risk needle-sharing activity.
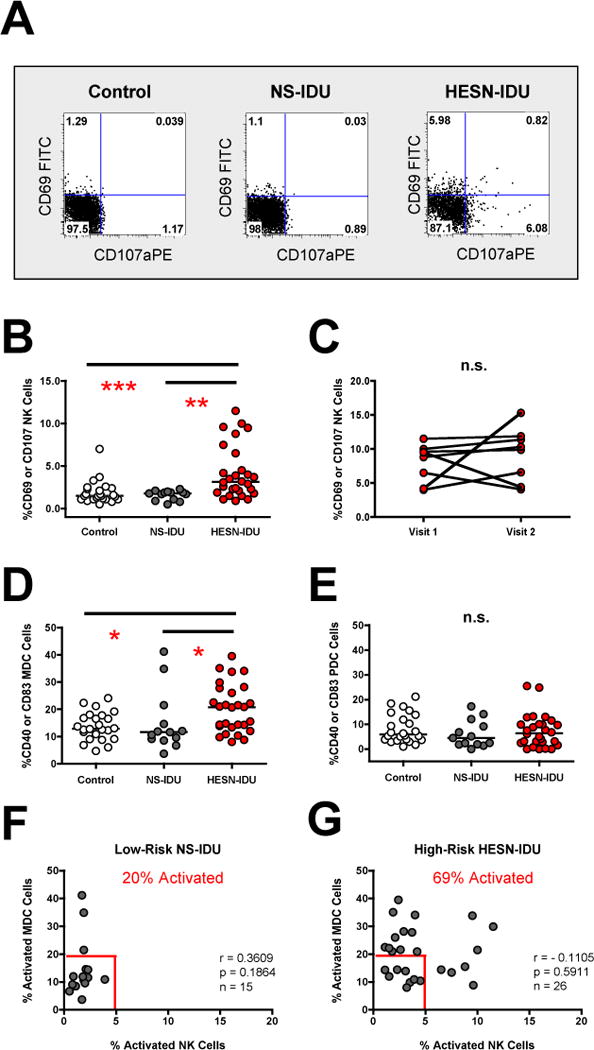
(A) PBMCs from a representative no-risk (control) donor, a low-risk non-sharing IDU (NS-IDU) subject and high-risk needle-sharing HESN-IDU subject were stained with fluorescently conjugated antibodies to NK phenotypic and activation markers. The frequency of constitutive CD107a (x-axis) and CD69 (y-axis) staining is shown on CD56+/CD3− gated lymphocytes with quadrant gates set based upon isotype control antibodies. (B) Composite graph of the constitutive CD69 activation and/or CD107a degranulation on CD56+/CD3− gated NK cells from no-risk control, low-risk NS-IDU and high-risk HESN-IDU subjects as depicted in panel A. (C) Constitutive NK CD69 activation and/or CD107a degranulation was measured longitudinally in 8 high-risk needle-sharing HESN-IDU subjects at multiple visits at least three months apart. (D–E) Composite graph of the constitutive CD40 activation and CD83 maturation on LIN−/HLA-DR+/CD11c+/BDCA-4− MDC cells (D) and LIN−/HLA-DR+/CD11c−/BDCA-4+ PDC cells (E) from no-risk control, low-risk NS-IDU and high-risk HESN-IDU subjects. (F–G) Composite graph of the constitutive NK cell activation (x-axis) and MDC activation (y-axis) in (F) low-risk NS-IDU subjects and (G) high-risk HESN-IDU subjects as described above. Frequency of individuals from each group staining positive for either NK or MDC activation is shown in red and gates were set based upon the 10th percentile of NK or MDC activation from control donors. Comparisons between three or more groups were performed using an unpaired, non-parametric Kruskal-Wallace ANOVA with a Dunn post-test. Statistical analysis of two groups was carried out using a Wilcoxon-Mann-Whitney test for two independent groups. Correlations between two variables were carried out using Spearman Correlation of untransformed data with a 95% confidence interval. In all cases, significant results have two-sided p values of p<0.05, p<0.01, p<0.001 denoted with a single, double or triple asterisk in graphs, respectively.
High-risk Needle-sharing Activity by HESN-IDU Subjects is Not Associated with Detectable HIV-specific CD8+ T cell or Antibody Responses
After having confirmed increased innate immune activation among high-risk HESN-IDU subjects from our cohort, we next investigated if HIV-specific humoral or cellular immune responses were detectable among HESN-IDU subjects. Despite previous evidence of detectable HIV-specific CD8+ T cell responses in some cohorts of HESN subjects [1–4, 6], we observed that none of the HESN-IDU subjects from our cohort possessed detectable CD8+ T cell responses to HIV-1 peptides (Figure 3A). HIV-1 infected subjects with detectable viremia in the absence of anti-retroviral therapy were used as positive controls for the HIV-specific peptide assay (Figure 3A, blue dots). Likewise, peptides specific for CMV, EBV and Flu (CEF) were used to show that CD8+ T cells from HESN-IDU subjects could respond to peptide stimulation from other endemic pathogens (Figure 3B).
Figure 3. High-risk Needle-sharing Activity by HESN-IDU Subjects is Not Associated with Detectable HIV-specific CD8+ T cell or Antibody Responses.
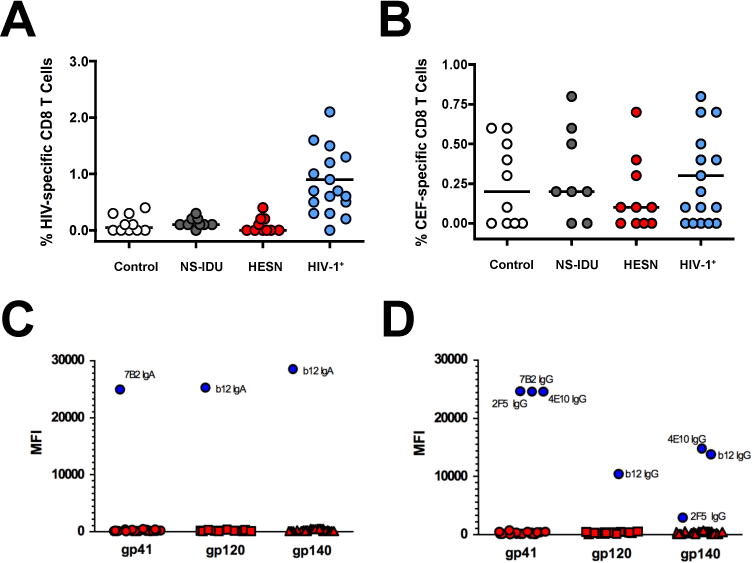
(A–B) Composite graphs from controls, NS-IDU subjects, HESN-IDU subjects, and HIV-1 infected reference subjects showing the (A) HIV-specific CD8+ T cell response to peptide pools from the HIV-1 Gag protein or the (B) non-HIV-specific CD8+ T cell response to combined peptide pools from Cytomegalovirus, Epstein-Barr and Influenza Viruses (CEF). (C–D) Plasma samples from 28 high-risk HESN-IDU subjects and 14 low-risk non-sharing IDU control subjects were analyzed for HIV-1 specific responses utilizing a custom HIV-1 binding antibody multiplex assay (BAMA). HIV-1 specific IgA (C) and IgG (D) plasma antibodies to gp41 and Consensus gp120 and gp140 envelope antigens are shown as representative data. HIV-specific monoclonal antibodies 7B2 mAb (1 μg/ml), 4e10 mAb (50 μg/ml), 2F5 mAb (16 μg/ml) and b12 mAb (20 μg/ml) were used as positive controls in addition to a HIV-IG titration curve (500 μg/ml titrated 6-fold, 10 places). Each sample was analyzed in two independent BAMA assays and HESN-IDU samples were defined as positive for a specific antigen if the sample MFI was greater than the average mean fluorescent intensity (MFI) plus 5 standard deviations of the panel of non needle-sharing IDU control subjects. Statistical analysis carried out as described in Figure 2.
We next investigated if HIV-specific IgA or IgG antibody responses could be identified in the plasma samples from high-risk HESN-IDU subjects or low-risk non-sharing IDU controls from our cohort. As shown in Figure 3C and D, there were no detectable levels of HIV-specific IgA or IgG responses to gp41, Consensus gp120 or Consensus gp140 from any of the high-risk HESN-IDU subjects or low-risk non-sharing IDU controls. Additionally, there were no HIV-1 specific IgA or IgG responses when these samples were tested against a panel of gp120 and gp140 envelope sequence from consensus HIV-1 clade A, B, C and M envelope proteins (data not shown). Responses to the Immunodominant epitope in gp41 from Clade B viruses, which represent the predominant HIV-1 viral strain in North America, were also negative (data not shown). Overall, our results indicate that the high-risk needle-sharing activity observed in HESN-IDU subjects from our cohort is associated with innate immune activation in the absence of detectable HIV-specific CD8+ T cell or antibody responses.
Constitutive NK activation in HESN-IDU subjects is not associated with exhaustion of innate cell function but correlated with plasma levels of IP-10
We next attempted to identify if any functional correlates or plasma cytokines were associated with the increased constitutive NK and MDC activation we observed in HESN-IDU subjects. We investigated NK function directly by incubating PBMC with K562 cells and measuring CD107a degranulation and/or cytokine production on CD56+/CD3− gated NK cells (see representative staining in Figure 4A and B). We observed that after PBMC incubation with K562 cells, NK cells from HESN-IDU subjects maintained strong CD107a degranulation and comparable IFN-gamma production when compared to low-risk NS-IDU subjects or no-risk non drug-user controls (Variables shown individually in Supplementary Figure 1D and E or together in Figure 4C). NK cells from HESN-IDU subjects also exhibited comparable MIP-1 beta and/or TNF-alpha production following PBMC incubation with K562 cells when compared to low-risk NS-IDU subjects or no-risk non drug-user controls (Figure 4D). When the variables were combined together as shown in Figure 4A and B, IFN-gamma production and/or CD107a degranulation by NK cells from HESN-IDU subjects was strongly correlated (rho=0.8308, p=0.0004, n=13) with the production of MIP-1 beta and/or TNF-alpha following K562-stimulation (Figure 4E).
Figure 4. Constitutive NK activation in HESN-IDU subjects is not associated with functional exhaustion of innate immune cells but is correlated with plasma levels of IP-10.
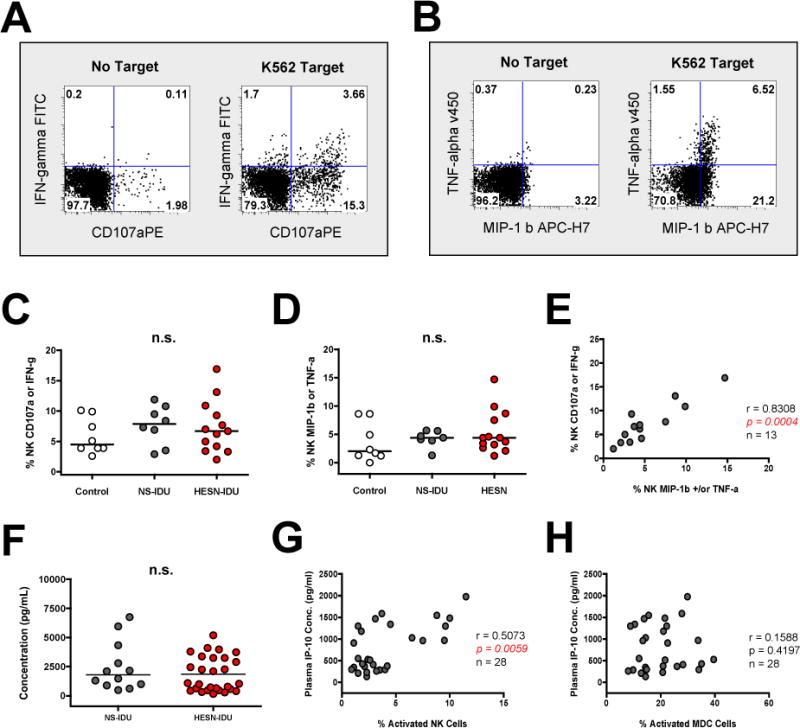
(A–B) PBMCs from a representative high-risk needle-sharing HESN-IDU subject were stained with fluorescently conjugated antibodies to NK phenotypic and functional markers following a four-hour incubation in the presence or absence of K562 tumor cells. The frequency of K562-target cell induced (A) CD107a degranulation (x-axis) or IFN-gamma production (y-axis) staining and (B) MIP-1 Beta (x-axis) or TNF-alpha production (y-axis) staining is shown on CD56+/CD3− gated lymphocytes with quadrant gates set based upon “no target” control cells incubated in the absence of target cells. (C–D) Composite graph of the K562-target cell induced (C) CD107a degranulation or IFN-gamma production and (D) MIP-1 Beta or TNF-alpha production on CD56+/CD3− gated NK cells from no-risk control, low-risk NS-IDU and high-risk HESN-IDU subjects as depicted in panel A. (E) Spearman correlation of K562-target cell induced CD107a degranulation or IFN-gamma production with K562-target cell induced MIP-1 Beta or TNF-alpha production. (F) Composite graph of plasma IP-10 levels as measured by Luminex analyisis for low-risk NS-IDU and high-risk HESN-IDU subjects. (G–H) Spearman correlation of plasma IP-10 levels (y-axis) with the percentage of activated (G) NK cells or (H) MDC cells (x-axis) in all HESN-IDU subjects. Statistical analysis carried out as described in Figure 2.
Finally, we investigated if plasma cytokines changes could be detected in relation to NK and MDC activation levels in HESN-IDU subjects when compared to low-risk non-sharing-IDU subjects. Using a comprehensive inflammatory luminex array, we did not detect significant differences between both groups among the 29 cytokines and chemokines tested including IP-10 (Figure 4F). This finding is in agreement with recent work showing a limited correlation of systemic cytokine levels with risk of HIV-1 acquisition [41]. However, when plasma samples from high-risk HESN-IDU subjects alone were correlated with the phenotypic and functional markers tested in the study, we observed that IP-10 was positively associated with NK activation (rho=0.5073, p=0.0059, n=28) (Figure 4G). In contrast, plasma levels of IP-10 were not associated with MDC activation in HESN-IDU subjects (Figure 4H). Together, these results suggest that constitutive NK cell activation in HESN-IDU subjects is not associated with exhaustion of innate immune cell function but is correlated with increased plasma levels of IP-10.
Discussion
Here we confirm previous reports of increased innate cell activation in HIV-1 exposed, sero-negative intra-venous drug users (HESN-IDU), and address for the first time the independent role of injected drugs and/or repeated injections in driving innate cell activation. Utilizing a high-risk cohort of needle-sharing HESN-IDU subjects and a low-risk cohort of non-sharing IDU controls from local needle exchange programs (NS-IDU), we establish that both NK cell and MDC activation were significantly increased in HESN-IDU subjects compared to NS-IDU controls. NK activation in HESN-IDU subjects was independent of drug use patterns but was durable over time and correlated with plasma levels of IP-10 by Luminex analysis. No detectable CD8+ T cell responses against HIV-1 peptides or antibody responses to HIV-1 envelope proteins were detected among HESN-IDU subjects suggesting that the adaptive immune response to HIV-1 is not associated with the lack of HIV-1 acquisition despite high-risk activity in our cohort of HESN-IDU subjects. Overall, our results indicate that heightened innate cell activation in HESN-IDU subjects is not a result of the IV-drugs and repeated injection practice itself, but to factors intrinsic to sharing needles.
We interpret that repeated exposure to microbial pathogens or other stimulation from contaminated injection equipment rather than an active co-infection at site of injection may contribute to innate immune activation in HESN-IDU subjects. The low level of innate immune cell activation among non-sharing NS-IDU subjects as well as the absence of high pro-inflammatory cytokines in plasma of HESN-IDU subjects argues against a sustained inflammation by an active infection at injection site. In contrast, we speculate that temporal yet repeated exposure to blood-borne viruses during needle-sharing such as Hepatitis C Virus, Hepatitis B Virus and HIV-1 may be contributing to innate immune activation. We have previously determined that the increased innate immune activation profile we observed among HESN-IDU subjects was independent of HCV status [22]. However, the high frequency of HCV sero-positivity among HESN-IDU subjects in our cohort (23/27 85%) suggests that persistent exposure to multiple viruses is likely encountered during prolonged needle sharing. Alternatively, NK activation may be driven by a direct response against heterologous donor cells exchanged via needle sharing. The MHC-I mis-match between injected cells from needle-sharing partners could act to sustain NK activation and be a reason for heightened CD107a degranulation observed on NK cells from HESN-IDU donors. Indeed, our previous work as shown that NK cells exhibit retained degranulation over extended periods of time following multiple target cell interactions with MHC-I mis-matched cells [42]. Alternatively, innate immune activation observed in high-risk HESN-IDU subjects from our cohort may be driven by unknown variables such a drug use patterns or risky sexual behavior. We undertook a comprehensive analysis of demographic info among low-risk NS-IDU subjects and high-risk HESN-IDU subjects as shown in Table 1. We observed that variables such as age, gender, ethnicity and frequency of Heroin usage were similar among the two groups. However, we did observe that the frequency of multiple drug use (cocaine/crack with heroin) was higher among HESN-IDU subjects (17/27 63%) than NS-IDU subjects (6/13 46%). We tested if the use of multiple drugs could stratify HESN-IDU subjects that exhibited high innate immune activation from those that had a low activation profile and observed no difference among the groups (Supplementary Figure 1F). Similarly, we observed that the frequency of unprotected sexual encounters could not distinguish HESN-IDU subjects that exhibited high innate immune activation from those with a low activation profile (Supplementary Figure 1G).
We have previously shown that in addition to NK activation, CD83 maturation of plasmacytoid dendritic cells (PDC) was increased in HESN-IDU subjects as compared to control donors that did not use IV drugs [22]. Here, we observed that MDC activation, but not PDC activation, was significantly increased in HESN-IDU subjects as compared to low-risk NS-IDU subjects or non drug-user controls. We interpret this discrepancy due to our more stringent definition of activation by inclusion of CD40 activation marker into our staining panel in addition to CD83 as well as our usage of additional phenotypic stains to further separate MDC (LIN−/HLA-DR+/CD11c+/BDCA-4−) from PDC (LIN−/HLA-DR+/CD11c−/BDCA-4+) when comparing methods between reports.
Recently, increased NK cell activation was found to be associated with increased HIV-1 acquisition in women receiving tenofovir microbicide gel in the CAPRISA-004 trial [43]. The CAPRISA-004 trial targeted at risk women practicing unprotected sexual intercourse in endemic areas of HIV-1 infection as opposed to our study where we investigated high-risk IV-drug users that shared needles in risk-pockets with high HIV prevalence. Differences in the route of HIV-1 exposure among our two studies may be particularly important with regard to the exposure of needle-sharing IV-drug users to heterologous cells which may drive NK activation. Importantly, the increased NK activation observed in HESN-IDU subjects was documented in multiple time points at least three months apart, indicating that the state of innate cell activation is a durable phenotype associated with high-risk needle-sharing. In spite of this sustained activation state, our data does not support the possibility of a loss of function by exhaustion state as normal innate immune cell functionality was documented in HESN-IDU subjects by NK degranulation/cytokine production assay. While we did not observe an anergic state among NK cells or MDCs in high-risk HESN-IDU subjects, we also did not observe an increase in activity as expected by the observed heightened innate immune activation among HESN-IDU subjects compared to low-risk non-sharing IDU or no-risk non drug-user controls. As our functional assays require overnight incubation of PBMC, it remains possible that short-term functional assays may show differences not detected in this study. Nevertheless, our findings here suggest that increased innate immune activation in conjunction with retained functionality are associated with a lack of HIV-1 acquisition despite high-risk activity in HESN-IDU subjects and highlights NK cells and MDCs as candidate cell types that may contribute to resistance from infection upon HIV-1 exposure.
Supplementary Material
Acknowledgments
We would like to thank the University of Pennsylvania Immunology Core Facility for running and analyzing the plasma samples utilizing the Millipore 29-Plex Human Cytokine/Chemokine Magnetic Bead Panel. We thank Dr. Nicole L. Yates for expertise with BAMA assay design, Drs. Hua-Xin Liao, Barton Haynes and M.A Moody for HIV-1 envelope reagents and the Duke Center for AIDS Research (NIH AI064518.)
This study was supported by grants from the National Institutes of Health (NIDA R01 DA028775, R01 AI073219, RO1 AI065279, Core grant P30 CA10815), the Philadelphia Foundation, and funds from the Pennsylvania Commonwealth Universal Research Enhancement Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Support for Shared Resources utilized in this study was provided by Cancer Center Support Grant (CCSG) CA010815 to The Wistar Institute.”
Sources of Funding: NIDA R01 DA028775, R01 AI073219, RO1 AI065279
Footnotes
The authors have no conflicts of interest.
Author Contributions.
Costin Tomescu (Completed all of the immunology assays, co-wrote the manuscript, completed statistical analysis), Kelly Seaton (Performed binding assays and analyzed data, edited the manuscript), Peter Smith and Mack Taylor (Coordinated subject recruitment, analyzed subject behavioral data, edited the manuscript), Georgia Tomaras (Binding assay design, analyzed BAMA data, edited the manuscript), David S. Metzger (Coordinated HESN-IDU subject cohort, analyzed subject behavioral data, edited the manuscript), Luis J. Montaner (Coordinated the study, analyzed all of the functional data, co-wrote the manuscript).
References
- 1.Beretta A, Furci L, Burastero S, Cosma A, Dinelli ME, Lopalco L, et al. HIV-1-specific immunity in persistently seronegative individuals at high risk for HIV infection. Immunol Lett. 1996;51:39–43. doi: 10.1016/0165-2478(96)02553-9. [DOI] [PubMed] [Google Scholar]
- 2.Clerici M, Giorgi JV, Chou CC, Gudeman VK, Zack JA, Gupta P, et al. Cell-mediated immune response to human immunodeficiency virus (HIV) type 1 in seronegative homosexual men with recent sexual exposure to HIV-1. J Infect Dis. 1992;165:1012–1019. doi: 10.1093/infdis/165.6.1012. [DOI] [PubMed] [Google Scholar]
- 3.Clerici M, Levin JM, Kessler HA, Harris A, Berzofsky JA, Landay AL, Shearer GM. HIV-specific T-helper activity in seronegative health care workers exposed to contaminated blood. JAMA. 1994;271:42–46. [PubMed] [Google Scholar]
- 4.Mazzoli S, Trabattoni D, Lo Caputo S, Piconi S, Ble C, Meacci F, et al. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat Med. 1997;3:1250–1257. doi: 10.1038/nm1197-1250. [DOI] [PubMed] [Google Scholar]
- 5.Pinto LA, Sullivan J, Berzofsky JA, Clerici M, Kessler HA, Landay AL, Shearer GM. ENV-specific cytotoxic T lymphocyte responses in HIV seronegative health care workers occupationally exposed to HIV-contaminated body fluids. J Clin Invest. 1995;96:867–876. doi: 10.1172/JCI118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranki A, Mattinen S, Yarchoan R, Broder S, Ghrayeb J, Lahdevirta J, Krohn K. T-cell response towards HIV in infected individuals with and without zidovudine therapy, and in HIV-exposed sexual partners. AIDS. 1989;3:63–69. doi: 10.1097/00002030-198902000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Alimonti JB, Kimani J, Matu L, Wachihi C, Kaul R, Plummer FA, Fowke KR. Characterization of CD8 T-cell responses in HIV-1-exposed seronegative commercial sex workers from Nairobi, Kenya. Immunol Cell Biol. 2006;84:482–485. doi: 10.1111/j.1440-1711.2006.01455.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaul R, Rowland-Jones SL, Kimani J, Fowke K, Dong T, Kiama P, et al. New insights into HIV-1 specific cytotoxic T-lymphocyte responses in exposed, persistently seronegative Kenyan sex workers. Immunol Lett. 2001;79:3–13. doi: 10.1016/s0165-2478(01)00260-7. [DOI] [PubMed] [Google Scholar]
- 9.Kaul R, MacDonald KS, Nagelkerke NJ, Kimani J, Fowke K, Ball TB, et al. HIV viral set point and host immune control in individuals with HIV-specific CD8+ T-cell responses prior to HIV acquisition. AIDS. 2010;24:1449–1454. doi: 10.1097/qad.0b013e3283391d40. [DOI] [PubMed] [Google Scholar]
- 10.Kaul R, Rowland-Jones SL, Kimani J, Dong T, Yang HB, Kiama P, et al. Late seroconversion in HIV-resistant Nairobi prostitutes despite pre-existing HIV-specific CD8+ responses. J Clin Invest. 2001;107:341–349. doi: 10.1172/JCI10714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Woodward A, Zhu H, Andrus T, McNevin J, Lee J, et al. Preinfection human immunodeficiency virus (HIV)-specific cytotoxic T lymphocytes failed to prevent HIV type 1 infection from strains genetically unrelated to viruses in long-term exposed partners. J Virol. 2009;83:10821–10829. doi: 10.1128/JVI.00839-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Begaud E, Chartier L, Marechal V, Ipero J, Leal J, Versmisse P, et al. Reduced CD4 T cell activation and in vitro susceptibility to HIV-1 infection in exposed uninfected Central Africans. Retrovirology. 2006;3:35. doi: 10.1186/1742-4690-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jennes W, Evertse D, Borget MY, Vuylsteke B, Maurice C, Nkengasong JN, Kestens L. Suppressed cellular alloimmune responses in HIV-exposed seronegative female sex workers. Clin Exp Immunol. 2006;143:435–444. doi: 10.1111/j.1365-2249.2006.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaren PJ, Ball TB, Wachihi C, Jaoko W, Kelvin DJ, Danesh A, et al. HIV-exposed seronegative commercial sex workers show a quiescent phenotype in the CD4+ T cell compartment and reduced expression of HIV-dependent host factors. J Infect Dis. 2010;202 (Suppl 3):S339–344. doi: 10.1086/655968. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn L, Trabattoni D, Kankasa C, Semrau K, Kasonde P, Lissoni F, et al. Alpha-defensins in the prevention of HIV transmission among breastfed infants. J Acquir Immune Defic Syndr. 2005;39:138–142. [PMC free article] [PubMed] [Google Scholar]
- 16.Quinones-Mateu ME, Lederman MM, Feng Z, Chakraborty B, Weber J, Rangel HR, et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17:F39–48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Garcia M, Climent N, Oliva H, Casanova V, Franco R, Leon A, et al. Increased alpha-defensins 1–3 production by dendritic cells in HIV-infected individuals is associated with slower disease progression. PLoS One. 2010;5:e9436. doi: 10.1371/journal.pone.0009436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trabattoni D, Caputo SL, Maffeis G, Vichi F, Biasin M, Pierotti P, et al. Human alpha defensin in HIV-exposed but uninfected individuals. J Acquir Immune Defic Syndr. 2004;35:455–463. doi: 10.1097/00126334-200404150-00003. [DOI] [PubMed] [Google Scholar]
- 19.Zapata W, Rodriguez B, Weber J, Estrada H, Quinones-Mateu ME, Zimermman PA, et al. Increased levels of human beta-defensins mRNA in sexually HIV-1 exposed but uninfected individuals. Curr HIV Res. 2008;6:531–538. doi: 10.2174/157016208786501463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravet S, Scott-Algara D, Bonnet E, Tran HK, Tran T, Nguyen N, et al. Distinctive NK-cell receptor repertoires sustain high-level constitutive NK-cell activation in HIV-exposed uninfected individuals. Blood. 2007;109:4296–4305. doi: 10.1182/blood-2006-08-040238. [DOI] [PubMed] [Google Scholar]
- 21.Scott-Algara D, Truong LX, Versmisse P, David A, Luong TT, Nguyen NV, et al. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J Immunol. 2003;171:5663–5667. doi: 10.4049/jimmunol.171.11.5663. [DOI] [PubMed] [Google Scholar]
- 22.Tomescu C, Duh FM, Lanier MA, Kapalko A, Mounzer KC, Martin MP, et al. Increased plasmacytoid dendritic cell maturation and natural killer cell activation in HIV-1 exposed, uninfected intravenous drug users. AIDS. 2010;24:2151–2160. doi: 10.1097/QAD.0b013e32833dfc20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulet S, Kleyman M, Kim JY, Kamya P, Sharafi S, Simic N, et al. A combined genotype of KIR3DL1 high expressing alleles and HLA-B*57 is associated with a reduced risk of HIV infection. AIDS. 2008;22:1487–1491. doi: 10.1097/QAD.0b013e3282ffde7e. [DOI] [PubMed] [Google Scholar]
- 24.Boulet S, Sharafi S, Simic N, Bruneau J, Routy JP, Tsoukas CM, Bernard NF. Increased proportion of KIR3DS1 homozygotes in HIV-exposed uninfected individuals. Aids. 2008;22:595–599. doi: 10.1097/QAD.0b013e3282f56b23. [DOI] [PubMed] [Google Scholar]
- 25.Montoya CJ, Velilla PA, Chougnet C, Landay AL, Rugeles MT. Increased IFN-gamma production by NK and CD3+/CD56+ cells in sexually HIV-1-exposed but uninfected individuals. Clin Immunol. 2006 doi: 10.1016/j.clim.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Naranbhai V, Altfeld M, Abdool Karim Q, Ndung’u T, Abdool Karim SS, Carr WH. Natural killer cell function in women at high risk for HIV acquisition: insights from a microbicide trial. AIDS. 2012;26:1745–1753. doi: 10.1097/QAD.0b013e328357724f. [DOI] [PubMed] [Google Scholar]
- 27.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 28.Tomescu C, Chehimi J, Maino VC, Montaner LJ. NK Cell Lysis of HIV-1-Infected Autologous CD4 Primary T Cells: Requirement for IFN-Mediated NK Activation by Plasmacytoid Dendritic Cells. J Immunol. 2007;179:2097–2104. doi: 10.4049/jimmunol.179.4.2097. [DOI] [PubMed] [Google Scholar]
- 29.Ghadially H, Keynan Y, Kimani J, Kimani M, Ball TB, Plummer FA, et al. Altered dendritic cell-natural killer interaction in Kenyan sex workers resistant to HIV-1 infection. AIDS. 2012;26:429–436. doi: 10.1097/QAD.0b013e32834f98ea. [DOI] [PubMed] [Google Scholar]
- 30.Tomescu C, Abdulhaqq S, Montaner LJ. Evidence for the innate immune response as a correlate of protection in human immunodeficiency virus (HIV)-1 highly exposed seronegative subjects (HESN) Clin Exp Immunol. 2011;164:158–169. doi: 10.1111/j.1365-2249.2011.04379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams CT, Metzger DS. Race and distance effects on regular syringe exchange program use and injection risks: a geobehavioral analysis. Am J Public Health. 2010;100:1068–1074. doi: 10.2105/AJPH.2008.158337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metraux S, Metzger DS, Culhane DP. Homelessness and HIV risk behaviors among injection drug users. J Urban Health. 2004;81:618–629. doi: 10.1093/jurban/jth145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boileau C, Bruneau J, Al-Nachawati H, Lamothe F, Vincelette J. A prognostic model for HIV seroconversion among injection drug users as a tool for stratification in clinical trials. J Acquir Immune Defic Syndr. 2005;39:489–495. doi: 10.1097/01.qai.0000153424.56379.61. [DOI] [PubMed] [Google Scholar]
- 34.Liu P, Overman RG, Yates NL, Alam SM, Vandergrift N, Chen Y, et al. Dynamic antibody specificities and virion concentrations in circulating immune complexes in acute to chronic HIV-1 infection. J Virol. 2011;85:11196–11207. doi: 10.1128/JVI.05601-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med. 2014;6:228ra239. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yates NL, Stacey AR, Nolen TL, Vandergrift NA, Moody MA, Montefiori DC, et al. HIV-1 gp41 envelope IgA is frequently elicited after transmission but has an initial short response half-life. Mucosal Immunol. 2013;6:692–703. doi: 10.1038/mi.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao F, Weaver EA, Lu Z, Li Y, Liao HX, Ma B, et al. Antigenicity and immunogenicity of a synthetic human immunodeficiency virus type 1 group m consensus envelope glycoprotein. J Virol. 2005;79:1154–1163. doi: 10.1128/JVI.79.2.1154-1163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaschen B, Taylor J, Yusim K, Foley B, Gao F, Lang D, et al. Diversity considerations in HIV-1 vaccine selection. Science. 2002;296:2354–2360. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 40.Liao HX, Sutherland LL, Xia SM, Brock ME, Scearce RM, Vanleeuwen S, et al. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology. 2006;353:268–282. doi: 10.1016/j.virol.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehman DA, Ronen K, Blish CA, Baeten JM, Jalalian-Lechak Z, Jaoko W, et al. Systemic cytokine levels show limited correlation with risk of HIV-1 acquisition. J Acquir Immune Defic Syndr. 2014;66:135–139. doi: 10.1097/QAI.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomescu C, Chehimi J, Maino VC, Montaner LJ. Retention of viability, cytotoxicity, and response to IL-2, IL-15, or IFN-{alpha} by human NK cells after CD107a degranulation. J Leukoc Biol. 2009;85:871–876. doi: 10.1189/jlb.1008635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naranbhai V, Abdool Karim SS, Altfeld M, Samsunder N, Durgiah R, Sibeko S, et al. Innate immune activation enhances hiv acquisition in women, diminishing the effectiveness of tenofovir microbicide gel. J Infect Dis. 2012;206:993–1001. doi: 10.1093/infdis/jis465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ballan WM, Vu BA, Long BR, Loo CP, Michaelsson J, Barbour JD, et al. Natural killer cells in perinatally HIV-1-infected children exhibit less degranulation compared to HIV-1-exposed uninfected children and their expression of KIR2DL3, NKG2C, and NKp46 correlates with disease severity. J Immunol. 2007;179:3362–3370. doi: 10.4049/jimmunol.179.5.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inngjerdingen M, Damaj B, Maghazachi AA. Expression and regulation of chemokine receptors in human natural killer cells. Blood. 2001;97:367–375. doi: 10.1182/blood.v97.2.367. [DOI] [PubMed] [Google Scholar]
- 46.Maghazachi AA, Skalhegg BS, Rolstad B, Al-Aoukaty A. Interferon-inducible protein-10 and lymphotactin induce the chemotaxis and mobilization of intracellular calcium in natural killer cells through pertussis toxin-sensitive and -insensitive heterotrimeric G-proteins. FASEB J. 1997;11:765–774. doi: 10.1096/fasebj.11.10.9271361. [DOI] [PubMed] [Google Scholar]
- 47.Taub DD, Sayers TJ, Carter CR, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155:3877–3888. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


