Abstract
In patients with obstructive sleep apnea, airway obstruction during sleep produces hypercapnia which in turn activates respiratory muscles that pump air into the lungs (e.g. the diaphragm) and that dilate and stabilize the upper airway (e.g., the genioglossus). We hypothesized that these responses are facilitated by glutamatergic neurons in the parabrachial complex (PB) that respond to hypercapnia and project to premotor and motor neurons that innervate the diaphragm and genioglossus muscles. To test this hypothesis we combined c-Fos immunohistochemistry with in situ hybridization for vGluT2 or GAD67 or with retrograde tracing from the ventrolateral medullary region that contains phrenic premotor neurons, the phrenic motor nucleus in the C3-C5 spinal ventral horn, or the hypoglossal motor nucleus. We found that hypercapnia (10% CO2 for 2 hours) activated c-Fos expression in neurons in the external lateral, lateral crescent (PBcr), and Kölliker-Fuse (KF) PB subnuclei and that most of these neurons were glutamatergic and virtually none GABAergic. Numerous CO2− responsive neurons in the KF and PBcr were labeled after retrograde tracer injection into the ventrolateral medulla or hypoglossal motor nuclei, and in the KF after injections into the spinal cord, making them candidates for mediating respiratory-facilitatory and upper airway stabilizing effects of hypercapnia.
Keywords: phrenic, diaphragm, respiratory, hypoglossal, c-Fos, carbon dioxide, AB_2341544, AB_631250, AB_2341545
Introduction
Patients with obstructive sleep apnea typically have small, collapsible pharyngeal airways. During wakefulness airway patency is maintained by enhanced airway dilator muscle activity, mainly of the genioglossus muscle (GG), a tongue protruder innervated by motoneurons residing in the hypoglossal motor nucleus. Loss of this neuromuscular compensation during sleep leads to partial or complete block of airflow. The consequent hypercapnia, hypoxia and negative intrapharyngeal airway pressure created by inspiratory effort against a blocked airway produce graded and progressive activation of respiratory muscles and often arousal from sleep. The neural circuitry that mediates these responses is not well understood.
The parabrachial complex (PB) is a key component of the central respiratory network (Chamberlin, 2004; Dutschmann and Dick, 2012) and thus is well positioned to monitor and respond to physiological signals of respiratory difficulty. The PB contains at least 11 distinct subnuclei with different chemical and connectional phenotypes. The respiratory portions of the PB which include the external lateral (PBel), lateral crescent (PBcr), and Kölliker-Fuse (KF) subnuclei receive intense inputs from the respiratory part of the nucleus of the solitary tract (NTS) and the retrotrapezoid nucleus (Ricardo and Koh, 1978; Herbert et al., 1990; Rosin et al., 2006) that collectively carry both peripheral and central respiratory chemoreceptor information as well as breathing-related mechanical sensation. Lateral PB and KF neurons express c-Fos in response to hypercapnia or hypoxia (Berquin et al., 2000); and PB lesions produce decrements in hypercapnic and hypoxic ventilatory responses in awake (Mizusawa et al., 1995) and anesthetized rats (Song and Poon, 2009). However, little is known about the projections of the PB neurons that are engaged by hypercapnia, particularly those that contribute to respiratory drive. The KF projects directly to respiratory motoneurons in the brainstem and spinal cord (Yokota et al., 2001; Yokota et al., 2004; Ezure and Tanaka, 2006; Yokota et al., 2011), and the KF and PBcr send intense projections to the ventrolateral medullary region that contains both neurons that generate the respiratory rhythm (the preBötzinger complex) as well as premotor neurons for the diaphragm and accessory breathing muscles (the rostral ventral respiratory group) (Herbert et al., 1990; Yokota et al., 2004; Ezure and Tanaka, 2006; Yokota et al., 2007). We hypothesized that during hypercapnia, these PBcr and KF neurons augment the motor output of the respiratory pump (diaphragm) and upper airway dilator (GG) muscles.
Therefore we sought to identify PB neurons that 1) respond to hypercapnia and 2) project to respiratory motor and premotor neurons. To match active neurons with their downstream targets we combined immunohistochemistry for c-Fos, a marker for neural activation, with retrograde tracing. We chose to examine hypercapnia-induced c-Fos expression in neurons projecting to three major PB targets that contain respiratory motor and premotor neurons and therefore could affect the strength of ventilatory responses to hypercapnia: the ventrolateral medulla/rostral ventral respiratory group (diaphragm premotor neurons), the phrenic motor nucleus and the hypoglossal motor nucleus (GG and other tongue motoneurons) (Herbert et al., 1990; Yokota et al., 2001; Yokota et al., 2004; Yokota et al., 2011).
Materials and Methods
Experiments were performed on adult male C57BL/6 mice (Jackson Labs, n=79) weighing between 23 and 33 gms. Mice were maintained in standard laboratory conditions including 12:12 hr light:dark cycle (lights on at 7:00) and 21±1°C room temperature. The mice had ad libitum access to food and water. Care of the mice met National Institutes of Health standards, as set forth in the Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the BIDMC Institutional Animal Care and Use Committees.
The mice were anesthetized with isoflurane (2 %) and 9-15 nanoliters of a 0.2% solution of cholera toxin b subunit (CTb; List Biol. Lab) were injected into the intended target following craniotomy or laminectomy as described previously (Lu et al., 2000; VanderHorst and Ulfhake, 2006). Wounds were closed and mice given postoperative analgesia (meloxicam; 5 mg/kg, sc; once per day for two days). Following the surgical procedure, the mice were maintained undisturbed in the housing room for at least 14 days. Mice were acclimated to the experimental environment for 6-9 hours per day for 4 days prior to hypercapnia exposure. Mice in their home cages were moved from the animal housing room to the laboratory and placed inside a secondary box with a light inside. Home cages were fitted with a special airtight top and mice were exposed to a hypercapnic mixture of compressed air (21% O2; 10% CO2; 69% N2) or a control mixture (21% O2; 0% CO2; 79% N2) for two hours. Air or hypercapnic exposures were performed between 14:00 and 19:00.
Mice were then deeply anesthetized with chloral hydrate (500mg/kg, ip) and transcardially perfused with saline, followed by 10% formalin. Brains were removed, postfixed overnight immersed in 20% sucrose and cut into four alternate series of 30 micron frozen sections.
Histology- antibody characterization
Antibodies used in this study (see Table 1) were as characterized as follows. Tissue staining with rabbit anti-alpha-calcitonin gene-related peptide (CGRP) was eliminated by preadsorption with rat alpha-CGRP (Wang et al., 2006). The c-Fos antibody from Oncogene Sciences stained a single band of 55 kDa on Western blots from rat brain (manufacturer's technical information). The c-Fos antibody from Santa Cruz Biotechnology, was raised against amino acids 3-16 near the N-terminus of c-Fos of human origin: FSGFNADYEASSSR and affinity purified. Both c-Fos antibodies stained only neuronal nuclei in the same patterns as previously reported at this time of day for rats that were untreated (Scammell et al., 2000) or exposed to CO2 (Berquin et al., 2000). The staining patterns with the CTb antibody depended solely upon the site of CTb injection; this antibody stains nothing in animals not injected with CTb.
Table 1.
Table of Primary Antibodies Used
| Antigen | Description of Immunogen | Source, Host Species, Cat. #, RRID | Concentration Used |
|---|---|---|---|
| CGRP (alpha-calcitonin gene-related peptide) | amino acids 83–119 of the CGRP precursor protein | Peninsula Labs, rabbit polyclonal, cat. # T4032, RRID: AB_2341544. | Diluted 1:1000 |
| c-Fos | amino acids 4–17 of human c-Fos | Oncogene Sciences, rabbit polyclonal, cat. # Ab5, RRID: none. Please note that this antibody is no longer commercially available. | Diluted 1:20,000 |
| c-Fos | amino acids 3-16 near the N-terminus of c-Fos of human origin: FSGFNADYEASSSR | Santa Cruz Biotechnology, goat polyclonal, cat. # sc-52-G, RRID: AB_631250. | Diluted 1:500 |
| CTb | Cholera toxin b subunit | List Biological Labs, goat polyclonal, cat. # 703, RRID: AB_2341545 | Diluted 1:10,000 |
Histology- immunohistochemistry
Sections were processed for detection of c-Fos alone; or c-Fos in combination with CTb or CGRP; or c-Fos in combination with in situ hybridization for vGluT2 or GAD67 mRNA (see below). All incubations were performed on free-floating tissue sections at room temperature. Sections were first incubated overnight in c-Fos antibody. When c-Fos staining was to be combined with CGRP, we used goat anti-Fos antibody (AB_631250; diluted 1:500 in PBS with 0.2% Triton X-100 and 2.5% normal donkey serum). After rinsing, sections were incubated in Alexa488 (green fluorescence) conjugated donkey anti goat-IgG (Invitrogen, A11055) at 1:500 in PBS containing 0.2% Triton-X and 2.5% normal donkey serum for three hours. After rinsing in PBS sections were next incubated overnight with rabbit anti-CGRP (AB_2341544; diluted 1:1000 in PBS with 0.2% Triton X-100 and 2.5% normal donkey serum). After rinsing the next day sections were incubated in Cy3 (red fluorochrome) conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Labs, code#111-165-003) at 1:500.
When c-Fos was to be visualized alone or in combination with CTb, tissue sections were first incubated overnight with rabbit anti-Fos (diluted 1:20,000 in PBS with 0.2% Triton X-100 and 2.5% normal donkey serum). After rinsing, sections were incubated in biotinylated donkey anti-rabbit IgG (Jackson ImmunoResearch Labs, diluted 1:500) for 3 hours and then ABC elite (Vector Labs, 1:1000) for 1 hour. After rinsing sections were reacted with 0.05% diaminobenzidine hydrochloride (DAB; Sigma) and 0.01% H2O2 in PBS containing 0.01–0.02% nickel sulfate, which renders a black reaction product. For immunohistochemical visualization of CTb sections were next incubated overnight with goat anti-CTb (AB_2341545; diluted 1:10,000 in PBS with 0.25% triton X-100). After rinsing, sections were incubated in biotinylated donkey anti-goat IgG (Jackson ImmunoResearch Labs, diluted 1:500) for 3 hours and then ABC elite (Vector Labs, 1:1000) for 1 hour. After rinsing, sections were reacted with 0.05% DAB and 0.01% H2O2.
Histology- in situ hybridization
Free-floating sections were incubated overnight with hybridization buffer containing probes for vGluT2 (1 μg/ml; template graciously donated by Dr. Qinchung Tong (Tong et al., 2007)) or fragmented probes by alkaline hydrolysis for GAD67; 0.5 μg/ml; full-length cDNA encoding rat GAD67 (3.2 kilobases; (Erlander et al., 1991)) from Dr. A.J. Tobin (University of California, Los Angeles, CA) at 60 °C. Subsequently, the sections were washed twice in 2x standard saline citrate (SSC) at 60 °C for 60 min, treated with RNase A (20 μg/ml), washed in 2x SSC at 60 °C for 60 min, and then washed twice in 1x SSC at 60 °C for 60 min. After washing in Tris-buffered saline (pH 7.6), the sections were incubated overnight in an alkaline-phosphatase conjugated anti-digoxigenin antibody (1:500; Roche). After washing, the sections were developed with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT) solution (Vector Labs) for 6 h. Subsequently, c-Fos protein was visualized as described above. Briefly, the sections were incubated overnight with an anti-Fos antibody (1:10,000), a biotinylated donkey antibody to rabbit IgG, and then with ABC-Elite for 1 h. The sections were reacted with 0.05% DAB and 0.01% H2O2.
Data analysis- PB subnuclear anatomy
CTb- and c-Fos-immunoreactive (-ir) cells in the PB were first plotted from 6 sections in each series of every fourth 30-μm-thick section. After the locations of all labeled neurons were mapped, the coverslips were removed from slides and the tissue stained for Nissl substance with thionin. The borders of subnuclei were drawn based on cytoarchitecture, with the exception of the PBcr (Fig. 1). The PBcr is not distinct cytoarchitectonically (Fulwiler and Saper, 1984), but is clearly outlined by retrograde tracing from the ventrolateral medulla in rats (Herbert et al., 1990; Chamberlin and Saper, 1992), in which the lateral crescent neurons are seen along the lateral margin of the unlabeled PBel (Chamberlin and Saper, 1992). Because we saw a similar retrograde labeling pattern in our mice, we considered all of the retrogradely labeled neurons along the border of the PBel after medullary injections to be part of the PBcr (see below). Some of these neurons wrapped around the dorsomedial border of PBel into what had been considered the central lateral subnucleus (PBcl). Because there is no clear cytoarchitectonic boundary at the interface between the PBcr and PBcl, but the vast majority of hypercapnia-activated PB neurons projecting to the medulla were continuous with the PBcr, in our cell counts of c-Fos-ir neurons for practical purposes we considered these two structures together as a single unit. The borders of the KF were drawn on the basis of cytoarchitecture. KF neurons are larger than those of other PB neurons, have a pyramidal shape, and distinct nucleoli as has been previously reported in rats (Fulwiler and Saper, 1984). It is worth noting that in both rats and mice the ventral spinocerebellar tract provides a convenient tissue landmark for the dorsolateral border of KF (Figs. 1, 3, and 6-9).
Figure 1.
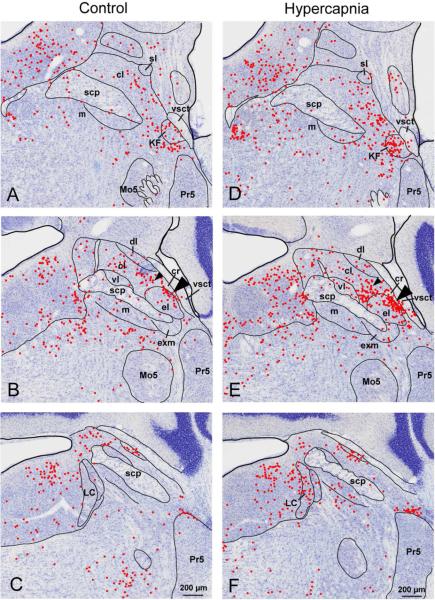
Distribution of c-Fos-ir neurons in the parabrachial nucleus (PB) in animals breathing normal air or hypercapnic air (10% CO2). Each red dot indicates one c-Fos-immunoreactive (-ir) neuronal nucleus. Shown are three representative sections through the PB from rostral to caudal (A-C and D-F). Note the increase in c-Fos expression in the hypercapnic condition vs control in the Kölliker-Fuse nucleus (KF) (A vs D), and in the outer portion of external lateral (el), lateral crescent (cr; arrowheads in B and E) and central lateral (cl) PB subnuclei. The smaller arrowheads in panels B and E shows the area where we found hypercapnia-activated, medullary-projecting neurons (see Fig. 6). Abbreviations: cl: central lateral; cr: lateral crescent; dl: dorsal lateral; el: external lateral; exm: external medial, KF: Kölliker Fuse, LC: locus coeruleus, m: medial, Mo5: motor trigeminal, Pr5: principal sensory trigeminal, scp: superior cerebellar peduncle, sl: superior lateral, vl: ventral lateral, vsct: ventral spinocerebellar tract
Figure 3.
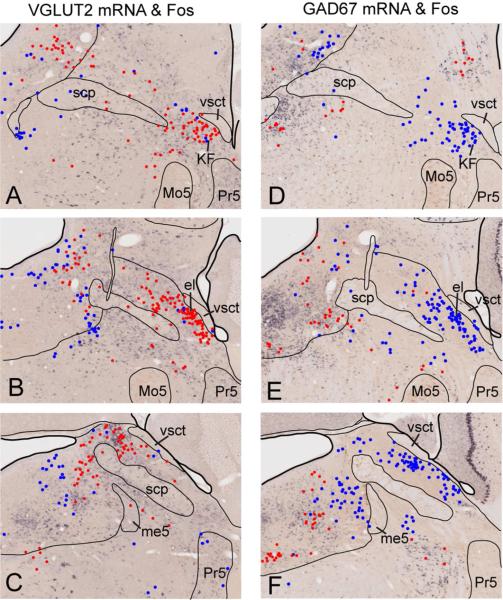
Parabrachial neurons that express c-Fos following hypercapnia are glutamatergic but not GABAergic. All dots represent c-Fos-ir neurons. Red are double-labeled for c-Fos and digoxygenin-tagged vGluT2 (A-C, rostral to caudal, representing glutamatergic neurons) or GAD67 (D-F, rostral to caudal, representing GABAergic neurons). Note that nearly all of the c-Fos-ir neurons in the PB also contain vGluT2 mRNA. Abbreviations: cl: central lateral; cr: lateral crescent; dl: dorsal lateral; el: external lateral; KF: Kölliker Fuse, LC: locus coeruleus, m: medial, me5: mesencephalic trigeminal tract, Mo5: motor trigeminal, Pr5: principal sensory trigeminal, scp: superior cerebellar peduncle, sl: superior lateral, vl: ventral lateral, vsct: ventral spinocerebellar tract
Figure 6.
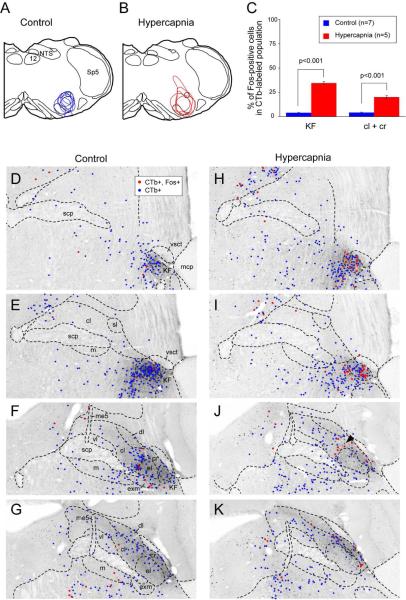
Distribution of hypercapnia-responsive, ventrolateral medullary-projecting parabrachial neurons. Line drawings depict CTb injection sites for the seven mice exposed to room air (control, A) and five hypercapnia-exposed mice (B). Dots show the distribution of retrogradely-labeled parabrachial neurons in one representative control (D-G) and hypercapnia treated (H-K) mouse. Blue dots illustrate neurons that were labeled only for CTb and red dots show neurons that were labeled for CTb- and c-Fos-immunoreactivity. Note the thin layer of doubly-labeled PBcr neurons in Fig. 6 J and K. The small arrowhead in panel J shows the double-labeled neurons that we take as PBcr neurons intruding into PBcl. Abbreviations: cl: central lateral; cr: lateral crescent; dl: dorsal lateral; el: external lateral; exm: external medial, KF: Kölliker Fuse, LC: locus coeruleus, m: medial, me5: mesencephalic trigeminal tract, Mo5: motor trigeminal, Pr5: principal sensory trigeminal, scp: superior cerebellar peduncle, sl: superior lateral, Sp5: spinal trigeminal nucleus, vl: ventral lateral, vsct: ventral spinocerebellar tract
Figure 9.
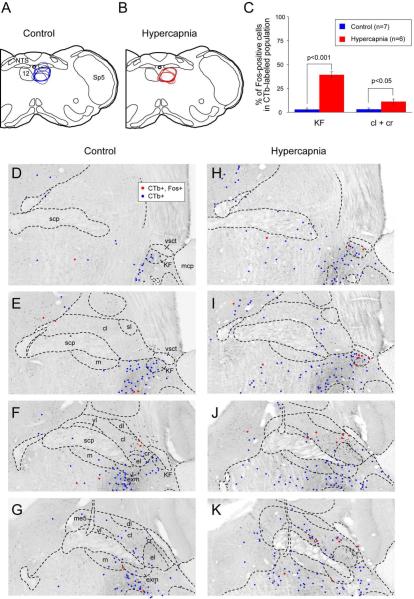
Distribution of hypercapnia-responsive, hypoglossally-projecting parabrachial neurons. Line drawings depict CTb injection sites for the seven control (A) and six hypercapnia-exposed (B) mice. Dots show the distribution of retrogradely-labeled parabrachial neurons in the control (D-G) and hypercapnic (H-K) conditions. Blue dots show neurons that were labeled only for CTb and red dots demonstrate neurons that were labeled for both CTb- and c-Fos-ir. Abbreviations: cl: central lateral; cr: lateral crescent; dl: dorsal lateral; el: external lateral; exm: external medial, KF: Kölliker Fuse, LC: locus coeruleus, m: medial, me5: mesencephalic trigeminal tract, Mo5: motor trigeminal, Pr5: principal sensory trigeminal, scp: superior cerebellar peduncle, sl: superior lateral, Sp5: spinal trigeminal nucleus, vl: ventral lateral, vsct: ventrolateral spinocerebellar tract
Results were expressed as mean +/− SEM. Differences between groups were assessed by one-way ANOVA and groups with p<0.05 were taken as significant.
Results
Fos distribution in the PB following hypercapnia
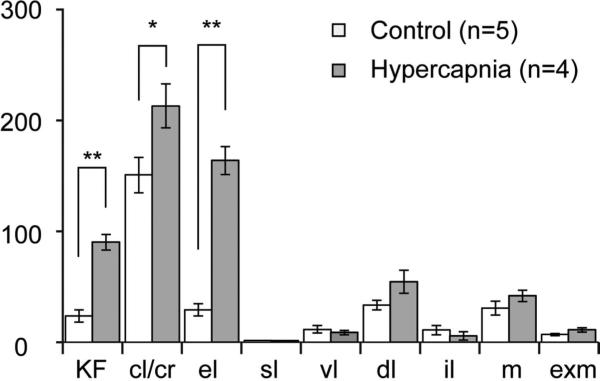
We examined the distribution of c-Fos-ir nuclei in the PB in control animals breathing air, and then compared that with the pattern of c-Fos expression after hypercapnia (Fig. 1). We then quantified the numbers of c-Fos expressing neurons in PB subnuclei. Subnuclear borders were delineated solely on the basis of cytoarchitecture, Under control conditions, c-Fos-ir nuclei appeared sparsely throughout the PB (Fig. 1 A-C). Following hypercapnia we found numerous c-Fos-ir nuclei in the KF, PBcr, PBcl, and PBel subnuclei (Fig. 1D-E). At rostral levels the most prominent group of activated neurons was located in the KF nestled against the medial edge of the ventral spinocerebellar tract (vsct; Fig. 1D). At mid-PB levels just caudal to the KF, c-Fos immunoreactivity was seen in the lateral part of PBel and in the PBcr just lateral to it. c-Fos-ir neurons extended medially over the surface of the PBel into the PBcl subnucleus (Fig 1E small arrowhead). Counts of c-Fos-ir neurons verified the observations of increased labeling following hypercapnia in the KF and in the PBel and PBcr/cl (Fig. 2).
Figure 2.
Mean numbers of c-Fos-ir neurons in the PB of mice breathing normal air or hypercapnic air (10% CO2). The gray bars illustrate the average numbers of c-Fos-ir neurons in each PB subnucleus (+/− SEM). **; P<0.001, *; P<0.05. Abbreviations: cl: central lateral; cr: lateral crescent; dl: dorsal lateral; el: external lateral; exm: external medial, il: internal lateral KF: Kölliker Fuse, m: medial, sl: superior lateral, vl: ventral lateral.
Neurochemical phenotype of hypercapnic-responsive PB neurons
Because most PB neurons contain either glutamate or GABA, we determined whether PB neurons responding to hypercapnia contained these neurotransmitters. Tissue from two groups of mice exposed to hypercapnia was labeled for vGluT2 (n=4) or GAD67 (n=4) mRNA in combination with c-Fos immunoreactivity (Figs. 3, 4). Nearly every c-Fos-ir neuron also contained vGluT2 mRNA; 1255 out of the 1353 (93%) PB cells exhibiting c-Fosimmunoreactivity following hypercapnia also expressed vGluT2 mRNA. By contrast, only a few PB cells (3%; 35 out of 1360 cells) exhibiting c-Fos immunoreactivity also expressed GAD67 mRNA (n=4 mice; 6 sections in each mouse).
Figure 4.
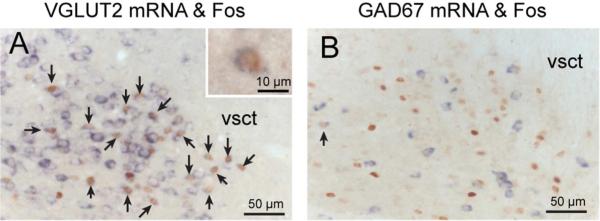
High magnification photomicrographs showing KF neurons responsive to hypercapnia are glutamatergic. Tissue from mice previously exposed to hypercapnia was labeled for c-Fos-immunoreactivity (brown) and vGluT2 mRNA (purple, left) or GAD67 mRNA (purple, right). Arrows denote double-labeled neurons. Essentially all of the neurons with a brown c-Fos-ir nucleus on the left are surrounded by a rim of purple VGluT2 mRNA, and none by GAD67 mRNA. Abbreviations: vsct: ventral spinocerebellar tract
Following hypercapnia, many c-Fos-ir neurons straddled the border between the outer portion of the PBel and the PBcr (large arrowhead, Fig. 1E). To determine which cell group these neurons belonged to, tissue sections from two hypercapnia-exposed mice were doubly stained immunohistochemically with antibody to CGRP (which is found in most PBel neurons) in combination with c-Fos antibody (Fig. 5). In each case, c-Fos labeling was seen in both CGRP-ir and CGRP-negative neurons, indicating that neurons of both types were CO2− responsive. Most of the c-Fos-ir cells that were also CGRP-ir were located in the PBel and most of the CGRP-negative ones in a thin layer beyond the PBel (Fig. 5), but there was a mixture of both types along the outer edge of the PBel (i.e., they were not cleanly separated anatomically).
Figure 5.
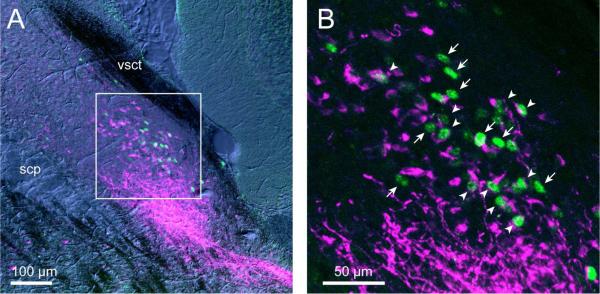
c-Fos-ir neurons after hypercapnia included both CGRP- and non-CGRP-ir populations. Low (A) and High (B) magnification confocal photomicrographs depict c-Fos-ir nuclei (green) and CGRP-ir (magenta) cell bodies of neurons in the PBel and adjacent PBcr subnuclei. Arrowheads indicate doubly labeled neurons (green nucleus and magenta cytoplasm) of the PBel subnucleus, whereas arrows show neurons with only a green nucleus, which are not CGRP-ir, and presumably belong to the PBcr group. Note that more of the doubly labeled neurons are located within the bulk of the CGRP-ir external lateral nucleus, and that most of the singly labeled c-Fos-ir nuclei are more superficially located along the surface of the CGRP-ir cell group, but that the two also show considerable overlap. Abbreviations: CGRP: calcitonin gene-related peptide; scp: superior cerebellar peduncle, vsct: ventral spinocerebellar tract.
Projections of hypercapnia-responsive PB neurons
Ventrolateral medulla
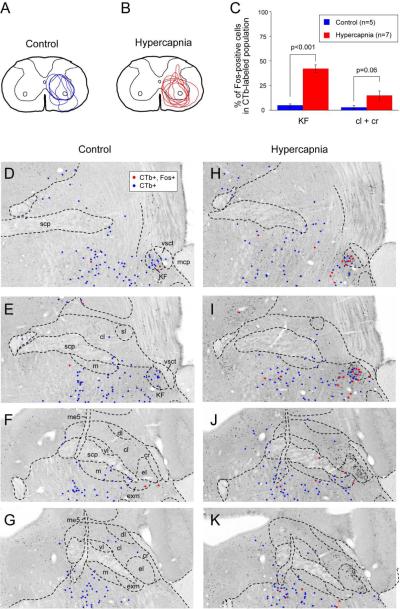
To determine whether PB neurons projecting to the ventrolateral medulla expressed c-Fos following hypercapnia exposure we combined retrograde tracing with c-Fos immunohistochemistry (n=12). Following injections of CTb into the ventrolateral medulla at the level where the NTS just touches the floor of the fourth ventricle rostral to the obex, retrogradely labeled neurons were found mainly in the KF, PBcl, PBcr, and medial PB subnuclei in a pattern similar to that previously described in rats (Fig. 6); (Herbert et al., 1990; Chamberlin and Saper, 1992). Following hypercapnia, 31-40% (out of a total of 110+/−12; n=5) of retrogradely labeled neurons in the KF were also c-Fos-ir indicating that hypercapnia activated neurons projecting to the ventrolateral medulla (Fig. 6, 7A-C). By contrast in controls, only 2-6% (out of a total of 140+/−14; n=7) neurons in the KF were double-labeled. In addition to the KF, doubly-labeled neurons were located in the PBcr area and wrapped around the medial edge of the PBel into the PBcl (Fig. 6 J, K). There were no doubly-labeled neurons in the PBcl subnucleus aside from this group just medial to PBel. Based on these observations, we consider this group of doubly-labeled neurons to be an extension of the PBcr (see Discussion), and within the PBcr defined in this way, 32.9+/−2.85% (n=5) of retrogradely labeled neurons were c-Fos-ir.
Figure 7.
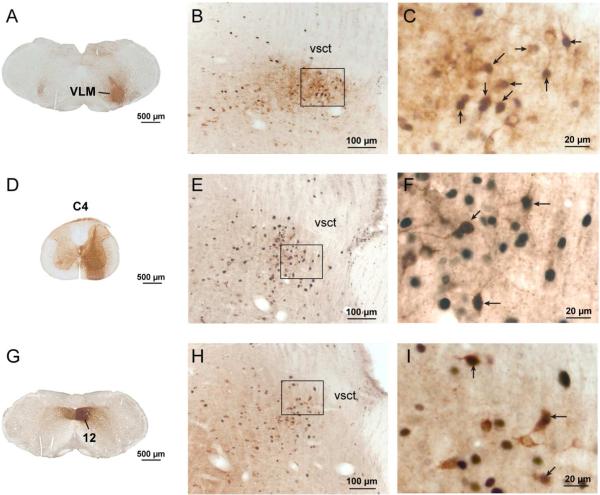
A series of photomicrographs illustrating the projections of the Kolliker-Fuse nucleus to respiratory-related sites. Panels A-C show c-Fos-activation with CO2 exposure in the projection to the ventrolateral medulla; D-F show projections to the phrenic motor level of the spinal cord; and G-I demonstrate projections to the hypoglossal motor nucleus. Injection sites into the brain (A, G) or spinal cord (D) of animals exposed to CO2 are shown on the left. The middle panels show a low magnification photomicrograph through the KF nucleus (B, E, H), and a higher magnification image on the right (C, F, I) showing the area in the box in B, E, H from a hypercapnic animal in each series. Note the large percentage of doubly-labeled neurons (indicated by arrows, brown cytoplasm demonstrating CTb, black nucleus stained for c-Fos) in each case. Few if any doubly labeled neurons were seen in control brains (Figs 6, 8, 9). Abbreviations: C4, fourth cervical spinal segment; VLM: ventrolateral medulla; 12 hypoglossal motor nucleus; vsct: ventral spinocerebellar tract.
Phrenic motor nucleus
Spinal cord injections (n=12) of CTb were made into the ventral horn of the spinal cord at the C3-C5 level where the phrenic motor neurons are located (Qiu et al., 2010)(Figs. 7D-F, 8). Within the PB, retrogradely labeled neurons were located mainly in the KF (Fig. 8) as is the case in rats (Fulwiler and Saper, 1984). Following hypercapnia 31-63 % (out of a total of 27+/−3; n=7) of spinally projecting KF neurons were c-Fos-ir vs. 3-11% out of a total of 27 +/− 4; n=5) in control cases.
Figure 8.
Distribution of hypercapnia-responsive, spinally-projecting parabrachial neurons. Line drawings depict CTb injection sites for the five control (A) and seven hypercapnia-exposed (B) mice. Dots show the distribution of retrogradely-labeled parabrachial neurons in the control (D-G) and hypercapnic (H-K) conditions. Blue dots illustrate neurons that were labeled only for CTb and red dots show neurons that were labeled for both CTb- and c-Fos-ir. Abbreviations: cl: central lateral; cr: lateral crescent; dl: dorsal lateral; el: external lateral; exm: external medial, KF: Kölliker Fuse, LC: locus coeruleus, m: medial, me5: mesencephalic trigeminal tract, Mo5: motor trigeminal, Pr5: principal sensory trigeminal, scp: superior cerebellar peduncle, sl: superior lateral, vl: ventral lateral, vsct: ventral spinocerebellar tract
Hypoglossal motor nucleus
Injections of CTb were centered in the hypoglossal motor nucleus (n=13) with virtually no spread dorsally into the dorsal motor vagal nucleus or the NTS (Fig. 7 G-I; 9). Retrogradely labeled neurons were prominent in the region just lateral to the injection site, an area known to contain hypoglossal premotor neurons (Chamberlin et al., 2007). Within the PB, retrogradely labeled neurons were located primarily in the KF but also in the PBcr and the PBcl (although the latter were again mainly along the margin of the PBel, which we interpret as an extension of the PBcr group; see Fig. 9). Hypercapnia produced significant increases in double-labeled neurons in both the KF and PBcr/cl (Fig. 9C). The percentage of hypoglossally-projecting KF neurons that displayed c-Fos following hypercapnia ranged from 30-53% (out of a total of 27+/−3; n=6). In air-breathing control mice 0-1 neurons out of a total of 19 +/− 3 (n=7) retrogradely-labeled neurons were also c-Fos-ir.
Discussion
Our findings demonstrate that many glutamatergic neurons in the PBcr and KF that project to respiratory motor and premotor regions are activated by hypercapnia in mice. These data identify for the first time the subnuclear distribution of PB neurons that show increases in c-Fos expression in animals exposed to hypercapnia (Teppema et al., 1997; Berquin et al., 2000), as well as demonstrate the respiratory targets of those neurons and showing that virtually all of them are glutamatergic (Yokota et al., 2004; Yokota et al., 2007; Yokota et al., 2011). It is likely that these pathways contribute importantly to the respiratory motor responses to hypercapnia (Mizusawa et al., 1995), such as occur in obstructive sleep apnea. In addition, because none of these pathways has been studied previously in mice, this work identifies the locations, neurotransmitters, respiratory-related activity, and connections of the KF and PBcr subnuclei in mice, and provides a basis for future work on the role of genetically targeted PB and KF neurons in respiratory control.
Technical Considerations
c-Fos has been well-validated as a marker for many types of activated neurons following stimuli of sufficient duration and magnitude (Sagar and Sharp, 1993). However, there are some limitations that should be considered in data interpretation. First, absence of c-Fos cannot be taken as absence of activity as not all activated neurons during a specific physiological response may express c-Fos. Second, c-Fos-ir neurons in our experiments may have responded to other stimuli that co-vary with hypercapnia. For example, PB neurons in the PBel-outer subdivision respond to raised blood pressure (Miller et al., 2012). As hypercapnia is typically accompanied by hypertension, our data do not allow us to distinguish between responses driven by hypercapnia and by subsequent responses such as hypertension. Nevertheless, the c-Fos-ir neurons during hypercapnic stimulation provide a starting point for understanding the physiological responses that are seen during hypercapnia. By combining this with retrogradely labeling from sites involved with respiratory control, we can identify candidate pathways by which elevated CO2 invokes respiratory responses, which can then be studied by genetically-targeted manipulations in mice.
Identification of PB subnuclei
In this study we defined the borders of PB subnuclei in mice by cytoarchitecture, consistent with earlier reports (see Kaur et al., 2013). Because the PBcr cannot be distinguished from the PBel or PBcl by cytoarchitectonic criteria alone, we used connectional, chemoarchitectonic, and functional information as well to deduce the subnuclear location of hypercapnia-activated PB neurons. In rats, the PBcr neurons project to the ventrolateral medulla, whereas the PBel neurons project to the amygdala; the combination of retrograde transport from these two targets clearly distinguishes the two cell populations (Chamberlin and Saper, 1992). In mice, the PBcr and PBel can also be visualized by tracing from the ventrolateral medulla and central nucleus of the amygdala, respectively. Neurons retrogradely labeled from the ventrolateral medulla can be followed along the lateral margin of the PBel, into the PBcl (Fig. 6). In both rats and mice ventrolateral medullary projecting neurons can be found throughout the mediolateral extent of PBcl. By contrast, the distribution of those medullary projecting neurons that are hypercapnia activated (c-Fos positive) is confined to the portion of PBcl just adjacent to PBel (Fig. 6J; red dots; small arrowhead). Therefore we conclude that these neurons belong to PBcr. Recent work has demonstrated a distinct group of neurons in rats that express the transcription factors FoxP2 and Lmx1b and that have a distribution identical with the pattern we have established for the PBcr + KF (Miller et al., 2012), i.e. extending from the KF dorsally into the lateral PB along the margins of PBel. It would be interesting to know whether the PBcr in mice would be defined by neurons expressing these same two transcription factors.
Sources of excitatory drive to PB hypercapnia-responsive neurons
Although there are no published data to indicate whether PB neurons are directly CO2 or acid sensitive, our preliminary data using in vitro slices indicate that PB neurons do not respond to either (Arrigoni and Chamberlin, unpublished observations). Therefore it is likely that PB neurons are driven by other CO2 sensitive neurons. There are several pathways that could contribute to PB activation following stimulation of peripheral and central CO2 chemoreceptors. Afferents from peripheral CO2 chemoreceptors in the carotid body terminate in the dorsolateral and caudal commissural parts of the NTS (Panneton and Loewy, 1980; Finley and Katz, 1992), which in turn project to the lateral PB and KF (Herbert et al., 1990). Neurons along the ventral medullary surface in the retrotrapezoid nucleus are CO2 sensitive, also receive excitatory inputs from the CO2-sensitive parts of the NTS (Takakura et al., 2006), and project to the PBel, PBcl, PBcr, and KF (Rosin et al., 2006; Bochorishvili et al., 2012). Finally, the PB may receive CO2 chemoreceptor information from medullary serotonin neurons or lateral hypothalamic orexin neurons, both of which are also directly CO2 sensitive (Wang et al., 2001; Rosin et al., 2006; Williams et al., 2007) and project to the lateral PB and KF.
Functional roles of hypercapnia-responsive PB neurons
Our previous work demonstrated that one function of hypercapnia-activated PB neurons is likely to be causing the arousal that occurs when breathing is compromised during sleep such as in obstructive sleep apnea. We found that deletion of vGluT2 from neurons in the PBel and adjacent PBcr attenuated the EEG arousal to hypercapnia (Kaur et al., 2013). We did not detect any changes in ventilatory responses to hypercapnia in those experiments, although they were not designed to examine this question specifically. The rapidly rising CO2 levels in those experiments awakened the control mice in 10-15 sec., leaving us too little time asleep with CO2 to examine the ventilatory response. We also did not have sufficient experiments involving the KF to examine the role of that subnucleus in driving ventilation. It will be important in future studies to examine more thoroughly the role of the KF and PBcr vs. PBel in ventilatory responses using more cell-type specific interventions.
Pathways mediating effects of hypercapnic-responsive PB neurons
Our data suggest that when PBcr and KF neurons are engaged by hypercapnia, they contribute to activation of ventrolateral medullary respiratory pattern generator neurons as well as phrenic and hypoglossal motor neurons. Because the hypercapnia-responsive neurons in the PB that project to the ventrolateral medulla and hypoglossal and phrenic motor nuclei are glutamatergic, they would be expected to increase the firing frequency of their target neurons. Activation of neurons in the rostral ventral respiratory group increases breathing rate and depth (McCrimmon et al., 1986) while activation of phrenic motor neurons would cause stronger diaphragmatic contraction that would increase negative intrathoracic pressure and the rate of air flow in the upper airway. This would tend to collapse the airway, but the activation of hypoglossal inputs to the genioglossus muscle would protrude the tongue, thus stiffening and dilating the oropharyngeal airway to counteract the collapsing force of negative airway pressure. Therefore, the glutamatergic, hypercapnia responsive PB neurons that we have identified would be expected to increase both respiratory rate and tidal volume while keeping the airway open, all reflexes that are critical in conditions of asphyxia, particularly in patients who suffer from sleep apnea.
By contrast to hypercapnia-activated PBcr and KF neurons which have brainstem targets, PBel neurons that respond to hypercapnia drive forebrain targets, including the lateral hypothalamus, substantia innominata, central nucleus of the amygdala, and bed nucleus of the stria terminalis (Schwaber et al., 1988; Yasui et al., 1989; Bernard et al., 1993; Kaur et al., 2013). One or more of these regions is likely to mediate hypercapnic EEG arousal. It will be important in future work to take advantage of genetic differences in different PB neurons to selectively disrupt PBel vs PBcr and KF neurons, structures that lie cheek-byjowl, in the parabrachial complex (Chamberlin and Saper, 1992) as well as to determine both the forebrain and brainstem sites activated by the PB during hypercapnia, and their interactions in supporting arousals that re-establish adequate ventilation.
Acknowledgments
This work was supported by grants from the National Institute of Health (Bethesda, MD, USA): P01 HL095491, NS079623
Footnotes
We have no conflicts of interest to report.
Authors made the following contributions to the work:
Shigefumi Yokota, Veronique VanderHorst and Satvinder Kaur performed experiments. Shigefumi Yokota and Nancy Chamberlin analysed and interpreted data. Nancy Chamberlin and Clifford Saper obtained funding, contributed to the conception and design of the project, to data analysis, interpretation and writing the manuscript. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: A phaseolus vulgaris Leucoagglutinin (PHA-L) study in the rat. J Comp Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- Berquin P, Bodineau L, Gros F, Larnicol N. Brainstem and hypothalamic areas involved in respiratory chemoreflexes: a Fos study in adult rats. Brain Res. 2000;857(1-2):30–40. doi: 10.1016/s0006-8993(99)02304-5. [DOI] [PubMed] [Google Scholar]
- Bochorishvili G, Stornetta RL, Coates MB, Guyenet PG. Pre-Botzinger complex receives glutamatergic innervation from galaninergic and other retrotrapezoid nucleus neurons. J Comp Neurol. 2012;520(5):1047–1061. doi: 10.1002/cne.22769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL. Functional organization of the parabrachial complex and intertrigeminal region in the control of breathing. Respir Physiol Neurobiol. 2004;143(2-3):115–125. doi: 10.1016/j.resp.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol (Lond) 2007;579(Pt 2):515–526. doi: 10.1113/jphysiol.2006.121889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of cardiovascular responses to electrical and glutamate microstimulation of the parabrachial nucleus in the rat. J Comp Neurol. 1992;326(2):245–262. doi: 10.1002/cne.903260207. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Dick TE. Pontine mechanisms of respiratory control. Compr Physiol. 2012;2(4):2443–2469. doi: 10.1002/cphy.c100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7(1):91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. Distribution and medullary projection of respiratory neurons in the dorsolateral pons of the rat. Neuroscience. 2006;141(2):1011–1023. doi: 10.1016/j.neuroscience.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Finley JCW, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992;572(1–2):108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res Rev. 1984;7:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293(4):540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, Chamberlin NL, Saper CB. Glutamatergic Signaling from the Parabrachial Nucleus Plays a Critical Role in Hypercapnic Arousal. The Journal of Neuroscience. 2013;33(18):7627–7640. doi: 10.1523/JNEUROSCI.0173-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20(10):3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Feldman JL, Speck DF. Respiratory motoneuronal activity is altered by injections of picomoles of glutamate into cat brain stem. J Neurosci. 1986;6(8):2384–2392. doi: 10.1523/JNEUROSCI.06-08-02384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL, Knuepfer MM, Wang MH, Denny GO, Gray PA, Loewy AD. Fos-activation of FoxP2 and Lmx1b neurons in the parabrachial nucleus evoked by hypotension and hypertension in conscious rats. Neuroscience. 2012;218:110–125. doi: 10.1016/j.neuroscience.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Shirato K. Role of parabrachial nucleus in ventilatory responses of awake rats. J Physiol (Lond) 1995;489:877–884. doi: 10.1113/jphysiol.1995.sp021100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneton WM, Loewy AD. Projections of the carotid sinus nerve to the nucleus of the solitary tract in the cat. Brain Res. 1980;191(1):239–244. doi: 10.1016/0006-8993(80)90326-1. [DOI] [PubMed] [Google Scholar]
- Qiu K, Lane MA, Lee KZ, Reier PJ, Fuller DD. The phrenic motor nucleus in the adult mouse. Exp Neurol. 2010;226(1):254–258. doi: 10.1016/j.expneurol.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;1553:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499(1):64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR. Early response genes as markers of neuronal activity and growth factor action. Adv Neurol. 1993;59:273–284. [PubMed] [Google Scholar]
- Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, Saper CB. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 2000;20(22):8620–8628. doi: 10.1523/JNEUROSCI.20-22-08620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaber JS, Sternini C, Brecha NC, Rogers WT, Card JP. Neurons containing calcitonin gene-related peptide in the parabrachial nucleus project to the central nucleus of the amygdala. J Comp Neurol. 1988;270(3):416–426. 398–419. doi: 10.1002/cne.902700310. [DOI] [PubMed] [Google Scholar]
- Song G, Poon CS. Lateral parabrachial nucleus mediates shortening of expiration and increase of inspiratory drive during hypercapnia. Respir Physiol Neurobiol. 2009;165(1):9–12. doi: 10.1016/j.resp.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol (Lond) 2006;572(Pt 2):503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388(2):169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell metabolism. 2007;5(5):383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderHorst VG, Ulfhake B. The organization of the brainstem and spinal cord of the mouse: relationships between monoaminergic, cholinergic, and spinal projection systems. J Chem Neuroanat. 2006;31(1):2–36. doi: 10.1016/j.jchemneu.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Wang H, Wei F, Dubner R, Ren K. Selective distribution and function of primary afferent nociceptive inputs from deep muscle tissue to the brainstem trigeminal transition zone. The Journal of Comparative Neurology. 2006;498(3):390–402. doi: 10.1002/cne.21062. [DOI] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-Stimulated Neurons of the Medullary Raphe Are Serotonergic. J Neurophysiol. 2001;85(5):2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2 Proceedings of the National Academy of Sciences. 2007;104(25):10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, Saper CB, Cechetto DF. Calcitonin gene-related peptide immunoreactivity in the visceral sensory cortex, thalamus, and related pathways in the rat. J Comp Neurol. 1989;290(4):487–501. doi: 10.1002/cne.902900404. [DOI] [PubMed] [Google Scholar]
- Yokota S, Niu JG, Tsumori T, Oka T, Yasui Y. Glutamatergic Kolliker-Fuse nucleus neurons innervate hypoglossal motoneurons whose axons form the medial (protruder) branch of the hypoglossal nerve in the rat. Brain Res. 2011;1404:10–20. doi: 10.1016/j.brainres.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Yokota S, Oka T, Tsumori T, Nakamura S, Yasui Y. Glutamatergic neurons in the Kolliker-Fuse nucleus project to the rostral ventral respiratory group and phrenic nucleus: a combined retrograde tracing and in situ hybridization study in the rat. Neurosci Res. 2007;59(3):341–346. doi: 10.1016/j.neures.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Yokota S, Tsumori T, Ono K, Yasui Y. Phrenic motoneurons receive monosynaptic inputs from the Kolliker-Fuse nucleus: a light-and electron-microscopic study in the rat. Brain Res. 2001;888(2):330–335. doi: 10.1016/s0006-8993(00)03106-1. [DOI] [PubMed] [Google Scholar]
- Yokota S, Tsumori T, Ono K, Yasui Y. Glutamatergic pathways from the Kölliker-Fuse nucleus to the phrenic nucleus in the rat. Brain Res. 2004;995(1):118–130. doi: 10.1016/j.brainres.2003.09.067. [DOI] [PubMed] [Google Scholar]