Abstract
Endometriosis is a disease characterized by the growth of endometrial tissue outside the uterus and is associated with chronic pelvic pain. Peritoneal fluid (PF) of women with endometriosis is a dynamic milieu, rich in inflammatory markers and pain-inducing prostaglandins PGE2/PGF2α and lipid peroxides, and the endometriotic tissue is innervated with nociceptors. Our clinical study showed the abundance of oxidatively-modified lipoproteins in the PF of women with endometriosis and the ability of antioxidant supplementation to alleviate endometriosis-associated pain. We hypothesized that oxidatively-modified lipoproteins present in the PF are the major source of nociceptive molecules that play a key role in endometriosis-associated pain. In this study, PF obtained from women with endometriosis or control women were used for (i) the detection of lipoprotein derived oxidation-sensitive pain molecules, (ii) the ability of such molecules to induce nociception, and (iii) the ability of antioxidants to suppress this nociception. LC-MS/MS showed the generation of eicosanoids by oxidized-lipoproteins similar to that seen in the PF. The oxidatively-modified lipoproteins induced hypothermia (intra-cerebroventricular) in CD-1 mice and nociception in the Hargreaves paw-withdrawal latency assay in Sprague-Dawley rats. Antioxidants, vitamin-E and N-acetylcysteine and the NSAID, indomethacin suppressed the pain inducing ability of oxidatively-modified lipoproteins. Treatment of human endometrial cells with oxidatively-modified lipoproteins or PF from women with endometriosis showed up-regulation of similar genes belonging to the opioid and inflammatory pathways. Our finding that oxidatively-modified lipoproteins can induce nociception has a broader impact not only in the treatment of endometriosis-associated pain but also in other diseases associated with chronic pain.
INTRODUCTION
Endometriosis is a highly debilitating inflammatory disease [37] that afflicts 10-15% of women of child-bearing age [4; 12]. It is characterized by the presence of endometrial cells outside the uterus and often presents with pain and/or infertility. Endometriosis is most commonly associated with dysmenorrhea, dyspareunia, non-cyclic pain and abdominal pain [73; 77]. Laparoscopic surgery to remove the endometriotic tissue is a major treatment option to relieve pain, but often results in recurrence of the disease [19; 30]. The relationship between the severity of the disease and the presence of pain symptoms is not well defined [43]. It has been hypothesized that the ectopic lesion releases chemotactic molecules that attract immune cells into the peritoneal cavity, accumulating in the peritoneal fluid (PF) [6; 34]. This triggers the secretion of more cytokines and growth factors, thus sustaining the growth of the lesion [5].
The cyclooxygenases (COX-1 and COX-2) and 12, 15, or 5-lipoxygenase derived lipid-mediators from arachidonic acid (AA), such as prostaglandin E2 (PGE2) and prostaglandin F2α [56] and 12- and 15-(S)-hydroperoxyeicosatetraenoic acids (HPETE), 5- and 15-(S)-hydroxyeicosatetroenoic acids (HETE), and leukotriene B4 are potent activators of nociceptors [21; 72] and participate in nociception, or the ability to feel pain [84]. Endometriotic tissues expresses COX-2 [11; 36] and PF of patients with endometriosis contain varying amounts of PGE2 and PGF2α [66; 82]. Chronic pain is often attributed to tissue inflammation and injury resulting from oxidative stress and oxidants such as superoxide and nitric oxide (NO) play a role in nociception [1; 81].
Non-steroidal anti-inflammatory drugs (NSAIDs) which inhibit COX enzymes and prevent the enzymatic oxidation of AA to generate prostanoids are the most commonly prescribed agents to alleviate pain in endometriosis [2; 75]. Interestingly, AA can undergo non-enzymatic (free-radical mediated) oxidation to generate prostaglandin-like products that may not be inactivated by NSAIDs [21]. The source or nature of these prostaglandin-like products, the likelihood of these molecules to play a role in nociception and agents that inhibit their production is not currently known. This discovery will be highly beneficial in the treatment of chronic pain.
Studies from our laboratory has shown the importance of oxidative stress in the etiology of endometriosis [46; 47; 68] and showed increased presence of oxidative and inflammatory stress markers in the PF of women with endometriosis [68]. Many of these oxidant-sensitive markers increase inflammatory response and endometriotic lesion growth in animal models of endometriosis [14; 47], which can be prevented by antioxidant (N-acetylcysteine or vitamin E) supplementation [57; 60; 67]. Interestingly, our clinical trial showed that antioxidant (vitamin E and C) supplementation also lowered pain responses in women with endometriosis [67] thus suggesting that the nociceptive molecules are oxidation-sensitive. We have shown that women with endometriosis have high levels of lipoproteins (abundant in AA), in their PF [46; 47; 68]. In the present study, we provide evidence that these lipoproteins undergo non-enzymatic oxidation and generate prostaglandin-like molecules that modulate nociception in animal models of pain. Antioxidants can suppress the generation of these nociceptive molecules.
MATERIALS AND METHODS
Human subject participants
Approximately, 50 women/group, ages 18-60 years undergoing tubal ligation (control women, without endometriosis) or undergoing laparoscopy for endometriosis were recruited from the Department of Obstetrics and Gynaecology, Cabell Huntington Hospital, Marshall University School of Medicine, Huntington, WV and Emory University School of Medicine, Atlanta, GA. This HIPAA compliant study was approved by the Institutional Review Board of the Marshall University School of Medicine and Emory University School of Medicine and was carried out according to the principles of the Declaration of Helsinki. All patients were consented prior to the study. All women completed a validated patient history form and an assessment form of pain using a visual analogue scale for assessment of endometriosis associated pain (dysmenorrhoea, non-menstrual pelvic pain, dyspareunia, and dyschesia) (adapted from the validated International Pelvic Pain Society’s Pelvic Assessment Form). Peritoneal fluid (devoid of blood contamination) was collected on ice during surgery, then immediately transferred to the research facility and centrifuged at 2000xg to remove any cellular debris. The supernatant was used immediately for studies or stored at -80°C freezer for future use. The inclusion criteria for the study included adult non-smoking women, age 18-60 years, with normal menstrual cycles and otherwise in normal health (except for pain and endometriosis) who had not been on any hormonal medication for at least 1 month before sample collection. Exclusion criteria included subjects with current medical illnesses such as diabetes, cardiovascular disease, hyperlipidemia, hypertension, systemic lupus erythematosis or rheumatologic disease, positive HIV/AIDS, active infection, current medications such as hormonal/anti-hormonal medications, anti-inflammatory medications including corticosteroids. Subjects were asked to stop multivitamins that contain high levels of antioxidants at least one week prior to the surgery. The menstrual phase was calculated from the last menstrual period data obtained from the patient history forms.
Low Density Lipoprotein (LDL) isolation
LDL was isolated from heparinized blood obtained from normal human volunteers by the single-spin ultracentrifugation technique using a Beckman Table Top TL100 ultracentrifuge [17]. Since lipo-polysaccharide (LPS) contaminations can induce inflammatory responses, careful precautions were used to avoid any auto-oxidation or LPS contamination. LPS free water and buffers were used for all isolation procedures and the LPS levels were determined using the LAL assay (Limulus Amebocyte Lysate assay, CapeCod Inc, Falmouth, MA). The protein concentration of the isolated LDL was measured using Lowry’s method [38].
Oxidation of LDL in the presence or absence of antioxidants or NSAIDs
Briefly, 100μg/mL of LDL was incubated with 5μM of copper sulfate (an oxidant) in 1mL of 1X phosphate buffered saline (PBS, pH 7.4). Lipid peroxidation was initiated by the reaction between copper and polyunsaturated lipids present in the lipoproteins. The oxidation was measured in a Shimadzu UV-VIS spectrophotometer by following the generation of conjugated-diene products (lipid peroxidation marker) which has a unique absorption at OD 234nm (Supplementary Figure 1A). The oxidation process was terminated at specific time points to generate various forms of oxidatively-modified LDL preparations, by the addition of 50μM of 1mM EDTA (a copper chelator). The LDL preparations included: (a) native LDL (L0), (b) minimally-modified LDL-L1 (usually terminated at the end of the lag time), (c) oxidized LDL-L2 (after the oxidation has reached its plateau) and (d) completely or fully oxidized LDL-L3 (after 24 hours of oxidation) [51-53]. Control LDL preparations were incubated without copper and stopped at various time points similar to the one that goes through oxidation (in the presence of copper) to serve as respective non-oxidized LDL controls. These various forms of LDL preparations represent LDL that has undergone oxidation at various levels and thus has undergone changes in both chemical and biochemical properties [51-54]. Oxidative modification of LDL increases its negative charge which is reflected by its increased electrophoretic mobility as shown in Supplementary Figure 1B [3]. These represent heterogeneous LDL molecules that are theoretically possible to be physiologically present [54]. The various LDL preparations were prepared fresh for use in nociception and all other studies.
For antioxidant studies, the above LDL preparations were generated in the presence of antioxidants, N-acetylcysteine (1mM) or vitamin E (50μM) or the COX inhibitor indomethacin (1μg) (NSAID). Briefly, 100μg/mL of LDL was incubated with 5μM of copper sulfate in the presence of these agents and the oxidation was followed at OD 234 nm in a UV-VIS spectrophotometer. These preparations were used in the cell culture experiments and in Hargreaves nociception assay.
Thiobarbituric acid related substances (TBARS)
TBARS are a measure of the extent of oxidation when lipids undergo peroxidation. TBARS were measured in all the LDL preparations at the end of oxidation, as an increase in optical density 540nm using a standardized protocol [83]. The amounts of TBARS were quantitated using commercially available malondialdehyde (Sigma-Aldrich, St. Louis, MO) as standard and expressed as nmoles/mg protein. Typically, TBARS levels of the various preparations range as follows: L0<5 nmoles; L1=15-20 nmoles; L2=35-40 nmoles; L3>35 nmoles.
Agarose and native gel electrophoresis for lipoproteins
Agarose gel electrophoresis (Beckman Coulter Inc., Brea, CA) was used to separate lipoproteins in the PF and plasma and the various oxidatively modified LDL preparations (L0, L1, L2 and L3) using manufacturer’s instructions. The lipoproteins were identified using Fat red O staining. Commercially available LDL and HDL samples were used as standards. 10% native polyacrylamide gel electrophoresis was used to detect the presence of apolipoprotein B (Mol weight >200 kd) in the PF samples. Proteins were detected using Coomassie brilliant blue after separation of the samples on a native PAGE.
Prostaglandin E2 (PGE2) and 8-Isoprostane (8-Iso) detection using EIA kits
In order to asses if the non-enzymatic oxidation of lipoproteins can generate PGE2-like molecules, PGE2 and 8-isoprostane levels were detected in both the PF and oxidatively modified LDL preparations using commercially available Enzyme Immunoassay (EIA) kits from Cayman Chemicals (Ann Arbor, MI). 100μl of the LDL preparations were either directly (no dilution) or after dilutions (10-1000 fold dilution in 1xPBS) and 50 μL of PF were used in the EIA assays. Manufacturer’s instructions were followed for both the measurements and post-analysis of PGE2 and 8-isoprostanes and expressed as pg/ml. T-test was used to compare the levels of PGE2 in the control PF to the levels in endometriotic PF. In addition, one-way ANOVA followed by Dunnett’s multiple comparison test was used to compare the levels of PGE2-like molecules generated in all the oxidatively modified LDL preparations (L1, L2, L3) to the levels generated in the native LDL (unoxidized LDL-L0) control preparation.
LC-MS/MS detection
Eicosanoids and 20:4n6 lipids were extracted from PF and LDL preparations using an acetone liquid/liquid extraction [9; 10; 24] with slight modifications. PF or LDL preparations (500μl) was extracted with 500μl saline (0.9% NaCl) and 2mL acetone with deuterium labelled internal standards (100pg PGE2-d9; 500pg PGF2α-d4, LTB4-d4, TxB2-d4, 5S-HETE-d8, 12S-HETE-d8; 10ng 20:4n6-d8) (Cayman Chemicals) at pH 3.0 followed with 2 mL of chloroform with 0.005% BHT. The extract was dried under nitrogen and re-dissolved in 12μl of acetonitrile:water (40:60). The LC-MS/MS system consisted of an ACUITY UPLC pump (Waters; Milford, MA), and a XEVO TQ-S triple quadruple mass spectrometer (Waters) with electrospray ion source. The autosampler temperature was 8°C. Ten μL of sample was injected onto an ACUITY UPLC HSS T3 column (1.8μM, 100Å pore diameter, 2.1×150 mm, Waters) with an ACUITY UPLC HSS T3 precolumn (1.8μM, 100Å pore diameter, 2.1×5 mm, Waters). The separation was performed as previously described [7; 9]. The flow rate was 0.45mL/min and the initial conditions were 39%B (0.1% formic acid in acetonitrile) and 61%A (0.1% formic acid in water). At 0.5min solvent B was increased to 40.5% over 6.88min, then increased to 70% over 1.62min, then increased to 75% over 3min, further increased to 98% over 1.5min and held for 5.3min. The solvents were then returned to initial conditions over 0.2min and held for 2min to re-equilibrate the column. The mass spectrometer was operated in negative ion mode. The capillary and cone voltage were 2.3kV and 30V, respectively. The desolvation and source temperature were 550C and 150C, respectively. The nebulizer gas was 7.0 bar and the desolvation and cone gas flows were 1000L/h and 150L/h, respectively. MassLynx V4.1 (Waters) was used for instrument control, data acquisition and sample analysis.
PGF2α, TxB2, 20:4n6, LTB4, and 5-HETE were quantified using PGF2α-d4, TxB2-d4, 20:4n6-d8, LTB4-d4, and 5S-HETE-d8 as an internal standard, respectively, 12-HETE and 15-HETE using 12S-HETE-d8, and all other PG were quantified using PGE2-d9 as previously validated [24]. Analytes were monitored in MRM mode as previously described [8-10; 24] using the following mass transitions: PGE2-351.18/271.13; 11β-PGE2-351.18/271.13, 8-isoPGE2-351.18/271.13, PGD2-351.06/271.14; 6-ketoPGF1α-369.26/163.07; PGF2α-353.07/193.04; TXB2-369.20/169.00; 20:4n6-303.07/259.21; LTB4-335.07/194.99; 5-HETE-319.20/115.20; 12-HETE-319.10/267.21; 15-HETE - 319.12/219.09; PGE2-d9-360.2042/280.17; PGF2α-d4-357.16-197.01; TXB2-d4-373.22-173.03; 20:4n6–d8-310.93/267.21; LTB4-d4-339.26/197.06; 5S-HETE-d8-326.86/116.03; 12S-and-HETE-d8-327.12/184.04. The collision energies used were (eV): PGE2-16; PGD2-16; 11β-PGE2-16, 8-isoPGE2-16,6-ketoPGF1α - 24; PGF2α-20; PGF2α-d4-22; TXB2-12; PGE2-d9-14; TXB2-d4-12; 20:4n6-12; LTB4-14; 20:4n6-d8-12; LTB4-d4-14; 5-HETE-10; 12-HETE-12; 15-HETE-10; 5S-HETE-d8-14; 12S-HETE-d8-12.
Human Pain: Neuropathic and Inflammatory RT2 PCR Array
Ishikawa cells (Sigma, St. Louis, MO), a human (39 year old female) established endometrial cell-line, was cultured in T75 flasks in complete media (DMEM/F12, Pen/Strep, FBS, glutamine). These cells were used since they express similar characteristics of mature endometrial epithelial cells [15; 35; 76]. About 80% confluent cells were treated with either PGE2 (50ng/ml), 25μg of various LDL preparations, or 100μl of PF from patients with and without endometriosis for 48 hours. The concentrations chosen were selected from preliminary unpublished studies. At the end of 48 hours, cells were collected using QIAzol reagent (Qiagen, Gaithersburg, MD) and RNA was isolated using Qiagen RNeasy Mini Kit. cDNA synthesis from 1μg of each sample was achieved using Qiagen RT2 First Strand Kit. Nociceptive and inflammatory pathway genes were analyzed in the cDNA samples using the commercial Human Pain: Neuropathic and Inflammatory RT2 PCR Array (PAHS-162ZA, Qiagen, Valencia, CA) on the BioRad MyiQ system. Ishikawa cells treated with 1% charcoal-stripped serum containing media alone (DMEM/F12, Pen/Strep, charcoal-stripped FBS, glutamine) were used as the control group. Fold change was determined using Pfaffl equation [2ˆ-(ddct)] for all groups compared to media control using the manufacturers (Qiagen) algorithm which uses T-test as the default statistics to compare differences between control and treated groups. A stringent 4-fold cutoff was used to identify differentially expressed genes in Ishikawa cells treated by various groups compared to the charcoal-stripped media treated cells (control group).
Body temperature assay (intracerebroventricular injections- hypothermia/hyperthermia)
Intracerebroventricular (i.c.v.) injection of PGE2 produces fever (hyperthermia) through an agonist action at the four subtypes of EP receptors (notably EP3 and EP1). Prostaglandins interact with these receptors to modulate body temperature (hypothermic or hyperthermic response) [23; 48]. To assess whether the oxidized LDL preparations function similarly, we assessed the effect of these lipids on body temperature in mouse models. An IACUC approval from Louisiana Health Sciences Center Institutional Review committee was obtained for this study. All investigators were certified to perform animal studies. Groups of male CD-1 mice (n =8; Charles River, Boston, MA) weighing 34-40g were acclimated to the testing room for 3 hrs. Baseline body temperatures were taken rectally three times separated by at least 10 min using a thermistor telethermometer (Cole-Parmer). Only the last determination was used for statistical comparison. After baseline testing, 5 μl of saline (control), 1ng/ml PGE2, LDL-L0 (100 μg/ml), or one of the oxidized LDL preparations (L1, L2, L3) was injected i.c.v. using a 30 gauge needle attached to a Hamilton microsyringe with PE10 tubing [27]. After injection, body temperature was assessed every 10 min for 1 hr. After the first hour, temperatures were assessed at 120 min, 160 min and 24 hours after initial injection. Baseline temperatures were subtracted from post-injection temperatures, and reported as mean change in body temperature. The mice were euthanized at the end of the study using IACUC approved procedures. Two-way ANOVA (repeated measures) followed by Bonferroni’s post-hoc test was used to assess the difference in body temperature between saline injection and the other treatments over time.
Hargreaves Paw Withdrawal pain assay
To assess whether the non-enzymatically oxidized lipoproteins are a potential source of pain inducing molecules, we performed a Hargreaves assay of paw withdrawal latency as a measure of in vivo pain response using known pain inducers (carrageenan and PGE2), and compared it to native LDL (L0) and its oxidized forms (L1 and L2). The Hargreaves Method [28] was performed to measure nociception (pain) in rodent models using the IITC Model 390 Plantar Test Analgesia Meter (Woodland Hills, CA). An IACUC approval from Marshall University Institutional Review committee was obtained for this study. All investigators were certified to perform animal studies. Male Sprague-Dawley rats (Hill-top Lab animals, Scottdale, PA), 7-8 weeks of age, were used for this study. Briefly, a beam of light was directed onto the midplantar (dorsal surface) region of the hindpaws. The operator turned off the light and recorded the time of withdrawal of the hindpaw from the surface. The active intensity was set at 25% and the cutoff time as 20 secs. Treatments were performed through a randomized blinded study. Each treatment sample (100μL) was injected into the dorsal surface of the hindpaw of the rats and readings (paw withdrawal time) were measured every 30 minutes for the first hour and subsequently every hour for a total of 8 hours. An additional measurement was performed after 24 hours, post-injection. Saline injected on the left paw was used as an internal control to account for volume and pain related behaviour associated with injection. Treatments included 3% carrageenan (an irritant that produces pain), 50ng/mL PGE2, 100μl of different oxidatively modified forms of LDL protein (100μg/mL), PF from women with and without endometriosis (100μL). Carrageenan served as a positive control to validate the use of the Hargreaves paw withdrawal pain assay. In order to investigate if antioxidants would mitigate the nociceptive response mediated by oxidatively-modified lipoproteins, LDLs prepared in the presence of antioxidants 1mM N-acetylcysteine, and 50μM vitamin E or the COX inhibitor 1μg indomethacin were also tested for nociception. The paw-withdrawal time was recorded in triplicates for each treatment per time point per rat. The number of rats used for each experiment varied according to the treatment and were kept at least n>6 for all treatments. The rats were euthanized at the end of the pain assessment using IACUC approved procedures. The data were averaged for each time point and % withdrawal latency was determined by comparing the treatment response time versus saline response time (equation 1). Thus, % withdrawal latency <100 is indicative of increased pain related behaviour associated with the stated treatment, whereas a % withdrawal latency >100 is indicative of a decrease in pain related behaviour with the stated treatment.
| Equation 1 |
Statistical Analysis
Prism© software (Graphpad, Inc., La Jolla, CA) was used for statistical analysis of human and cell culture studies. T-test was used for detecting differences in eicosanoids (LC-MS), prostaglandins, 8-isoprostanes (EIA) and electrophoretic mobilities of isolated lipoproteins between control and endometriotic PF. One-way ANOVA followed by Dunnett’s multiple comparison test was used to find differences in PGE2 levels between unoxidized LDL (L0) and the oxidized preparations (L1-L3). Two way ANOVA followed by Bonferroni’s post hoc test was used to detect differences in body temperature (icv) between treatments and saline (control) over time.
Statistical analysis on results generated by the Hargreaves pain assay was implemented in R (v 3.0.2) (www.r-project.org). Significance was defined at p-values < 0.05 (two-sided). Linear mixed effects (LME) models on log10 transformed data were used to identify significant differences in pain induction between the treatment groups of interest and the internal control (saline) through time. All models originally contained an interaction term with the time. Paired t-tests were used to compare differences between treatment and saline at each time as a follow-up exploratory analysis following LME; however it should be stated that the use of the paired t-test may increase probability of type 1 error.
Prism© software (Graphpad, Inc., La Jolla, CA) was used for preparation of graphs.
RESULTS
Human Studies
Presence of cyclooxygenase and lipoxygenase generated eicosanoids in PF of women with endometriosis
EIA analysis was done on the PF samples from endometriosis and control subjects. There was an increased presence of enzymatically derived PGE2 (p=0.07) (Figure 1A) and free-radical or non-enzymatically derived 8-isoprostanes (p=0.005, T-test) (Figure 1B) in the PF of women with endometriosis (n=43) compared to control (n=36) subjects. LC-MS/MS separation of the PF from a representative number of patients (n=6/group) was done to confirm the presence of eicosanoids (both enzymatic and non-enzymatically generated). Figure 1C showed higher levels of 12, 15 or 5-lipoxygenase derived eicosanoids such as 12, 15-HETEs (p<0.05, T-test) or 5-HETEs, and though not significant, cyclooxygenase derived eicosanoids such as PGE2 and PGD2 in endometriosis patients compared to control subjects.
Figure 1. Presence of eicosanoids in peritoneal fluid.
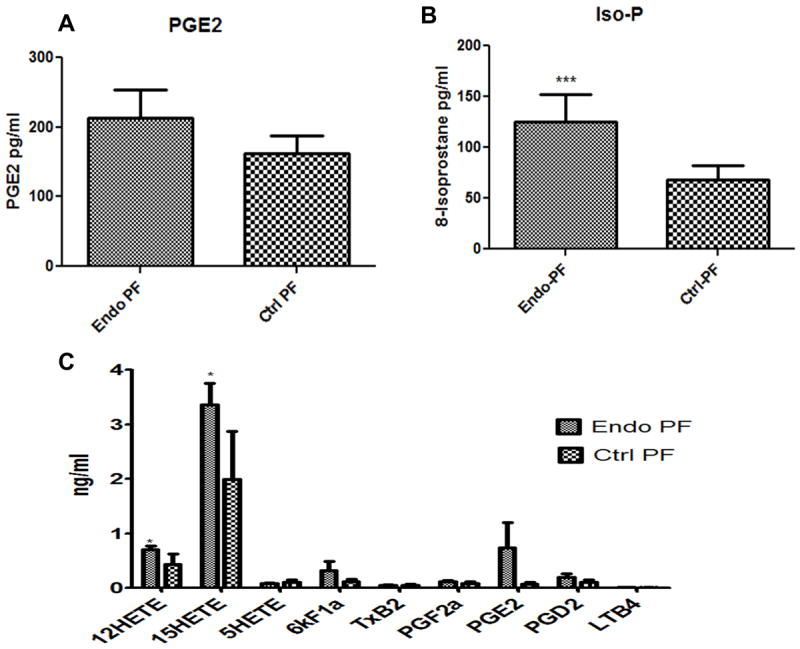
(A, B) Enzyme Immmunoassay-EIA showed the presence of COX generated PGE2 and free-radical generated 8-isoprostanes in the PF. The levels of PGE2 (p<0.07) and 8-isoprostanes (p<0.005) were higher in the PF from endometriosis patients (n=43) compared to control women (n=36). Results are expressed as pg/ml ± SEM. (C) LC-MS/MS separation of PF lipids showed the presence of higher levels of both COX generated prostanoids (PGE2, PGD2) as well as 12, 15, 5-LOX generated 12, 15, 5-HETEs in the PF from endometriosis (n=6) patients compared to control women (n=6). T-test was used for statistical analysis. P<0.05 was considered significant.
Evidence for presence of modified lipoproteins in the PF of women with endometriosis
Supplementary Figure 2A shows a typical agarose gel separation of lipoproteins (LDL, VLDL and HDL) present in plasma (PL), LDL isolated from the plasma (Pl-LDL), peritoneal fluid (PF) and LDL isolated from peritoneal fluid (PF-LDL). As shown earlier, oxidative modification of LDL increases its negative charge which is reflected by its increased electrophoretic mobility (Supplementary Figure 1B) [3]. We have earlier shown that while 90% of the PF samples from subjects with endometriosis were positive for the presence of lipoproteins (LDL and HDL), only 45% from normal subjects were positive for lipoproteins [46; 47]. We had also previously shown that plasma LDL and PF-LDL of subjects with endometriosis had significantly greater electrophoretic mobilities of 0.87±0.08 and 1.37±0.15cm, respectively (p<0.005, T-test) compared to 0.6±0.02 and 0.8±0.06cm in control subjects, respectively [46; 47]. The presence of lipoproteins in the PF of subjects with endometriosis did not appear to be due to contamination by blood. Supplementary Figure 2B showed an intact apolipoprotein B (apoB) in the LDL isolated from the PF.
Non-enzymatic oxidation of LDL generates prostaglandin-like molecules
Figure 2A shows that the non-enzymatically modified LDL preparations generated PGE2-like molecules measurable using PGE2 Enzyme Immunoassay (EIA) kits (Cayman chemicals, Ann Arbor, MI). Compared to native LDL (L0), the levels of PGE2-like molecules increased with the extent of LDL oxidation, (2-3 fold=L1, 5-6 fold=L2 and 10-12 fold=L3) (p<0.0001, one-way ANOVA, Dunnett’s multiple comparison test), i.e. the more the length of oxidation time, the more formation of PGE2-like molecules were observed. LC-MS/MS studies confirmed the generation of prostanoids PGE2, PGD2 and 11β-PGE2 and lipoxygenase products 12, 15 and 5-HETEs in the non-enzymatically modified LDLs (Figure 2B). The prostanoid levels increased whereas LOX products decreased with increase in extent of LDL oxidation (Figure 2C).
Figure 2. Detection of prostaglandin-like molecules by LC-MS/MS.
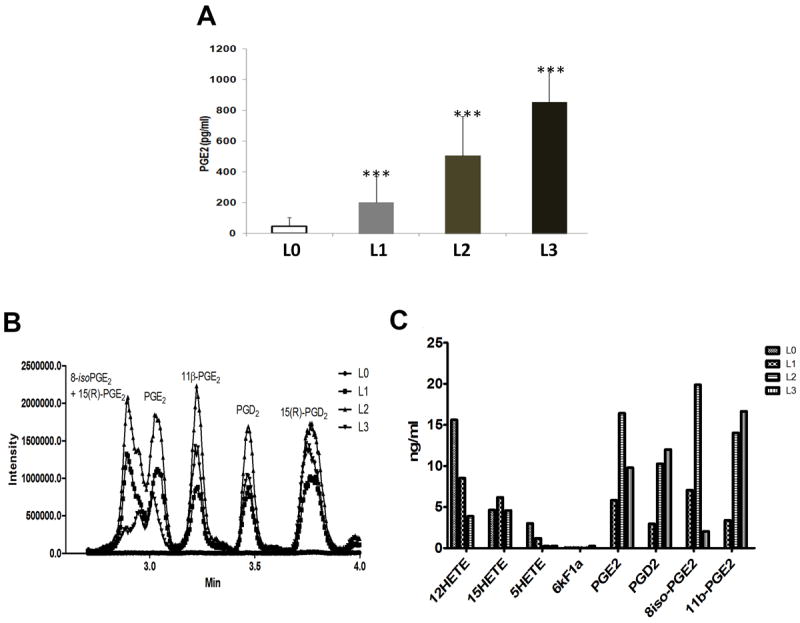
(A) Oxidatively-modified LDL preparations were used to detect the presence of prostaglandin-like molecules using PGE2 EIA kit. The levels of prostaglandin-like molecules as measured by EIA increased as the level of oxidation of LDL increased, with L0 (control group) having the least level compared to L3 (n=10-12). (B and C) LC-MS/MS was used to detect eicosanoids generated by non-enzymatic oxidation of LDLs. Both enzymatic (PGE2, PGD2), and non-enzymatically generated oxidation products were generated during the oxidation of lipoproteins. One-way ANOVA followed by Dunnetts’ multiple comparison test was used for statistical analysis. P<0.05 was considered significant.
Studies in Rodent model
ICV Injections of LDL in mice model induce thermal response
Intracerebroventricular (i.c.v.) injection of PGE2 (Figure 3), produced a significant (Two way ANOVA, Bonferroni posttest) increase in body temperature (38° to 40°) within 30 minutes (p<0.001) of injection compared to saline injection. This response lasted until 60-90 minutes (p<0.01 at 90 min). In contrast, L0 (native LDL) and its three oxidatively modified LDLs (L1-L3) all produced an initial drop (hypothermia) in body temperature (-2 to -4 degree Celsius, 38° to 35°) around 30-40 minutes. L0 and L3 injected mice returned to baseline temperatures (38°C) within 90 minutes however the L1 and L2 injected mice maintained higher temperature for more than 100-120 minutes (p<0.05 for L1 and p<0.01 for L2 at 120 min and p<0.01 for L2 at 160 min). These results are indicative of these lipids functioning similar to prostanoids (PGE2) in modulating body temperature either through EP receptors or others such as opioid receptors [23].
Figure 3. Oxidatively-modified LDLs and body temperature.
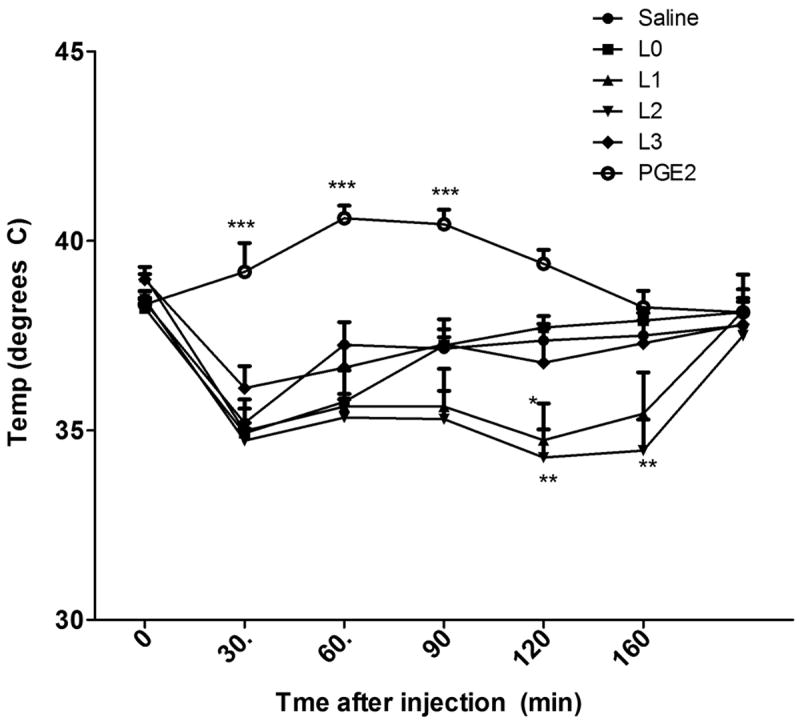
Baseline rectal temperatures were assessed in five groups of CD-1 mice (n=10). Mice were then injected i.c.v. with native LDL (L0), minimally modified LDL (L1), oxidized LDL (L2), fully oxidized LDL (L3), or PGE2 and temperature assessed every ten min for sixty min and recorded as a difference from baseline (0 = 38.4°C, SEM = 0.2). PGE2 produced a significant increase in body temperature (38° to 42°C) from 20 min to 50 min after injection, whereas native LDL (L0) and its oxidized preparations (L1, L2, L3) produced a decrease in body temperature (38° to 35°) from 10 min through 60 min. Two-way ANOVA followed by Bonferroni posttest was used to assess differences in body temperature between saline injection and other treatments over time.
Oxidatively-modified lipoproteins induce pain related behaviour
In the Hargreaves paw withdrawal assay, all treatments (carrageenan, PGE2, LDL preparations) injected on the dorsal surface of the right paw of SD rats were compared against the respective internal saline control (injected on the dorsal surface of the left paw). A paw withdrawal latency <100% indicates an increase in pain related behaviour induced by the stated treatment. Figure 4A illustrates a typical % withdrawal latency ± SEM (as compared to saline) curve following injection with known pain inducers, carrageenan or PGE2. Carrageenan, an irritant (n=5) served as a positive control to validate the use of the Hargreaves pain assay. Compared to saline, significant reductions in withdrawal latencies (indicative of increased pain sensitivity) were observed at 60min, 180min and 240min post-injection with carrageenan (p: 0.013, 0.043 and 0.002, respectively). Compared to saline, treatment with 50ng/mL PGE2 (n=22) induced significant reductions in withdrawal latency at 30min, 60min, 120min, and 180min post-injection (p: 0.0002, 0.011, 0.025, and 0.0008 respectively); however, no differences in withdrawal latency were observed beyond 180min. Figure 4B illustrates the % withdrawal latency ± SEM (as compared to saline) following injection with native-LDL (L0; n=29), minimally modified-LDL (L1; n=44) or oxidized-LDL (L2; n=37). Compared to saline, treatment with L1 induced significant reductions in withdrawal latency at 240min (p: 0.0008) and L2 at 60min, 240min, 300min and 360min post-injection (p: 0.005, 0.045, 0.014, and 0.0472, respectively). L0 indicated a reduction in withdrawal latency at 120min compared to saline.
Figure 4. Oxidatively-modified LDLs induce nociception in Hargreaves assay.
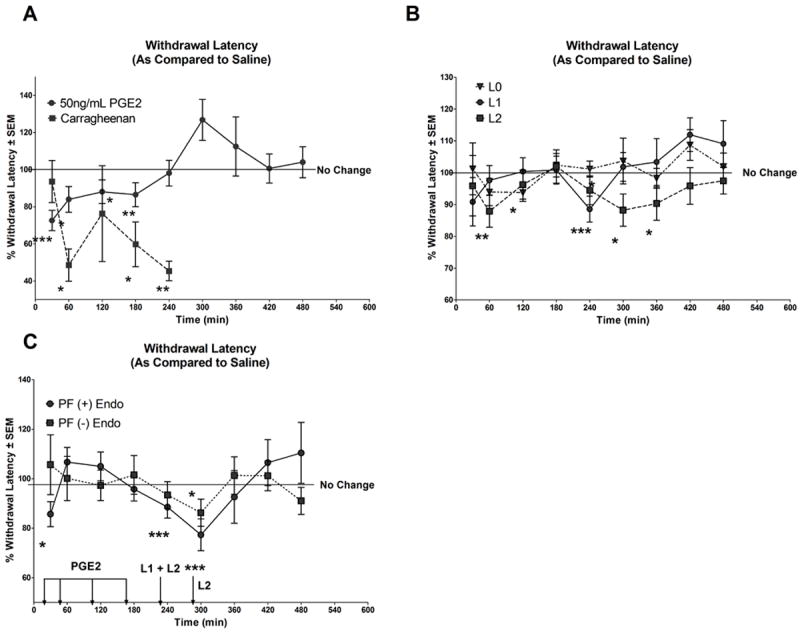
Paw withdrawal latency <100 in the Hargreaves assay indicates increased pain related behaviour induced by the respective treatment. (A) Illustrates the % withdrawal latencies ± SEM (as compared to saline) following injection with known pain inducer carrageenan (3%) or PGE2 (50ng/mL). Significant reductions in withdrawal latency were observed at 60, 180 and 240 minutes (min) post-carrageenan injection. Significant reductions in withdrawal latency were observed post-PGE2 treatment at 30, 60, 120, and 180 minutes. (B) Illustrates the % withdrawal latencies ± SEM (as compared to saline) following injection with 100μg/ml L0, L1 or L2. Compared to saline, significant decreases in withdrawal latency were observed at 120min and 240min for L0 and L1 respectively and at 60, 240, 300 and 360min for L2. (C) Illustrates the % withdrawal latencies ± SEM (as compared to saline) following injection with 100μl PF (+) Endo or PF (-) Endo. Relative to saline, significant reductions in withdrawal latency were observed at 30, 240 min and 300 min following treatment with PF (+) Endo and at 240min following treatment with PF (-) Endo. Abbrev: PGE2: Prostaglandin E2; L0: Native LDL; L1: Minimally modified LDL; L2: oxidized LDL. PF (+) Endo: Peritoneal fluid from women with endometriosis; PF (-) Endo: Peritoneal fluid from control subjects; *: <0.05; **: <0.01; ***: <0.005. Arrows and labelled treatments above the arrow represent points of significant induction of pain related behaviour by the stated treatment at the respective time points. The line presented at 100% indicates no change in response between treatment and saline. Statistical significance was determined using linear mixed effects models (LME) as described in the methods section.
Raw paw withdrawal response times (seconds) for each treatment group and the respective saline control over the course of 8 hours is provided in Supplementary Figure 3. Statistical analysis using LME models comparing treatments to saline (internal control) and with “time” as the interaction term, showed no significant interaction for saline versus treatment and time for Carrageenan, L0 and L2. However, comparing PGE2 to saline showed a significant interaction (p: 0.0002) between time and treatment versus saline indicating a different time course of pain related behaviour for PGE2 versus saline. Similarly, comparing L1 to saline, showed a significant interaction (p: 0.0039) indicative of a different time course of pain related behaviour for L1 versus saline.
Peritoneal fluid from women with endometriosis induces pain related behaviour
Figure 4C illustrates the % withdrawal latency ± SEM (as compared to saline) following injection with PF from women with endometriosis (+Endo; n=8 subjects, in quadruplicates) or women without endometriosis (-Endo; n=8, in triplicates). Compared to saline, treatment with PF from women with endometriosis (+Endo) indicated significant reductions in withdrawal latency (i.e. increased pain related behaviour) at 30min (p: 0.011) (time at which PGE2 induces pain related behaviour, indicated by an arrow in the figure), as well as at 240min and 300min post-injection (p: 0.003 and 0.005, respectively) (times at which L1 and L2 induce pain related behaviour, indicated with arrows in the figure, respectively). No such reduction in paw withdrawal latency was observed for PF (-Endo) except at 240 min.
Raw paw withdrawal response times (seconds) following injection with PF from women with or without endometriosis and the respective saline control over the course of 8 hours are provided in Supplementary Figure 3. Statistical analysis using LME models comparing treatments to saline (internal control) and with “time” as the interaction term, showed no interaction between time and treatment when comparing PF from women without endometriosis (-Endo) and saline, but a significant interaction (p: 0.0027) between time and PF (+) Endo versus saline was observed.
Effect of antioxidants, N-acetylcysteine or vitamin E and the NSAID, indomethacin on induction of pain related behaviour by oxidatively-modified LDL
We hypothesized that if non-enzymatic oxidation of LDL generated oxidation-sensitive factors of nociception, then oxidation of LDLs in the presence of N-acetylcysteine (1mM), vitamin E (50μM) or indomethacin (1μg) should decrease the generation of these nociceptive factors. LDLs oxidized in the presence of these agents were tested for their nociceptive ability using the Hargreaves assay. Figure 5A illustrates the % withdrawal latency ± SEM (as compared to saline) following injection with minimally-modified-LDL-L1 prepared in the presence of antioxidants or NSAID, i.e. L1+1mM N-acetylcysteine (n=23), L1+50μM vitamin E (n=34), and L1+1μg indomethacin (n=14). Compared to saline, treatment with L1+1mM N-acetylcysteine indicated a significant reduction in withdrawal latency at 60min and 360min post-injection (p: 0.005 and 0.045, respectively); however, no pain related behaviour was observed at 240min post injection (the point in time where L1 alone induced a significant reduction in withdrawal latency (represented by arrow in the figure). Relative to saline, L1+50μM vitamin E indicated significant reductions in withdrawal latency at 120min, 180min and 240min post injection. There were no significant differences in withdrawal latency between L1 + 1μg indomethacin and saline. Figure 5B illustrates the % withdrawal latency ± SEM (as compared to saline) following injection with oxidized-LDL-L2 prepared in the presence of antioxidant or NSAID, i.e. L2+1mM N-acetylcysteine (n=18), L2+50μM vitamin E (n=19), and L2+1μg indomethacin (n=10). Compared to saline, L2+1mM N-acetylcysteine indicated significant reductions in withdrawal latency at 30min, 180min and 240min post injection (p: 0.040, 0.042 and 0.017, respectively). Compared to saline, treatment with either L2+50μM vitamin E or L2+1μg indomethacin did not indicate any significant reductions in withdrawal latency, suggesting no pain related behaviour response.
Figure 5. Withdrawal latencies of oxidatively-modified LDLs generated in the presence of N-acetylcysteine, Vitamin E or Indomethacin.
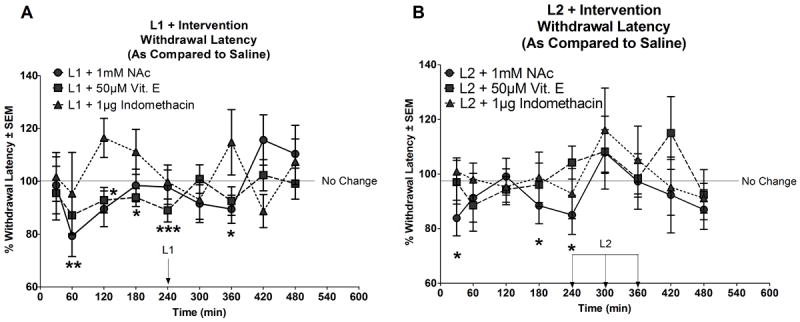
(A) Illustrates the % withdrawal latencies ± SEM (as compared to saline) following injection with 100μg/mL L1 + 1mM NAc, L1 + 50μM Vit. E or L1 + 1μg indomethacin. Compared to saline, significant reductions in withdrawal latency were observed at 30 and 360min following treatment with L1 + 1mM NAc and 120, 180 and 240min following treatment with L1 + 50μM Vit. E. The reductions were lower compared to L1 alone (the time at which L1 alone induced pain related behaviour-indicated as an arrow in the figure). No differences in withdrawal latency were observed between saline treatment and L1 + 1μg indomethacin. (B) illustrates the % withdrawal latencies ± SEM (as compared to saline) following injection with 100μg/mL L2 + 1mM NAc, L2 + 50μM Vit. E or L2 + 1μg indomethacin. Compared to saline, L2 + 1mM NAc indicated significant reductions in withdrawal latency at 30, 180 and 240 min post injection (arrows represent points of significant induction of pain related behaviour by L2 alone). No differences in withdrawal latency was observed between saline and L2 + 50μM Vit E or L2 + 1μg indomethacin. *: <0.05; **:< 0.01; ***<0.005. The line presented at 100% indicates no change in response between treatment and saline. Statistical significance was determined using linear mixed effects models (LME) as described in the methods section.
Raw paw withdrawal response times (seconds) for each treatment group and the respective saline controls over the course of 8 hours are provided in Supplementary Figure 4. Statistical analysis using LME models comparing treatments to saline (internal control) and with “time” as the interaction term, showed significant interaction (p: 0.037) between L1+1mM N-acetylcysteine and time versus saline but no such interactions with either L1+50μM vitamin E or L1+1μg indomethacin to saline.
Human Cell culture Studies
Oxidatively-modified LDL similar to PGE2 modulate genes involved in nociception
A PCR array for neuropathic and inflammatory genes (Qiagen, Valencia, CA) was used to assess the similarities in regulation of genes involved in nociception by the oxidatively-modified lipoprotein preparations (L0-L3) and PGE2. Ishikawa endometrial cells were either treated with LDL preparations or PGE2. A list of differentially expressed genes obtained from the Human Pain: Neuropathic and Inflammatory Array is provided in the Supplemental Section (Supplementary Table 1). The clustergram in Figure 6A shows the relative expression of the 84 genes in the treatment groups compared to the Control group (cells treated with 1% charcoal-stripped media alone). Figure 6B shows a breakdown of the up- and down-regulation of genes within each treated group based on the fold change. Stringent cut-offs of 4-fold changes compared to the control group were used. In most cases, the expression of these genes increased with the increasing level of LDL oxidation (L3>L0), supporting the concept of increased nociception or inflammation with increasing non-enzymatic oxidation of LDLs. A table of notable genes altered due to various treatments is given in Figure 6C. Blue indicates a value that is < a 4-fold decreased expression compared to the control, while red values indicate a ≥4-fold increased expression from the control. Amongst the genes responsible for pain conduction, there was a distinct up-regulation in voltage-gated sodium channel (SCN10A, 11A, 3A and 9A) and opioid receptor (OPRD1 and M1) genes. About half of the genes associated with synaptic transmission (serotonin-HTR2A and glutamate receptors-GRIN2B and GRM5, calcium channels-CACNA1B) experienced similar up-regulation as a function of LDL oxidation level. The expression of key inflammatory genes such as interleukins-IL-2, IL-6, and fractalkine receptor-1-CX3CR1, also increased in cells treated with increasing oxidation of LDL. PGE2 treated cells compared to other treatments had a higher expression of PGE receptor 3 (PTGER3), the major receptors involved in PGE2 mediated pain and fever.
Figure 6. Human Pain-Nociceptive and inflammatory PCR array.
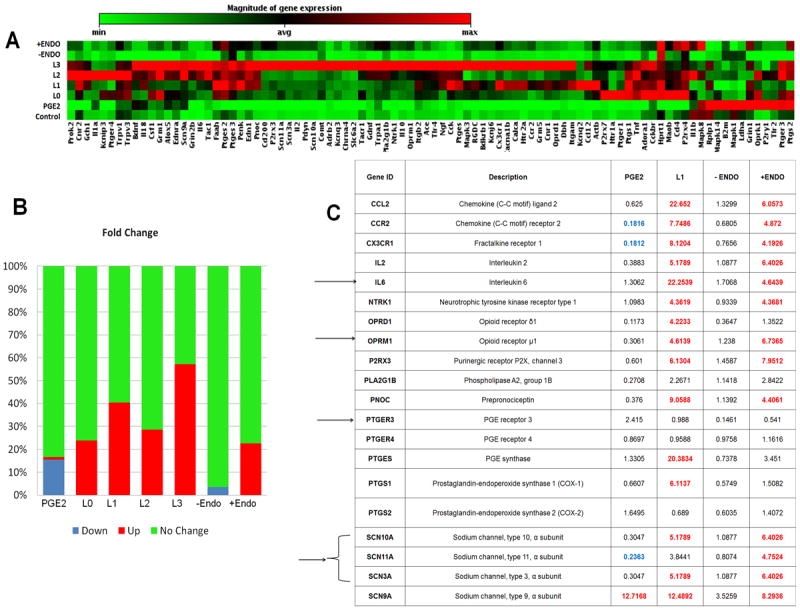
Human Pain-Nociceptive and Inflammatory PCR array on Ishikawa endometrial cell lines that were treated with either PGE2 (50ng/ml) or the different forms of oxidatively modified LDLs (25μg/ml), or PF (100μl) from patients with endometriosis (+ Endo) or control women (-Endo) for 48 hours. (A) Clustergram of gene expression in cells treated with PGE2, oxidatively modified LDLs and peritoneal fluid. The array includes 84 inflammatory and nociceptive genes as well as housekeeping genes and quality control standards. (B) Represents the percent of genes that were classified as up-regulated, down-regulated, or no change compared to the control treatment based on a 4-fold cutoff of the fold change. (C) Represents notable genes based on fold change. Potential genes involved in the oxidatively-modified LDL mediated nociceptive pathway are highlighted with an arrow. While PGE2 and PF from women without endometriosis (-Endo) had very little change in gene expression, oxidatively-modified LDLs and endometriotic fluid from patients with endometriosis (+Endo) showed similar trends in induction of several nociceptive and inflammatory genes. [The supplementary Table 1 lists all the genes measured in the pain array]. Statistical analysis (T-test) was performed using the algorithm provided by the manufacturer (Qiagen).
The array was also used to study the changes in pain and inflammatory related genes in Ishikawa cells treated with PF obtained from women with (+Endo) and without (-Endo) endometriosis. Many genes that were differentially expressed by oxidatively-modified LDL treatments were similarly modulated in cells treated with PF from patients with endometriosis (Figure 6C) compared to control subjects.
DISCUSSION
The relationship between endometriosis and existence of pelvic pain symptoms is still not well understood. Hence, treatment still remains a major challenge. Prostaglandins synthesized by COX-1 and COX-2 are key mediators of pain and nociception which are induced by cytokines and oxidative stress [4; 37]. Lowering COX-2 levels is correlated to decreased endometriotic lesion size and number of implants [32; 39]. Peripheral inflammation increases pain sensitivity and COX-2 expression in the periphery and the central nervous system [80]. Though NSAIDs are anti-inflammatory and anti-angiogenic in patients with endometriosis and animal models of endometriosis [2; 22] and used as the first line of treatment for pain, there is, still no evidence that these agents are completely effective in relieving pain associated with endometriosis.
Oxidative stress which is implicated in the etiology of several types of pain including chronic pelvic pain [70], abdominal pain [16] and fibromyalgia [49], induces NFkB and COX-2 and generates pro-inflammatory cytokines, TNF, IL-1β, NGF, nitric oxide (NO) and prostanoids. Oxidants such as superoxide [81] and NO donors (glyceryl trinitrite, sodium nitroprusside, SIN-1) can induce pain in humans [1; 56] and NOS inhibitors (NG-methyl-L-arginine) can reduce inflammatory hyperalgesia in a PGE2-dependent manner [1]. 8-Isoprostanes are elevated in the prostatic fluid of patients with chronic pelvic pain and in the serum and plasma of patients with muscle injury [70; 71]. We and others have demonstrated the potential for an important role for oxidative stress (lipid peroxides) in the etiology of endometriosis [4; 14; 69]. In addition to cytokines, chemokines, and growth factors, there is an abundance of oxidative stress markers in the PF of women with endometriosis [14; 59; 68]. We and others have shown the presence of lipoproteins, especially LDL in the PF of women with endometriosis [46; 47; 58; 79]. Isolated LDL from the PF of women with endometriosis exhibited a higher electrophoretic mobility (oxidation increases the negative charge on the protein), lower levels of associated lipid antioxidants such as vitamin E and an increased ex-vivo oxidizability, thus suggesting that these lipoproteins are already in a slightly oxidized form (similar to the L1=minimally-modified LDL) [46; 47]. There is an abundance of transition metals such as copper and iron present in the PF of women with endometriosis that may contribute to the oxidation process [18; 79]. Our clinical study demonstrated that vitamin E supplementation not only reduced inflammatory and oxidative stress markers in the women with endometriosis, but also reduced their pain symptoms [67], suggesting the possible role for oxidation sensitive nociception in these women.
Oxidatively-modified lipoproteins can cross-react with antibodies generated against prostaglandins, PGE2 and PGF2 [62]. This finding adds to the speculation that oxidatively-modified lipoproteins can mimic prostaglandin effects. This study demonstrated that non-enzymatic oxidatively modified lipoproteins (shown to be present in the PF of women of endometriosis) are similar to prostaglandins in their ability to modulate body temperature, induce nociception and alter the expression of inflammatory and nociceptive genes. LC-MS/MS studies revealed that even non-enzymatic oxidation of LDLs generated both COX and LOX derived oxidation products of polyunsaturated lipids (PGE2, PGD2 and 11β-PGE2 and 12, 15 and 5-HETEs). Hence, suppressing the generation of these molecules will probably necessitate the use of an antioxidant with or without conjunction with a COX inhibitor (NSAIDs).
It is known that the systemic inflammatory response to PGE2 depends on its dose as well as its distribution. Centrally, as well as at low doses, PGE2 causes fever, whereas peripherally and at higher doses, it elicits a hypothermic response [45; 65]. Non-prostaglandin eicosanoids such as seen in the oxidized LDL preparations can also play a role in thermoregulation [33]. In our study, at the doses tested, i.c.v injection of LDL preparations resulted in hypothermia in contrast to the PGE2 mediated hyperthermic reaction (body temperature). It has been shown that LPS as well as oxidants such as NO can cause hypothermia [25; 31; 44]. In Hargreaves assay of nociception we observed a time-sensitive pain related behaviour by PGE2 which peaked around 30 min and had no pain related behaviour beyond 180min. Oxidized LDL preparations had varying pain related behaviour responses which correlated with the extent of oxidation (L2>L1>L0). Interestingly, the oxidized LDL preparations had a dual pain related behaviour response, with an initial early response (around 30-60 min), possibly due to the presence of PGE2 followed by a later response (around 240-300 min) possibly due to the presence of other oxidized products. This dual response was similar to the pain related behaviour response seen in the presence of PF from women with endometriosis (+Endo). These studies suggested both central and peripheral effects of oxidized LDL metabolites.
Antioxidants when given alone or in combination with analgesics lowered oxidative stress mediated pain [13; 64]. Oxidized lipoproteins (L1-L2) generated in the presence of antioxidants N-acetylcysteine (aqueous antioxidant) and Vitamin E (lipid-soluble antioxidant) or the COX inhibitor (NSAID), indomethacin had no major effect on the initial pain related behaviour responses (i.e. PGE2 mediated) but had a reduced capacity to induce pain related behaviour at later time points (i.e. oxidized lipids mediated). The discrepancy in overall responses can be attributed to the dose of the agents tested as well as the extent to which these agents were able to inhibit the oxidation of LDL. Overall, our results support the notion that the use of antioxidants along with NSAIDs or other analgesics can reduce pain related behaviour induced by oxidatively-modified LDL and may serve as potential therapeutic options for endometriosis-associated pain or other pain conditions. Though there were few instances where a null response or negative effect of dietary antioxidants [29; 78] [50] or other agents such as raloxifene actually increasing chronic pelvic pain [74] was observed, majority of the studies with antioxidants had positive effects in ameliorating pelvic pain in endometriosis [61; 63].
The Nociceptive and Inflammatory Pathway Gene Array revealed potential candidate genes that might be involved in nociception due to oxidatively-modified lipoproteins. We found that these lipoproteins do interact with similar receptors as PGE2, but perhaps to a different extent. For example there was very little change seen in the expression of PGE2 receptors (e.g. PTGE3) when cells were treated with oxidatively-modified lipoproteins compared to PGE2 but had higher induction of the opioid receptors, OPRD1 and OPRM1. DNA microarray analysis of tissues obtained from patients with deep-endometriosis identified OPRM1 as one of the top three potential candidate genes involved in pain pathways [40; 42] and both GnRH agonists and continuous oral progestin treatments reduced the expression of OPRM1 in these patients [41]. Other genes that were differentially expressed in oxidized lipoprotein treated cells included, voltage-gated sodium channels (SCN10A, 3A and 9A) [20] which are known to be upregulated by COX derived prostanoids after inflammation [26], and the TRP family of ligand-gated ion channels (TRPV1 and TRPA1) [55]. Inflammatory genes (IL-6, IL-2, CX3CR1) were also activated suggesting a role for these pathways in nociception. Interestingly, most of the genes that were modulated by oxidatively-modified lipoproteins were similar to those induced by PF from women with endometriosis treated cells. The major limitations of our study include the use of non-endometriosis animal model to test pain responses, use of single doses of the tested agents and the use of a single nociceptive assay (Hargreaves). However, our findings are significant enough to support the presence of the oxidized lipid molecules in the PF of women with endometriosis and their potential role in endometriosis associated pain. The observation that oxidatively-modified lipoproteins are able to induce pain receptors and have the ability to modulate nociception makes them ideal candidates as therapeutic targets for pain in conditions such as the endometriosis-associated pain. Future studies using other animal models and in humans should explore the therapeutic use of targeting these agents in nociception.
Supplementary Material
(A) Lipoproteins undergo oxidation at different levels [53]. The figure is a graphical representative of LDL that has undergone copper-mediated non-enzymatic oxidation. LDL preparations were collected at different time points during oxidation to represent, L0: native LDL at zero time, L1: minimally oxidized LDL, collected at the end of the lag-time, when the LDL begins to undergo oxidation, L2: oxidized LDL, collected at the end of LDL oxidation and L3: fully oxidized LDL, collected at the end of 24 hours of oxidation. (B) Representative figure of agarose gel electrophoresis showing increase in negative charge with increasing oxidation of LDL (L3>L2>L1>L0).
(A) A representative figure of the agarose gel electrophoresis showing the presence of LDL, VLDL and HDL in the plasma, and the presence of LDL and HDL in peritoneal fluid. We have earlier published that while 90% of the PF samples from subjects with endometriosis were positive for the presence of lipoproteins, only 45% from normal subjects were positive for lipoproteins. We have shown earlier that the LDL isolated from PF was slightly more oxidized (increased negative charge) compared to plasma isolated LDL [46; 47]. (B) The image on the right is a representative native gel electrophoresis followed by Coomassie blue staining of lipoproteins. It shows the presence of intact apoB in the isolated LDL from PF.
Paw withdrawal latencies (seconds) ± SEM across time (0-8hours) are shown. (A) Paw withdrawal latencies (seconds) ± SEM following injection of 3% carrageenan (right paw) and saline (left paw). Relative to saline, significant reductions in paw withdrawal latencies were observed at 60min, 180min and 240 min post carrageenan injection. (B) Paw withdrawal latencies (seconds) ± SEM following injection of 50ng/mL PGE2 (right paw) and saline (left paw). Compared to saline, significant decreases in paw withdrawal latencies were observed at 30min, 60min, 120min and 180 min post PGE2 injection. (C-E) Paw withdrawal latencies (seconds) ± SEM following injection of 100μL L0, L1 or L2 respectively (right paw) and saline (left paw). Compared to saline, a significant reduction in paw withdrawal latency was observed at 120min post L0 injection, at 240min post L1 injection and at 60min, 240min, 300min and 360min post L2 injection. (F-G) Paw withdrawal latencies (seconds) ± SEM following injection of 100μL PF from subjects with (+Endo) or without (-Endo) endometriosis (right paw) and saline (left paw). Compared to saline, significant decreases in paw withdrawal latencies were observed at 30min, 240min and 300min post PF (+Endo) injection and only around 240min post PF (-Endo) injection. Statistical significance was determined using linear mixed effects models (LME) as described in the method section. * p-value< 0.05; ** p-value< 0.01; *** p-value<0.005.
Paw withdrawal latencies (seconds) ± SEM across time (0-8hours) are shown. (A-C) Paw withdrawal latencies (seconds) ± SEM following injection of 100μL L1 + 1mM NAc or 50μM Vit.E or 1μg Indomethacin (right paw) and saline (left paw). Compared to saline, L1 + 1mM NAc indicated significant reductions in paw withdrawal latencies at observed at 60min and 360min post injection and at 120 min, 180 min and 240min for 50μM Vit E post injection. No significant differences in withdrawal latencies were observed for L2 + 1μg Indomethacin post injection. (D-F) Paw withdrawal latencies (seconds) ± SEM following injection of 100μL L2 + 1mM NAc or 50μM Vit.E or 1μg Indomethacin (right paw) and saline (left paw). Compared to saline, L2 + 1mM NAc indicated significant reductions in withdrawal latency at 30 min, 180 min and 240 min post injuction. No significant differences in withdrawal latencies were observed with either L2 +50μM Vit.E or L2 +1μg Indomethacin compared to saline. Statistical significance was determined using linear mixed effects models (LME) as described in the method section. * p-value< 0.05; *** p-value<0.005.
Acknowledgments
The authors would like to thank Ms. Sandy White for coordinating the patient sample collection and Mardochee Isme for technical assistance. The authors like to acknowledge the intellectual contributions by Dr. Sampath Parthasarathy (University of Central Florida, Orlando, FL), and Dr. Richard Egleton, (Marshall University School of Medicine, Huntington, WV). The authors would like to acknowledge the support of Dr. Robert Nerhood and Dr. David Jude, past and present Chairman of Department of Obstetrics & Gynecology, Marshall University School of Medicine. The authors would like to thank Dr. Elsa Mangiarua for proofreading the manuscript. This study was partially supported by funds available from the Institutional Development Award (IDeA) NIGMS-NIH, P20GM103434 and NCRR-NCATS, UL1TR000117. For LC-MS/MS studies we thank Ms. Amanda Marquardt for her excellent technical assistance and NIH funded COBRE Mass Spec Core Facility Grant 5P30GM103329-02 at University of North Dakota. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agency, NIH.
LIST OF ABBREVIATIONS
- AA
Arachidonic Acid
- COX-1
Cyclooxygenase-1
- COX-2
Cyclooxygenase-2
- EIA
Enzyme immunoassay
- LC-MS/MS
Liquid Chromatography-Tandem Mass Spectrometry
- LDL
Low Density Lipoprotein
- NSAID
Non-steroidal anti-inflammatory drugs
- PF
Peritoneal Fluid
- PGE2
Prostaglandin E2
- SEM
Standard error of mean
- TBARS
Thiobarbituric Acid reactive substances
Footnotes
Author Disclosure Statement
No competing financial interests exist. The authors indicate no conflicts of interest.
References
- 1.Aley KO, McCarter G, Levine JD. Nitric oxide signaling in pain and nociceptor sensitization in the rat. J Neurosci. 1998;18(17):7008–7014. doi: 10.1523/JNEUROSCI.18-17-07008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen C, Hopewell S, Prentice A, Gregory D. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. 2009;(2) doi: 10.1002/14651858.CD004753.pub3. CD004753. [DOI] [PubMed] [Google Scholar]
- 3.Arrio B, Bonnefont-Rousselot D, Catudioc JD, Packer L. Electrophoretic mobility changes of oxidized human low density lipoprotein measured by laser Doppler electrophoresis. Biochemistry and molecular biology international. 1993;30(6):1101–1114. [PubMed] [Google Scholar]
- 4.Augoulea A, Alexandrou A, Creatsa M, Vrachnis N, Lambrinoudaki I. Pathogenesis of endometriosis: the role of genetics, inflammation and oxidative stress. Arch Gynecol Obstet. 2012 doi: 10.1007/s00404-012-2357-8. [DOI] [PubMed] [Google Scholar]
- 5.Barcz E, Kaminski P, Marianowski L. Role of cytokines in pathogenesis of endometriosis. Med Sci Monit. 2000;6(5):1042–1046. [PubMed] [Google Scholar]
- 6.Bedaiwy MA, Falcone T. Peritoneal fluid environment in endometriosis. Clinicopathological implications. Minerva Ginecol. 2003;55(4):333–345. [PubMed] [Google Scholar]
- 7.Brose SA, Baker AG, Golovko MY. A fast one-step extraction and UPLC-MS/MS analysis for E2/D 2 series prostaglandins and isoprostanes. Lipids. 2013;48(4):411–419. doi: 10.1007/s11745-013-3767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brose SA, Golovko MY. A rapid oxygen exchange on prostaglandins in plasma represents plasma esterase activity that is inhibited by diethylumbelliferyl phosphate with high affinity. Rapid communications in mass spectrometry : RCM. 2012;26(20):2472–2476. doi: 10.1002/rcm.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brose SA, Golovko MY. Eicosanoid post-mortem induction in kidney tissue is prevented by microwave irradiation. Prostaglandins Leukot Essent Fatty Acids. 2013;89(5):313–318. doi: 10.1016/j.plefa.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brose SA, Thuen BT, Golovko MY. LC/MS/MS method for analysis of E(2) series prostaglandins and isoprostanes. J Lipid Res. 2011;52(4):850–859. doi: 10.1194/jlr.D013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchweitz O, Staebler A, Wulfing P, Hauzman E, Greb R, Kiesel L. COX-2 overexpression in peritoneal lesions is correlated with nonmenstrual chronic pelvic pain. Eur J Obstet Gynecol Reprod Biol. 2006;124(2):216–221. doi: 10.1016/j.ejogrb.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–519. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cameron NE, Jack AM, Cotter MA. Effect of alpha-lipoic acid on vascular responses and nociception in diabetic rats. Free radical biology & medicine. 2001;31(1):125–135. doi: 10.1016/s0891-5849(01)00564-0. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho LF, Samadder AN, Agarwal A, Fernandes LF, Abrao MS. Oxidative stress biomarkers in patients with endometriosis: systematic review. Arch Gynecol Obstet. 2012;286(4):1033–1040. doi: 10.1007/s00404-012-2439-7. [DOI] [PubMed] [Google Scholar]
- 15.Castelbaum AJ, Ying L, Somkuti SG, Sun J, Ilesanmi AO, Lessey BA. Characterization of integrin expression in a well differentiated endometrial adenocarcinoma cell line (Ishikawa) J Clin Endocrinol Metab. 1997;82(1):136–142. doi: 10.1210/jcem.82.1.3658. [DOI] [PubMed] [Google Scholar]
- 16.Chi CH, Shiesh SC, Lin XZ. Total antioxidant capacity and malondialdehyde in acute abdominal pain. The American journal of emergency medicine. 2002;20(2):79–82. doi: 10.1053/ajem.2002.30102. [DOI] [PubMed] [Google Scholar]
- 17.Chung BH, Wilkinson T, Geer JC, Segrest JP. Preparative and quantitative isolation of plasma lipoproteins: rapid, single discontinuous density gradient ultracentrifugation in a vertical rotor. J Lipid Res. 1980;21(3):284–291. [PubMed] [Google Scholar]
- 18.Defrere S, Gonzalez-Ramos R, Lousse JC, Colette S, Donnez O, Donnez J, Van Langendonckt A. Insights into iron and nuclear factor-kappa B (NF-kappaB) involvement in chronic inflammatory processes in peritoneal endometriosis. Histol Histopathol. 2011;26(8):1083–1092. doi: 10.14670/HH-26.1083. [DOI] [PubMed] [Google Scholar]
- 19.Deguara CS, Pepas L, Davis C. Does minimally invasive surgery for endometriosis improve pelvic symptoms and quality of life? Curr Opin Obstet Gynecol. 2012;24(4):241–244. doi: 10.1097/GCO.0b013e328355626f. [DOI] [PubMed] [Google Scholar]
- 20.Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. Sodium channels in normal and pathological pain. Annual review of neuroscience. 2010;33:325–347. doi: 10.1146/annurev-neuro-060909-153234. [DOI] [PubMed] [Google Scholar]
- 21.Durand T, Bultel-Ponce V, Guy A, Berger S, Mueller MJ, Galano JM. New bioactive oxylipins formed by non-enzymatic free-radical-catalyzed pathways: the phytoprostanes. Lipids. 2009;44(10):875–888. doi: 10.1007/s11745-009-3351-1. [DOI] [PubMed] [Google Scholar]
- 22.Efstathiou JA, Sampson DA, Levine Z, Rohan RM, Zurakowski D, Folkman J, D’Amato RJ, Rupnick MA. Nonsteroidal antiinflammatory drugs differentially suppress endometriosis in a murine model. Fertil Steril. 2005;83(1):171–181. doi: 10.1016/j.fertnstert.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 23.Furuya K, Murakami M, Makimura N, Matsuda H, Ikou K, Saito K, Kawakami Y, Shibazaki T, Fukui U, Mizumoto Y, Tokuoka S, Nagata I, Kikuchi Y. Immunological and endocrinological studies on lymphocyte subpopulation and medical treatment for infertility in patients with endometriosis. Mol Cell Endocrinol. 2003;202(1-2):195–199. doi: 10.1016/s0303-7207(03)00083-2. [DOI] [PubMed] [Google Scholar]
- 24.Golovko MY, Murphy EJ. An improved LC-MS/MS procedure for brain prostanoid analysis using brain fixation with head-focused microwave irradiation and liquid-liquid extraction. J Lipid Res. 2008;49(4):893–902. doi: 10.1194/jlr.D700030-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Goteri G, Lucarini G, Pieramici T, Filosa A, Pugnaloni A, Montik N, Biagini G, Tranquilli AL, Fabris G, Ciavattini A, Lo Muzio L. Endothelial cell survivin is involved in the growth of ovarian endometriotic cysts. Anticancer Res. 2005;25(6B):4313–4318. [PubMed] [Google Scholar]
- 26.Gould HJ, 3rd, England JD, Soignier RD, Nolan P, Minor LD, Liu ZP, Levinson SR, Paul D. Ibuprofen blocks changes in Na v 1.7 and 1.8 sodium channels associated with complete Freund’s adjuvant-induced inflammation in rat. J Pain. 2004;5(5):270–280. doi: 10.1016/j.jpain.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Haley TJ, McCormick WG. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br J Pharmacol Chemother. 1957;12(1):12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 29.Kashanian M, Lakeh MM, Ghasemi A, Noori S. Evaluation of the effect of vitamin E on pelvic pain reduction in women suffering from primary dysmenorrhea. J Reprod Med. 2013;58(1-2):34–38. [PubMed] [Google Scholar]
- 30.Koga K, Osuga Y, Takemura Y, Takamura M, Taketani Y. Recurrence of endometrioma after laparoscopic excision and its prevention by medical management. Front Biosci (Elite Ed) 2013;5:676–683. doi: 10.2741/e648. [DOI] [PubMed] [Google Scholar]
- 31.Koga K, Osuga Y, Tsutsumi O, Okagaki R, Momoeda M, Yano T, Fujiwara T, Takai Y, Kugu K, Morita Y, Taketani Y. Increased concentrations of soluble tumour necrosis factor receptor (sTNFR) I and II in peritoneal fluid from women with endometriosis. Mol Hum Reprod. 2000;6(10):929–933. doi: 10.1093/molehr/6.10.929. [DOI] [PubMed] [Google Scholar]
- 32.Komiyama SI, Aoki D, Katsuki Y, Nozawa S. Proliferative activity of early ovarian clear cell adenocarcinoma depends on association with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2006 doi: 10.1016/j.ejogrb.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 33.Kozak W, Fraifeld V. Non-prostaglandin eicosanoids in fever and anapyrexia. Front Biosci. 2004;9:3339–3355. doi: 10.2741/1486. [DOI] [PubMed] [Google Scholar]
- 34.Kyama CM, Mihalyi A, Simsa P, Falconer H, Fulop V, Mwenda JM, Peeraer K, Tomassetti C, Meuleman C, D’Hooghe TM. Role of cytokines in the endometrial-peritoneal cross-talk and development of endometriosis. Front Biosci (Elite Ed) 2009;1:444–454. doi: 10.2741/e40. [DOI] [PubMed] [Google Scholar]
- 35.Lessey BA, Ilesanmi AO, Castelbaum AJ, Yuan L, Somkuti SG, Chwalisz K, Satyaswaroop PG. Characterization of the functional progesterone receptor in an endometrial adenocarcinoma cell line (Ishikawa): progesterone-induced expression of the alpha1 integrin. J Steroid Biochem Mol Biol. 1996;59(1):31–39. doi: 10.1016/s0960-0760(96)00103-3. [DOI] [PubMed] [Google Scholar]
- 36.Lousse JC, Defrere S, Colette S, Van Langendonckt A, Donnez J. Expression of eicosanoid biosynthetic and catabolic enzymes in peritoneal endometriosis. Hum Reprod. 2010;25(3):734–741. doi: 10.1093/humrep/dep408. [DOI] [PubMed] [Google Scholar]
- 37.Lousse JC, Van Langendonckt A, Defrere S, Ramos RG, Colette S, Donnez J. Peritoneal endometriosis is an inflammatory disease. Front Biosci (Elite Ed) 2012;4:23–40. doi: 10.2741/e358. [DOI] [PubMed] [Google Scholar]
- 38.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biological, Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- 39.Machado DE, Berardo PT, Landgraf RG, Fernandes PD, Palmero C, Alves LM, Abrao MS, Nasciutti LE. A selective cyclooxygenase-2 inhibitor suppresses the growth of endometriosis with an antiangiogenic effect in a rat model. Fertil Steril. 2010;93(8):2674–2679. doi: 10.1016/j.fertnstert.2009.11.037. [DOI] [PubMed] [Google Scholar]
- 40.Matsuzaki S. DNA microarray analysis in endometriosis for development of more effective targeted therapies. Front Biosci (Elite Ed) 2011;3:1139–1153. doi: 10.2741/e317. [DOI] [PubMed] [Google Scholar]
- 41.Matsuzaki S, Canis M, Pouly JL, Botchorishvili R, Dechelotte PJ, Mage G. Both GnRH agonist and continuous oral progestin treatments reduce the expression of the tyrosine kinase receptor B and mu-opioid receptor in deep infiltrating endometriosis. Hum Reprod. 2007;22(1):124–128. doi: 10.1093/humrep/del368. [DOI] [PubMed] [Google Scholar]
- 42.Matsuzaki S, Canis M, Vaurs-Barriere C, Boespflug-Tanguy O, Dastugue B, Mage G. DNA microarray analysis of gene expression in eutopic endometrium from patients with deep endometriosis using laser capture microdissection. Fertil Steril. 2005;84(Suppl 2):1180–1190. doi: 10.1016/j.fertnstert.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 43.Milingos S, Protopapas A, Kallipolitis G, Drakakis P, Loutradis D, Liapi A, Antsaklis A. Endometriosis in Patients with Chronic Pelvic Pain: Is Staging Predictive of the Efficacy of Laparoscopic Surgery in Pain Relief? Gynecol Obstet Invest. 2006;62(1):48–54. doi: 10.1159/000092023. [DOI] [PubMed] [Google Scholar]
- 44.Monroy M, Kuluz JW, He D, Dietrich WD, Schleien CL. Role of nitric oxide in the cerebrovascular and thermoregulatory response to interleukin-1 beta. Am J Physiol Heart Circ Physiol. 2001;280(4):H1448–1453. doi: 10.1152/ajpheart.2001.280.4.H1448. [DOI] [PubMed] [Google Scholar]
- 45.Morimoto A, Long NC, Nakamori T, Murakami N. The effect of prostaglandin E2 on the body temperature of restrained rats. Physiology & behavior. 1991;50(1):249–253. doi: 10.1016/0031-9384(91)90528-v. [DOI] [PubMed] [Google Scholar]
- 46.Murphy AA, Santanam N, Morales AJ, Parthasarathy S. Lysophosphatidyl choline, a chemotactic factor for monocytes/T- lymphocytes is elevated in endometriosis. J Clin Endocrinol Metab. 1998;83(6):2110–2113. doi: 10.1210/jcem.83.6.4823. [DOI] [PubMed] [Google Scholar]
- 47.Murphy AA, Santanam N, Parthasarathy S. Endometriosis: a disease of oxidative stress? Semin Reprod Endocrinol. 1998;16(4):263–273. doi: 10.1055/s-2007-1016286. [DOI] [PubMed] [Google Scholar]
- 48.Oka T. Prostaglandin E2 as a mediator of fever: the role of prostaglandin E (EP) receptors. Front Biosci. 2004;9:3046–3057. doi: 10.2741/1458. [DOI] [PubMed] [Google Scholar]
- 49.Ozgocmen S, Ozyurt H, Sogut S, Akyol O. Current concepts in the pathophysiology of fibromyalgia: the potential role of oxidative stress and nitric oxide. Rheumatol Int. 2006;26(7):585–597. doi: 10.1007/s00296-005-0078-z. [DOI] [PubMed] [Google Scholar]
- 50.Parazzini F, Vigano P, Candiani M, Fedele L. Diet and endometriosis risk: a literature review. Reprod Biomed Online. 2013;26(4):323–336. doi: 10.1016/j.rbmo.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 51.Parthasarathy S, Auge N, Santanam N. Implications of lag time concept in the oxidation of LDL. Free Radic Res. 1998;28(6):583–591. doi: 10.3109/10715769809065814. [DOI] [PubMed] [Google Scholar]
- 52.Parthasarathy S, Raghavamenon A, Garelnabi MO, Santanam N. Oxidized low-density lipoprotein. Methods in molecular biology. 2010;610:403–417. doi: 10.1007/978-1-60327-029-8_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parthasarathy S, Santanam N, Auge N. Oxidized low-density lipoprotein, a two-faced Janus in coronary artery disease? Biochem Pharmacol. 1998;56(3):279–284. doi: 10.1016/s0006-2952(98)00074-4. [DOI] [PubMed] [Google Scholar]
- 54.Parthasarathy S, Santanam N, Ramachandran S, Meilhac O. Potential role of oxidized lipids and lipoproteins in antioxidant defense. Free Radic Res. 2000;33(3):197–215. doi: 10.1080/10715760000301381. [DOI] [PubMed] [Google Scholar]
- 55.Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, Murphy RC, Hargreaves KM. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest. 2010;120(5):1617–1626. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petho G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiological reviews. 2012;92(4):1699–1775. doi: 10.1152/physrev.00048.2010. [DOI] [PubMed] [Google Scholar]
- 57.Pittaluga E, Costa G, Krasnowska E, Brunelli R, Lundeberg T, Porpora MG, Santucci D, Parasassi T. More than antioxidant: N-acetyl-L-cysteine in a murine model of endometriosis. Fertil Steril. 2010 doi: 10.1016/j.fertnstert.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 58.Polak G, Barczynski B, Kwasniewski W, Bednarek W, Wertel I, Derewianka-Polak M, Kotarski J. Low-density lipoproteins oxidation and endometriosis. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/624540. 624540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polak G, Wertel I, Barczynski B, Kwasniewski W, Bednarek W, Kotarski J. Increased levels of oxidative stress markers in the peritoneal fluid of women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2013;168(2):187–190. doi: 10.1016/j.ejogrb.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 60.Porpora MG, Brunelli R, Costa G, Imperiale L, Krasnowska EK, Lundeberg T, Nofroni I, Piccioni MG, Pittaluga E, Ticino A, Parasassi T. A promise in the treatment of endometriosis: an observational cohort study on ovarian endometrioma reduction by N-acetylcysteine. Evidence-based complementary and alternative medicine : eCAM. 2013;2013 doi: 10.1155/2013/240702. 240702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Practice Committee of the American Society for Reproductive M. Treatment of pelvic pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927–935. doi: 10.1016/j.fertnstert.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 62.Proudfoot JM, Beilin LJ, Croft KD. PGF2-isoprostanes formed during copper-induced oxidation of low-density lipoproteins are the prostaglandins that cross-react with PGE2 antibodies. Biochem Biophys Res Commun. 1995;206(2):455–461. doi: 10.1006/bbrc.1995.1064. [DOI] [PubMed] [Google Scholar]
- 63.Ray KL, Mitchell BL, Santanam N. Power over pain: a brief review of current and novel interventions for endometriosis-associated pain. Journal of Endometriosis and Pelvic Pain Disorders. 2014;6(4) doi: 10.5301/je.5000199. [DOI] [Google Scholar]
- 64.Rokyta R, Holecek V, Pekarkova I, Krejcova J, Racek J, Trefil L, Yamamotova A. Free radicals after painful stimulation are influenced by antioxidants and analgesics. Neuro Endocrinol Lett. 2003;24(5):304–309. [PubMed] [Google Scholar]
- 65.Romanovsky AA, Simons CT, Szekely M, Kulchitsky VA. The vagus nerve in the thermoregulatory response to systemic inflammation. Am J Physiol. 1997;273(1 Pt 2):R407–413. doi: 10.1152/ajpregu.1997.273.1.R407. [DOI] [PubMed] [Google Scholar]
- 66.Sacco K, Portelli M, Pollacco J, Schembri-Wismayer P, Calleja-Agius J. The role of prostaglandin E2 in endometriosis. Gynecol Endocrinol. 2012;28(2):134–138. doi: 10.3109/09513590.2011.588753. [DOI] [PubMed] [Google Scholar]
- 67.Santanam N, Kavtaradze N, Murphy A, Dominguez C, Parthasarathy S. Antioxidant supplementation reduces endometriosis-related pelvic pain in humans. Transl Res. 2013;161(3):189–195. doi: 10.1016/j.trsl.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santanam N, Murphy AA, Parthasarathy S. Macrophages, oxidation, and endometriosis. Ann N Y Acad Sci. 2002;955:183–198. doi: 10.1111/j.1749-6632.2002.tb02779.x. discussion 119-200, 396-406. [DOI] [PubMed] [Google Scholar]
- 69.Santanam N, Song M, Rong R, Murphy AA, Parthasarathy S. Atherosclerosis, oxidation and endometriosis. Free Radic Res. 2002;36(12):1315–1321. doi: 10.1080/1071576021000049908. [DOI] [PubMed] [Google Scholar]
- 70.Shahed AR, Shoskes DA. Oxidative stress in prostatic fluid of patients with chronic pelvic pain syndrome: correlation with gram positive bacterial growth and treatment response. Journal of andrology. 2000;21(5):669–675. [PubMed] [Google Scholar]
- 71.Sinzinger H, Lupattelli G, Chehne F, Oguogho A, Furberg CD. Isoprostane 8-epi-PGF2alpha is frequently increased in patients with muscle pain and/or CK-elevation after HMG-Co-enzyme-A-reductase inhibitor therapy. J Clin Pharm Ther. 2001;26(4):303–310. doi: 10.1046/j.1365-2710.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- 72.Smith HS. Arachidonic acid pathways in nociception. The journal of supportive oncology. 2006;4(6):277–287. [PubMed] [Google Scholar]
- 73.Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–346. doi: 10.1093/humupd/dmq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stratton P, Sinaii N, Segars J, Koziol D, Wesley R, Zimmer C, Winkel C, Nieman LK. Return of chronic pelvic pain from endometriosis after raloxifene treatment: a randomized controlled trial. Obstet Gynecol. 2008;111(1):88–96. doi: 10.1097/01.AOG.0000297307.35024.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Streuli I, de Ziegler D, Santulli P, Marcellin L, Borghese B, Batteux F, Chapron C. An update on the pharmacological management of endometriosis. Expert Opin Pharmacother. 2013;14(3):291–305. doi: 10.1517/14656566.2013.767334. [DOI] [PubMed] [Google Scholar]
- 76.Sugihara K, Kobayashi Y, Suzuki A, Tamura N, Motamedchaboki K, Huang CT, Akama TO, Pecotte J, Frost P, Bauer C, Jimenez JB, Jr, Nakayama J, Aoki D, Fukuda MN. Development of pro-apoptotic peptides as potential therapy for peritoneal endometriosis. Nature communications. 2014;5:4478. doi: 10.1038/ncomms5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taylor RN, Hummelshoj L, Stratton P, Vercellini P. Pain and endometriosis: Etiology, impact, and therapeutics. Middle East Fertility Society journal. 2012;17(4):221–225. doi: 10.1016/j.mefs.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trabert B, Peters U, De Roos AJ, Scholes D, Holt VL. Diet and risk of endometriosis in a population-based case-control study. Br J Nutr. 2011;105(3):459–467. doi: 10.1017/S0007114510003661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turgut A, Ozler A, Goruk NY, Tunc SY, Evliyaoglu O, Gul T. Copper, ceruloplasmin and oxidative stress in patients with advanced-stage endometriosis. European review for medical and pharmacological sciences. 2013;17(11):1472–1478. [PubMed] [Google Scholar]
- 80.Vardeh D, Wang D, Costigan M, Lazarus M, Saper CB, Woolf CJ, Fitzgerald GA, Samad TA. COX2 in CNS neural cells mediates mechanical inflammatory pain hypersensitivity in mice. J Clin Invest. 2009;119(2):287–294. doi: 10.1172/JCI37098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309(3):869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 82.Wu MH, Lu CW, Chuang PC, Tsai SJ. Prostaglandin E2: the master of endometriosis? Exp Biol Med (Maywood) 2010;235(6):668–677. doi: 10.1258/ebm.2010.009321. [DOI] [PubMed] [Google Scholar]
- 83.Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods in molecular biology. 1998;108:101–106. doi: 10.1385/0-89603-472-0:101. [DOI] [PubMed] [Google Scholar]
- 84.Zeilhofer HU. Prostanoids in nociception and pain. Biochem Pharmacol. 2007;73(2):165–174. doi: 10.1016/j.bcp.2006.07.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Lipoproteins undergo oxidation at different levels [53]. The figure is a graphical representative of LDL that has undergone copper-mediated non-enzymatic oxidation. LDL preparations were collected at different time points during oxidation to represent, L0: native LDL at zero time, L1: minimally oxidized LDL, collected at the end of the lag-time, when the LDL begins to undergo oxidation, L2: oxidized LDL, collected at the end of LDL oxidation and L3: fully oxidized LDL, collected at the end of 24 hours of oxidation. (B) Representative figure of agarose gel electrophoresis showing increase in negative charge with increasing oxidation of LDL (L3>L2>L1>L0).
(A) A representative figure of the agarose gel electrophoresis showing the presence of LDL, VLDL and HDL in the plasma, and the presence of LDL and HDL in peritoneal fluid. We have earlier published that while 90% of the PF samples from subjects with endometriosis were positive for the presence of lipoproteins, only 45% from normal subjects were positive for lipoproteins. We have shown earlier that the LDL isolated from PF was slightly more oxidized (increased negative charge) compared to plasma isolated LDL [46; 47]. (B) The image on the right is a representative native gel electrophoresis followed by Coomassie blue staining of lipoproteins. It shows the presence of intact apoB in the isolated LDL from PF.
Paw withdrawal latencies (seconds) ± SEM across time (0-8hours) are shown. (A) Paw withdrawal latencies (seconds) ± SEM following injection of 3% carrageenan (right paw) and saline (left paw). Relative to saline, significant reductions in paw withdrawal latencies were observed at 60min, 180min and 240 min post carrageenan injection. (B) Paw withdrawal latencies (seconds) ± SEM following injection of 50ng/mL PGE2 (right paw) and saline (left paw). Compared to saline, significant decreases in paw withdrawal latencies were observed at 30min, 60min, 120min and 180 min post PGE2 injection. (C-E) Paw withdrawal latencies (seconds) ± SEM following injection of 100μL L0, L1 or L2 respectively (right paw) and saline (left paw). Compared to saline, a significant reduction in paw withdrawal latency was observed at 120min post L0 injection, at 240min post L1 injection and at 60min, 240min, 300min and 360min post L2 injection. (F-G) Paw withdrawal latencies (seconds) ± SEM following injection of 100μL PF from subjects with (+Endo) or without (-Endo) endometriosis (right paw) and saline (left paw). Compared to saline, significant decreases in paw withdrawal latencies were observed at 30min, 240min and 300min post PF (+Endo) injection and only around 240min post PF (-Endo) injection. Statistical significance was determined using linear mixed effects models (LME) as described in the method section. * p-value< 0.05; ** p-value< 0.01; *** p-value<0.005.
Paw withdrawal latencies (seconds) ± SEM across time (0-8hours) are shown. (A-C) Paw withdrawal latencies (seconds) ± SEM following injection of 100μL L1 + 1mM NAc or 50μM Vit.E or 1μg Indomethacin (right paw) and saline (left paw). Compared to saline, L1 + 1mM NAc indicated significant reductions in paw withdrawal latencies at observed at 60min and 360min post injection and at 120 min, 180 min and 240min for 50μM Vit E post injection. No significant differences in withdrawal latencies were observed for L2 + 1μg Indomethacin post injection. (D-F) Paw withdrawal latencies (seconds) ± SEM following injection of 100μL L2 + 1mM NAc or 50μM Vit.E or 1μg Indomethacin (right paw) and saline (left paw). Compared to saline, L2 + 1mM NAc indicated significant reductions in withdrawal latency at 30 min, 180 min and 240 min post injuction. No significant differences in withdrawal latencies were observed with either L2 +50μM Vit.E or L2 +1μg Indomethacin compared to saline. Statistical significance was determined using linear mixed effects models (LME) as described in the method section. * p-value< 0.05; *** p-value<0.005.


