Abstract
Objective
There is growing evidence that cerebellum plays a crucial role in cognition and emotional regulation. Cerebellum is likely to be involved in the physiopathology of both bipolar disorder and schizophrenia. The objective of our study was to compare cerebellar size between patients with bipolar disorder patients with schizophrenia and healthy controls in a multicenter sample. In addition, we studied the influence of psychotic features on cerebellar size in bipolar patients.
Method
One hundred and fifteen bipolar I patients, thirty-two patients with schizophrenia and fifty-two healthy controls underwent 3 Tesla MRI. Automated segmentation of cerebellum was performed using FreeSurfer software. Volumes of cerebellar cortex and white matter were extracted. Analyses of covariance were conducted and age, sex and intracranial volume were considered as covariates.
Results
Bilateral cerebellar cortical volumes were smaller in patients with schizophrenia compared to patients with bipolar I disorder and healthy controls. We found no significant difference of cerebellar volume between bipolar patients with and without psychotic features. No change was evidenced in white matter.
Conclusion
Our results suggest that reduction of cerebellar cortical volume is specific to schizophrenia. Cerebellar dysfunction in bipolar disorder, if present, appears to be more subtle than a reduction in cerebellar volume.
Keywords: Schizophrenia, Bipolar disorder, Psychosis, cerebellum, MRI
INTRODUCTION
Although the role of the cerebellum in motor functioning has been well established, the full range of cerebellar functions remains uncertain. In the human brain, the cerebellum and the prefrontal cortex are the two most developed parts in comparison with higher primates (1). Cerebellum represents 10% of brain volume but contains 50% of its neurons (2). In the past decade, consistent work has been achieved to study the implication of cerebellum in both cognitive and affective functions (3). Indeed, descriptions of the cerebellar cognitive affective syndrome in patients with lesions limited to the cerebellum (4) provide an interesting insight on the role of cerebellum in cognition and emotional regulation. This syndrome includes deficits in executive functions, language disruption, affective disorders and psychotic features. Additionally, the cerebellum is connected to the cerebral cortex by a cortico-cerebellar-thalamic-cortical circuit. This loop facilitates automatic modulation of mood and behavior. Moreover, a damage to the cerebellar part of the loop affects cognitive and emotional processing (5).
Studies of neurological lesions and neuroimaging studies enlightened the potential involvement of the cerebellum in the pathophysiology of psychiatric disorders such as schizophrenia and bipolar disorder (6).
In schizophrenia, the implication of the cerebellum has been studied by several groups (see a full review in (1). Volumetric studies mostly show decreases of cerebellar volume in patients with schizophrenia compared to healthy controls (1). Functional imaging provides further evidence of abnormal activations in patients with schizophrenia, while patients perform a broad range of tasks involving memory, attention, social cognition and emotions (1). Recently, abnormalities in cerebellar connectivity (white matter fractional anisotropy) have been evidenced in first-episode schizophrenia (7). However, most of the studies included small samples and yielded heterogeneous results.
Fewer studies proposed that the cerebellum may also be implicated in the physiopathology of bipolar disorder: most of neuroimaging studies in bipolar disorder focused on the prefrontal and limbic regions (8, 9). However, bipolar disorder is related to cognitive impairments consistent with an alteration of cerebello-striatal-prefrontal network (10). Several studies reported evidence of a cerebellar dysfunction in patients with bipolar disorder. Lauterbach et al., (11) described mania in patients with cerebellar lesions. Most of the studies reported a reduced volume of the cerebellum in bipolar disorder, but with heterogeneous results (12, 13, 14, 15, 16, 17, 18). Hoppenbrouwers et al., (6) emphasize that the implication of the cerebellum in the pathophysiology of bipolar disorder should be taken with caution due to comorbid substance abuse. Indeed, alcohol abuse is very prevalent in bipolar disorder (19) and may therefore induce a confusion bias due to an alcohol-related brain atrophy (20, 21).
Since Kraepelin, it has been proposed that schizophrenia and bipolar disorder may share common pathophysiological features. Whole-brain neuroimaging studies reported an overlap in brain changes in patients with schizophrenia and patients with bipolar disorder (22, 23, 24, 25). They suggested that these changes may be more subtle in bipolar disorder. Finding brain regions specifically altered in schizophrenia or in bipolar disorder may provide insights on specific neuroanatomical substrates of these two disorders. Moreover, both epidemiological and genetic evidences suggest that psychotic bipolar disorder (26) may be considered as an intermediate subtype between schizophrenia and non-psychotic bipolar disorder (27, 28). However, a relatively small number of neuroimaging studies have specifically compared psychotic bipolar patients to non-psychotic to investigate specific differences between these two phenotypes.
Aims of the study
The first objective of our study was thus to compare cerebellar volumes between patients with bipolar I disorder, patients with schizophrenia and healthy subjects. Additionally, we wanted to study, in a second analysis, the influence of psychotic features on cerebellar size in a large international sample of patients with bipolar I disorder.
Material and methods
Participants
Participants were recruited in France (APHP Henri Mondor hospitals, Créteil and Fernand Widal Hospital, France), United States (University of Pittsburgh Medical Center, Pittsburgh) and Germany (Central Institute of Mental Health, Mannheim). Participants were aged from 18 to 65.
All participants underwent clinical assessment by trained raters, using the Diagnostic Interview for Genetic Study (DIGS) at the French sites or the Structured Clinical Interview (SCID) for DSM-IV at US and German sites. The DIGS has been translated and well validated in French (29). In all sites, diagnoses were defined in accordance with DSM-IV classification.
Healthy controls were recruited through advertisements in the local press, had no DSM-IV axis-I psychiatric disorder and no first-degree family history of schizophrenia, schizo-affective or bipolar disorder.
Patients with bipolar disorder met DSM-IV criteria for bipolar type I disorder (BPI) and were recruited in France, Germany and United States. DIGS and SCID were used to determine if they had psychotic features during a major episode.
Patients with schizophrenia met DSM-IV criteria for schizophrenia or schizo-affective disorder and were recruited at the French sites. Schizo-affective disorder is considered as part of the schizophrenia spectrum disorder. Although the clinical entity and the diagnosis of schizo-affective disorder remains controversial (30, 31), we choose to include patients with schizophrenia and patients with schizo-affective disorder in the same group.
Subjects were not included if MRI was contraindicated or if pregnant
All participants gave informed and written consent to participation. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and the respective local ethics committees approved the protocol. Patients with BPI and controls partially overlap with the sample of a manuscript about diffusion MRI in bipolar disorder (32).
After MRI acquisition, subjects with any significant cerebral anatomic anomaly were excluded
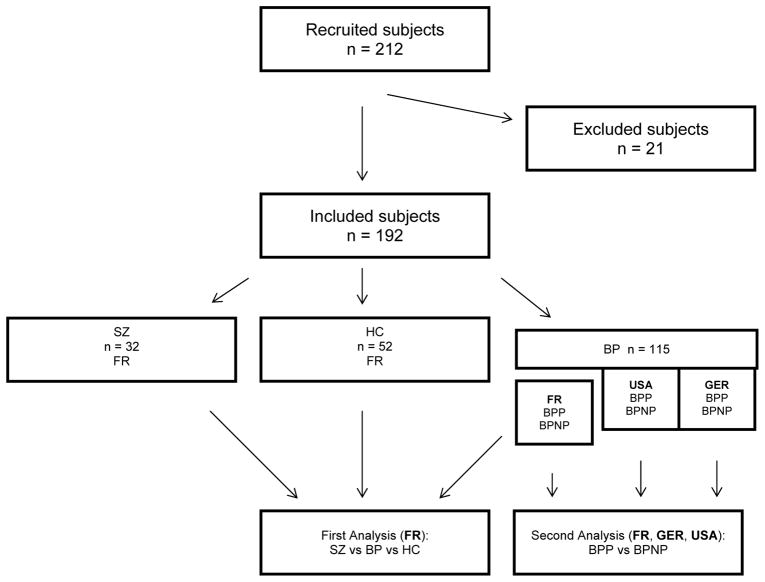
Two hundred and twenty subjects were recruited. One hundred and ninety-nine subjects were included in the statistical analysis and 21 participants were excluded (Figure 1). Ten subjects (3 healthy controls, 4 patients with schizophrenia, 3 patients with BPI) were excluded because the cerebellum was not entirely scanned during the acquisition. One subject was excluded because there was an error during segmentation process. Seven BPI patients recruited at the French sites were excluded because information relative to the “psychotic feature” was missing. Three subjects were excluded because they were statistical outliers. Outliers were defined as cases with standardized residuals greater than +/− 4 standard deviations and were not included in the analysis.
Figure 1.
Flow-chart
Abreviations:
FR, Subjects recruited in France; GER, Subjects recruited in Germany; USA, Subjects recruited in the USA
SZ, patients with schizophrenia; BP, patients with bipolar disorder; BPP, bipolar patients with psychotic features;
BPNP, bipolar patients without psychotic features
One hundred and twelve subjects were included in France, 47 subjects were included in USA and 40 in Germany. Population of the study is presented in Table 1. Five patients, all recruited in France and included in the group of patients with schizophrenia, met the criteria of schizo-affective disorder.
Table 1.
Demographic and clinical characteristics of participants
| Patients with BPI with psychotic features (n = 53) | Patients with BPI without psychotic features (n = 62) | Patients with schizophrenia (n=32)* | Healthy controls (n = 52) | ANOVA (F) Chi-Square Test (χ2) Fisher’s Exact Test (FE) |
||
|---|---|---|---|---|---|---|
| F/χ2 (df) | p - value | |||||
| Mean age (standard deviation) | 35.4 (10.7) | 37.3 (10.6) | 31.4 (10.2) | 37.2 (11.8) | F = 2.45 (3) | 0.065 |
| Male sex (%) | 25 (47) | 26 (42) | 21 (66) | 21 (40) | χ2 = 6.00 (3) | 0.11 |
| Recruitment site (number of subjects) | France (n = 17)a
b USA (n = 24)b Germany (n = 12)b |
France (n = 11)a
b USA (n = 23)b Germany (n =28)b |
France (n = 32)a | France (n = 52)a | - | - |
| Mean age of onset (standard deviation) | 20 (8) | 22 (8) | 22 (5)** | NA | F = 1.08 (2) | 0.34 |
| Use of antipsychotic (%) | 31 (58) | 22 (35) | 32 (100) | NA | χ2= 36.04 (2) | < 0.001 *** |
| Use of lithium (%) | 24 (43) | 18 (29) | 2 (6) | NA | FE | < 0.001 |
| Use of mood stabilizer other than lithium (%) | 23 (43) | 42 (68) | 7 (22)** | NA | FE | 0.001 |
| Use of antidepressant (%) | 21 (40) | 35 (56) | 6 (19)** | NA | FE | 0.016 |
| History of alcohol abuse or dependence (%) | 14 (26) | 9 (15) | 0 (0) | NA | FE | 0.006 |
Percentages have been rounded and may not total 100.
Abbreviations: BPI, bipolar I patients; df, degrees of freedom
Including 5 patients with schizo-affective disorder
Number of missing data for patients with schizophrenia: Mean age of onset: 7; Use of mood stabilizer: 7; Use of antidepressant: 7; patients with schizophrenia were not included in supplemental analyses on medication effects.
Post-hoc Tukey test was performed after the Chi-Square test: Patients with schizophrenia were significantly using more antipsychotics than BPI with psychotic features and BPI without psychotic features.
Patients included in the first analysis (n = 112): comparison between bipolar patients (regardless of psychotic features), patients with schizophrenia and healthy controls.
Patients included in the second analysis (n = 115): comparison between bipolar patients with psychotic features and bipolar patients without psychotic features
Magnetic Resonance Imaging acquisition
All imaging data were acquired using very similar protocols in the three sites in France, Germany and the United States.
Participants underwent an MRI in the same model of scanner: 3T Siemens Tim Trio equipped with a standard 12-channel head coil. MR data were obtained using a 3DT1-weighted sequence (voxel size = 1×1mm, slice thickness = 1 or 1.1mm, TR = 2300 ms, FOV = 256mm).
Magnetic Resonance Imaging processing
T1 images from the 3 sites were processed on the same Linux workstation. All images were inspected by a radiologist to seek for clinically relevant abnormalities. BrainVISA/Anatomist software (http://brainvisa.info) was used by an examiner blind of the diagnosis to check more specifically the posterior fossa (CL and JH). Four patients presented cysts of posterior fossa with no clinically relevant cerebellar atrophy. Three were recruited in France (healthy controls) and one (patient with schizophrenia) was recruited in Germany. All four patients were included in the analysis. Results were not significantly altered when they were excluded.
We used the FreeSurfer 5.3.0 software free package (http://surfer.nmr.mgh.harvard.edu) on a Linux Workstation for segmentation and volumetric analysis of the cerebellum (Figure 2).
Figure 2.
Fully automated cerebellar segmentation with FreeSurfer - Sagittal and Coronal views
The automated procedure for volumetric measures of subcortical structures and cerebellum has been previously described (33). All images were transformed into Talairach space, corrected for inhomogeneities. FreeSurfer automatically provides segments and labels for brain structures and assigns a neuroanatomic label to each voxel in an MRI on the basis of probabilistic information estimated from manually labeled training set.
This technique, performing segmentation and labeling of brain structures was reported to be as effective as manual tracing (33). It has been successfully used to perform cerebellar parcellation in samples of patients with neurological (34, 35, 36, 37) and psychiatric disorders.
Although FreeSurfer automatically performs full segmentation of all sub-cortical structures, only four labels were considered: right and left gray and white matter cerebellar volumes.
In addition to the recommended segmentation check proposed by FreeSurfer, an evaluator unaware of diagnosis checked visually all segmented images to ensure that there was no error in the label process. Intracranial volumes (ICV) were evaluated with Statistical Parametric Mapping (SPM 8) software using the “New segment” procedure.
Statistical analyses
Chi-2 tests, Fisher’s exact tests (for categorical variables) and ANOVA (for quantitative variables) were used to compare both demographic and clinical variables of our sample (Table 1). Post-hoc Tukey tests were performed to compare groups of subjects. Univariate analyses of covariance (ANCOVA) were used to compare cerebellar cortex and white matter volumes (left/right) between the different groups.
We performed two main analyses. The first one (ANCOVA) compared cerebellar volumes between healthy controls, patients with BPI and patients with schizophrenia (subjects included at the French site). The second analysis (mixed-model ANCOVA) compared cerebellar volumes between BPI patients with psychotic features and BPI patients without psychotic features from a larger international sample of patients recruited in France, Germany and the United States.
We performed these two separate analyses in order to study (i) cerebellar size differences between patients with schizophrenia, BPI patients and healthy controls and (ii) the influence of psychotic features among BPI patients on cerebellar size. To answer these two questions we used two different samples (although partially overlapping). Since patients with schizophrenia were only recruited in France, we were not able to compare all subjects in the same analysis because disease-effect (for patients with schizophrenia) could interfere with site-effect. Comparing BPI patients with and without psychotic features to healthy controls could lead to the double dipping of our data, because the French group of healthy controls would have been used in both analyses.
In both analyses, age, sex and ICV (fixed effects) were considered as covariates. The site of inclusion (random effect) was included in the second analysis. Before performing pairwise comparisons between patients and controls in both analyses, the following assumptions were checked: (i) standardized residuals were normally distributed, as assessed by Shapiro-Wilk test (p>0,05) and a QQ-plot; (ii) homoscedasticity and homogeneity of variances, as assessed by visual inspection of a scatterplot and Levene’s Test of Homogeneity of Variance. Outliers were defined as cases with standardized residuals greater than +/− 4 standard deviations and were not included in the analysis.
We investigated in a supplementary analysis if alcohol abuse, ICV and age of onset (AOO) were potential confounders in patients with BPI. Considering that the mean AOO of bipolar disorder is estimated to be of 21 (38), early-onset BPI patients were defined as patients with an AOO of 21 or below, and late-onset BPI patients were defined as patients with an AOO of 22 or above.
To ensure the robustness of our results, we wanted to study the influence of medication on cerebellar size. Considering our sample of patients with BPI, we performed an ANCOVA using sex, ICV, age and site as covariates to compare (i) patients with and without antipsychotic medication, and (ii) patients with and without lithium medication. Last, we explored the relationship between cerebellar size and medication load using a partial correlation analysis controlled for age, sex, ICV and site. The medication load score was computed following a method described elsewhere (39).
All statistical analyses were performed using SPSS 20.0. Interpretations of statistical significance were made if p < 0.05 after Bonferroni correction for multiple testing was applied.
RESULTS
Demographic and clinical characteristics
Considering the following groups: healthy controls, BPI patients without psychotic features, BPI patients with psychotic features and patients with schizophrenia, there was no significant statistical difference between mean ages and sex ratio in these groups. Others demographic and clinical characteristics are described and compared in Table 1.
Cerebellar volume comparison between healthy subjects, BPI patients and patients with schizophrenia
In this first analysis, we considered patients recruited in France (n = 112). We compared healthy controls (n = 52), patients with BPI (n = 28), regardless of psychotic features, and patients with schizophrenia (n = 32).
We found a significant difference between the three groups for left and right cerebellar cortical volumes and no significant difference in white matter volumes (Table 2).
Table 2.
Estimated means (mm3) of cerebellar volume in patients with schizophrenia, patients with bipolar I disorder and healthy controls. (Subjects recruited at the French site)
| Structure | Side | SZ | BPI | HS | ANCOVA | ||
|---|---|---|---|---|---|---|---|
| M (CI 95%) | M (CI 95%) | M (CI 95%) | p | df | Effect-size for diagnosis, sex, age, ICV (Eta-squared effect size) | ||
| Cerebellar Cortex (mm3) | Left | 47616 (46004 – 49229) | 51614 (49816 – 53412) | 50678 (49470 – 51885) | 0.003* | 2 | 0.11; 0.21; 0.34; 0.001 |
| Right | 49518 (47817 – 51219) | 53136 (51240 – 55032) | 52471 (51198 – 53744) | 0.009* | 2 | 0.09; 0.22; 0.27; 0.001 | |
| Cerebellar White Matter (mm3) | Left | 14846 (14251 – 15442) | 15548 (14885 – 16212) | 15590 (15145 – 16036) | 0.131 | 2 | 0.03; 0.17; 0.10; 0.002 |
| Right | 15379 (14740 – 16019) | 15667 (14954 – 16380) | 15824 (15345 – 16303) | 0.5 | 2 | 0.011; 0.2; 0.06; 0.008 | |
SZ, patients with schziophrenia; BPI, patients with bipolar I disorder; HS, healthy subjects
Df, degrees of freedom
M, mean (mm3); CI, confidence interval; ANCOVA with age, sex and intracranial volume as covariates.
Means are calculated with following covariates: Intracranial volume = 1.526152 and age = 35.164114
p values < 0,05 allowing contrast analyses between the three groups (Table 3)
The contrast analysis for cerebellar right and left cortex yielded significant (Bonferroni corrected) decreases in both volumes for patients with schizophrenia compared to patients with BPI and healthy subjects. Volume of cerebellar cortex and white matter were not statistically different, when comparing healthy controls and BPI patients. Results are summarized in Table 3.
Table 3.
Contrast analyses of cerebellar size when comparing patients with schizophrenia, patients with bipolar I disorder and healthy controls. (Subjects recruited at the French site).
| Structure | Side | SZ vs BPI | SZ vs HC | HC vs BPI |
|---|---|---|---|---|
| p | p | p | ||
| Cerebellar Cortex | Left | 0.005* | 0.011* | n.s. |
| Right | 0.020* | 0.023* | n.s. | |
| Cerebellar White Matter | Left | n.s. | n.s. | n.s. |
| Right | n.s. | n.s. | n.s. | |
SZ, patients with schziophrenia; BPI, patients with bipolar I disorder; HC, healthy controls
n.s.: non significant ANOVA omnibus p values.
p values < 0,05 that retained their significance after Bonferroni correction for multiple comparisons
When considering cerebellar global volume (including left and right cerebellar cortex and white matter), cerebellum was smaller in patients with schizophrenia compared to patients with BPI (p = 0.022) and healthy subjects (p = 0.020) after Bonferroni correction.
Cerebellar volume comparison between bipolar patients without psychotic features and bipolar patients with psychotic features
A second analysis was then performed, including BPI patients with and without psychotic features recruited in France (n = 28), Germany (n = 40) and USA (n = 47). We sought to compare BPI patients with psychotic features (n = 53) and BPI patients without psychotic features (n = 62). We did not find any significant difference between the two groups for right cerebellar cortex (p = 0.48), right cerebellar white matter (p = 0.33), left cerebellar cortex (p = 0.42) and left cerebellar white matter (p = 0.41).
Supplementary analyses: influence of potential confounding factors
We performed the two analyses while removing separately 21 patients with history of alcohol abuse or dependence, 4 patients with cysts of posterior fossa and 5 patients with schizo-affective disorder, which didn’t change our results.
As considering ICV as a covariate remain controversial (40), we performed the analyses without considering ICV. All previously significant results remained significant.
Age of onset did not appear to influence cerebellar volume in BPI patients: we did not find any statistical difference in cerebellar volume (considering age, ICV, sex and site as covariates) when comparing early onset BPI patients to later onset patients.
We performed an ANCOVA considering sex, age, ICV and site as covariates to study the influence of treatments on cerebellar size.
When comparing BPI patients with (n = 42) and without (n = 73) lithium medication, we found no difference in the volume of left cerebellar cortex (p = 0.75), left cerebellar white matter (p = 0.80), right cerebellar cortex (p = 0.94) and right cerebellar white matter (p = 0.61).
When comparing BPI patients with (n = 53) and without (n = 62) antipsychotic treatment, we found no difference in the volume of left cerebellar cortex (p = 0.33), left cerebellar white matter (p = 0.50), right cerebellar cortex (p = 0.18) and right cerebellar white matter (p = 0.68).
Last, we found no correlation (correlation coefficient (CC) calculated with a partial correlation analysis) between medication load (when controlling for age, sex, site and ICV) and the volume of left cerebellar cortex (CC = −0.29; p = 0.76), left cerebellar white matter (CC = −0.61; p =0.52), right cerebellar cortex (CC = −0.24; p = 0.80) and right cerebellar white matter (CC = −0.09; p = 0.34).
DISCUSSION
Our results provide evidence of cortical and global cerebellar volume reductions in patients with schizophrenia compared to patients with BPI and healthy controls. We found no cerebellar volume differences between BPI patients and healthy controls and no influence of psychotic features on cerebellar size among BPI patients, using an automated segmentation process in a relatively large international sample.
We found significant reductions in the cerebellar cortex volume in patients with schizophrenia compared to BPI patients and healthy controls. A wide range of cortical and sub-cortical regions has been implicated in the pathophysiology of schizophrenia. Interestingly, the cerebellum is widely connected to other brain regions found to be reduced in volume in patients with schizophrenia. Such cortical reductions may be related to the malfunction between cerebellum and other brain regions such as prefrontal cortex which are connected through the cortical-cerebellar-thalamic-cortical circuit. This dysconnectivity has been evidenced in structural and functional studies (41, 42).
It is noteworthy that we found a reduction of the cerebellar cortex but not of cerebellar white matter volume in patients with schizophrenia. This result is in accordance with those of neuropathological studies describing a decrease in linear density and decreased size of Purkinje cells in cerebellar cortex in patients with schizophrenia (43, 44). Eastwood et al., (45) provided evidence for synaptic pathology in the cerebellar cortex of patients with schizophrenia. The authors measured the expression of three synaptic proteins distributed in Purkinje and granule cells (synaptophysin, complexin I and complexin II) in the cerebellum of patients with schizophrenia, which was reduced compared to healthy controls. Moreover, in his review Andreasen et al., (1) underlined this evidence of an imbalance in the Purkinje cell inhibitory input to the deep nuclei which could explain cognitive impairment in schizophrenia.
The volume of the cerebellum in patients with schizophrenia has been extensively studied in volumetric MRI studies. Nine studies (46, 47, 48, 49, 50, 51, 41, 52, 53) showed vermal, hemispheric or global cerebellar size reductions in patients with schizophrenia (including first episode patients) compared to healthy controls. Five studies (54, 55, 56, 57, 58) yielded no significant differences whereas one study (59) reported vermal increase in patients with schizophrenia compared to controls. Different reasons could explain these inconsistent findings. Most of the studies used relatively small sample size which reduces the probability that statistically significant results reflect a true effect (60). In addition, the authors often used manual, semi-automated ROI methods or less frequently Voxel-based morphometry (VBM) to assess cerebellar size. Manual and semi-automated segmentation can be applied only on small samples and are dependent on the evaluator. VBM was not originally designed for cerebellum and may be impeded by the surrounding cerebral tissue (35).
Cerebellar modifications have been investigated in bipolar disorder to a lesser extent. One study (17) showed smaller left and right cerebellum in the patient group, four studies showed size differences in the vermal area (12, 14, 15, 18) and one (13) showed no significant volume modification.
To our knowledge, only two studies tried to compare cerebellar size in patients with bipolar disorder and schizophrenia. In both cases, various sub-cortical structures were assessed instead of just focusing on the cerebellum, which has an influence on alpha-risk. Rimol et al., (23) found a decrease of the left and right cerebellar cortex volume when comparing patients with schizophrenia and patients with bipolar disorder with healthy controls. However the influence of history of alcohol abuse or dependence, which could be a confounding factor (61), or psychotic features in bipolar patients. In a voxel-based-morphometry (VBM) study, McDonald et al., (62) compared patients with bipolar disorder with psychotic features and patients with schizophrenia without alcohol abuse or dependence within the 12 months prior to assessment and found a global cerebellar cortical reduction in patients with schizophrenia but no reduction in bipolar patients.
We did not find any reduction of cerebellar volume in bipolar patients whether they had experienced psychotic symptoms during mood episodes or not, despite a relatively large sample size. This suggests that cerebellar cortex volume reductions may be specific to schizophrenia rather than an overlapping feature between the two disorders. Furthermore, this suggests a different role for the cerebellum between bipolar disorder and schizophrenia.
There is an ongoing debate within the similarities and differences between schizophrenia and bipolar disorder, in terms of pathophysiology, etiology and clinical features. Although there is evidence for shared features between these two disabling conditions (26), neuroimaging studies (23) enlightened only partially overlapping brain alterations in schizophrenia and bipolar disorder. Our study provides strong evidence of different morphological alterations of cerebellum in these two conditions.
Cognitive impairments are considered as a core feature of schizophrenia (63). It is now established that the cerebellum is involved in cognition (3); Andreasen et al., (1) introduced the “dysmetria of thought concept” which suggests an impaired coordination of mental processes leading to discoordinated language and disordered thought in schizophrenia. Cognitive impairments have been described to a lesser extent in bipolar disorder, may be dependent of the considered clinical subtype of bipolar disorder and appear after onset of the disease (64, 24).
Cognitive impairments suggest that the nature of cerebellar dysfunction may be different in schizophrenia compared to bipolar disorder. This is in accordance with a cerebellar cortical volume reduction specific to schizophrenia.
Another hypothesis to explain such a difference may be that patients with bipolar disorder may exhibit more subtle changes in the morphology of the cerebellum. Fully automated segmentation of the cerebellum and volumetric measurement could be not specific enough to highlight differences with healthy controls and between psychotic and non-psychotic bipolar disorder. Moreover, a growing body of evidence, mostly using diffusion weighted imaging, suggests that bipolar disorder is associated with white matter abnormalities. Thus, future microstructural assessment of the cerebellar white matter using diffusion MRI rather than assessment of the cerebellar white matter volume may be helpful to understand the involvement of a cerebellar dysfunction in bipolar disorder. Further, functional imaging studies (9) may be more effective to underline cerebellar changes in bipolar patients. Finally, studying the grey matter loss in cerebellum over time could also help define cerebellar changes in bipolar disorder through the course of the illness (65).
Furthermore, exploration of cerebellar anatomy in psychosis may benefit from recent software developments allowing cerebellar parcellation estimated by intrinsic functional connectivity (http://surfer.nmr.mgh.harvard.edu). In addition, diffusion weighted MRI can be used to study cortico-cerebellar interactions such as cerebellar projections to the prefrontal cortex (66). This method could also be applied across the psychotic dimension to compare patients with schizophrenia, bipolar patients and healthy controls to study the cortico-cerebellar-thalamic circuit.
We found no influence of lithium, antipsychotic medication or medication load on cerebellar size. These results are in accordance with the existing literature (67).
Several strengths support our study. First, we constituted a large multicenter sample which gave us good statistical power, thus avoiding overestimation of effect sizes (68). This also allowed us to study the influence of psychotic features on cerebellar size. Second, alcohol dependence was controlled in our sample. Chronic consumption of alcohol is well known to affect the cerebellum (69) and can lead to its size reduction (70, 61). This appears to be a confounding factor since patients with bipolar disorder have increased risk of alcohol dependence as compared with healthy controls (19). To our knowledge, no study tried to specifically compare the difference in cerebellar volume between patients with schizophrenia and bipolar patients when controlling alcohol abuse or dependence, which has a major impact on cerebellar size. Third, whereas most similar studies used manual tracing, we chose a fully automated segmentation method in order to avoid evaluator bias. Finally, the majority of volumetric studies considered vermis and cerebellar hemispheres instead of cortical and white matter volumes.
Several limits should be considered before interpreting our results. First, our study is cross-sectional and interpretations of our results must be done accordingly. Second, we were not able to investigate cerebellar volume differences in the same analysis, because patients with schizophrenia were only included in France.
In conclusion, our study shows that cortical cerebellar volume and global cerebellar volume are significantly smaller in patients with schizophrenia compared to bipolar patients and healthy controls. We did not find any influence of psychotic features on cerebellar size in patients with bipolar disorder. These results suggest that cerebellar abnormalities are more subtle in bipolar disorder than in schizophrenia and that cerebellar cortical size reduction may be specific to schizophrenia.
Significant outcomes.
Cortical volume of cerebellum is smaller in patients with schizophrenia, compared to bipolar patients and healthy controls.
Psychotic features have no influence on cerebellar size among bipolar I patients.
Our results support a categorical and non-dimensional approach of schizophrenia and bipolar disorder
Limitations.
The design of our study was cross-sectional
We were not able to investigate cerebellar volume differences in the same analysis, because patients with schizophrenia were only included in France.
Acknowledgments
We thank all subjects for their participation in this study; Soufiane Carde, MD, François-Eric Vederine, MD, Edouard Duchesnay, and the staff of participating centers for provided help regarding collection of data; David Kupfer, MD, provided logistical and administrative support to this study.
Financial support
This study was supported by public funding from the Alliance pour les Sciences de la Vie et de la Sante (J.H.); by grant ANR MNP 2008 from the French Agence Nationale pour la Recherche (M.L., J.H.); by grants SFB636/C6 and We3638/3-1 from the German Deutsche Forschungsgemeinschaft (M.W.); by grant R01 MH076971 from the National Institute of Mental Health (M.P.); and by a grant from the French Agence Regionale de Santé Ile-de-France (S.S.).
Footnotes
Ethical standards
The authors assert that all procedures contributing to this work comply with the Ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Declaration of interest
None.
References
- 1.Andreasen NC, Pierson R. The Role of the Cerebellum in Schizophrenia. Biol Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li W-K, Hausknecht MJ, Stone P, Mauk MD. Using a million cell simulation of the cerebellum: Network scaling and task generality. Neural Netw. 2013;47:95–102. doi: 10.1016/j.neunet.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon N. The cerebellum and cognition. Eur J Paediatr Neurol. 2007;11:232–234. doi: 10.1016/j.ejpn.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 5.Andersen K, Andersen BB, Pakkenberg B. Stereological quantification of the cerebellum in patients with Alzheimer’s disease. Neurobiol Aging. 2012;33:197.e11–e197.e20. doi: 10.1016/j.neurobiolaging.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Hoppenbrouwers SS, Schutter DJLG, Fitzgerald PB, Chen R, Daskalakis ZJ. The role of the cerebellum in the pathophysiology and treatment of neuropsychiatric disorders: A review. Brain Res Rev. 2008;59:185–200. doi: 10.1016/j.brainresrev.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Rigucci S, Rossi-Espagnet C, Ferracuti S, et al. Anatomical substrates of cognitive and clinical dimensions in first episode schizophrenia. Acta Psychiatr Scand. 2013;128:261–270. doi: 10.1111/acps.12051. [DOI] [PubMed] [Google Scholar]
- 8.Bora E, Fornito A, Yücel M, Pantelis C. Voxelwise Meta-Analysis of Gray Matter Abnormalities in Bipolar Disorder. Biol Psychiatry. 2010;67:1097–1105. doi: 10.1016/j.biopsych.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Houenou J, d’Albis M-A, Vederine F-E, Henry C, Leboyer M, Wessa M. Neuroimaging biomarkers in bipolar disorder. Front Biosci Elite Ed. 2012;4:593–606. doi: 10.2741/e402. [DOI] [PubMed] [Google Scholar]
- 10.Green MJ, Cahill CM, Malhi GS. The cognitive and neurophysiological basis of emotion dysregulation in bipolar disorder. J Affect Disord. 2007;103:29–42. doi: 10.1016/j.jad.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Lauterbach EC. Bipolar disorders, dystonia, and compulsion after dysfunction of the cerebellum, dentatorubrothalamic tract, and substantia nigra. Biol Psychiatry. 1996;40:726–730. doi: 10.1016/0006-3223(96)82516-9. [DOI] [PubMed] [Google Scholar]
- 12.DelBello MP, Strakowski SM, Zimmerman ME, Hawkins JM, Sax KW. MRI Analysis of the Cerebellum in Bipolar Disorder: A Pilot Study. Neuropsychopharmacology. 1999;21:63–68. doi: 10.1016/S0893-133X(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 13.Brambilla P, Harenski K, Nicoletti M, et al. MRI study of posterior fossa structures and brain ventricles in bipolar patients. J Psychiatr Res. 2001;35:313–322. doi: 10.1016/s0022-3956(01)00036-x. [DOI] [PubMed] [Google Scholar]
- 14.Mills NP, DelBello MP, Adler CM, Strakowski SM. MRI Analysis of Cerebellar Vermal Abnormalities in Bipolar Disorder. Am J Psychiatry. 2005;162:1530–1533. doi: 10.1176/appi.ajp.162.8.1530. [DOI] [PubMed] [Google Scholar]
- 15.Monkul ES, Hatch JP, Sassi RB, et al. MRI study of the cerebellum in young bipolar patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:613–619. doi: 10.1016/j.pnpbp.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Womer FY, Wang F, Chepenik LG, et al. Sexually Dimorphic Features of Vermis Morphology in Bipolar Disorder. Bipolar Disord. 2009;11:753–758. doi: 10.1111/j.1399-5618.2009.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baldaçara L, Nery-Fernandes F, Rocha M, et al. Is cerebellar volume related to bipolar disorder? J Affect Disord. 2011;135:305–309. doi: 10.1016/j.jad.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 18.Kim D, Byul Cho H, Dager SR, et al. Posterior cerebellar vermal deficits in bipolar disorder. J Affect Disord. 2013;150:499–506. doi: 10.1016/j.jad.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardoso BM, Kauer Sant’Anna M, Dias VV, Andreazza AC, Ceresér KM, Kapczinski F. The impact of co-morbid alcohol use disorder in bipolar patients. Alcohol. 2008;42:451–457. doi: 10.1016/j.alcohol.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Chanraud S, Pitel A-L, Müller-Oehring EM, Pfefferbaum A, Sullivan EV. Remapping the brain to compensate for impairment in recovering alcoholics. Cereb Cortex N Y N 1991. 2013;23:97–104. doi: 10.1093/cercor/bhr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. NeuroImage. 2013;65:176–193. doi: 10.1016/j.neuroimage.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis VA, van Os J, Murray RM. The Kraepelinian Dichotomy Evidence From Developmental and Neuroimaging Studies. J Neuropsychiatry Clin Neurosci. 2000;12:398–405. doi: 10.1176/jnp.12.3.398. [DOI] [PubMed] [Google Scholar]
- 23.Rimol LM, Hartberg CB, Nesvåg R, et al. Cortical Thickness and Subcortical Volumes in Schizophrenia and Bipolar Disorder. Biol Psychiatry. 2010;68:41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 24.Frangou S. A Systems Neuroscience Perspective of Schizophrenia and Bipolar Disorder. Schizophr Bull. doi: 10.1093/schbul/sbu017. Published Online First: 8 March 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dazzan P. The promise of neuroanatomical markers in psychosis. Acta Psychiatr Scand. 2013;128:235–237. doi: 10.1111/acps.12076. [DOI] [PubMed] [Google Scholar]
- 26.Bellivier F, Geoffroy PA, Scott J, Schurhoff F, Leboyer M, Etain B. Biomarkers of bipolar disorder: specific or shared with schizophrenia? Front Biosci Elite Ed. 2013;5:845–863. doi: 10.2741/e665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ketter TA, Wang PW, Becker OV, Nowakowska C, Yang Y. Psychotic bipolar disorders: dimensionally similar to or categorically different from schizophrenia? J Psychiatr Res. 2004;38:47–61. doi: 10.1016/s0022-3956(03)00099-2. [DOI] [PubMed] [Google Scholar]
- 28.Tesli M, Espeseth T, Bettella F, et al. Polygenic risk score and the psychosis continuum model. Acta Psychiatr Scand. 2014;130:311–317. doi: 10.1111/acps.12307. [DOI] [PubMed] [Google Scholar]
- 29.Preisig M, Fenton BT, Matthey ML, Berney A, Ferrero F. Diagnostic interview for genetic studies (DIGS): inter-rater and test-retest reliability of the French version. Eur Arch Psychiatry Clin Neurosci. 1999;249:174–179. doi: 10.1007/s004060050084. [DOI] [PubMed] [Google Scholar]
- 30.Pagel T, Franklin J, Baethge C. Schizoaffective disorder diagnosed according to different diagnostic criteria – systematic literature search and meta-analysis of key clinical characteristics and heterogeneity. J Affect Disord. 2014;156:111–118. doi: 10.1016/j.jad.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Pagel T, Baldessarini RJ, Franklin J, Baethge C. Heterogeneity of schizoaffective disorder compared with schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2013;128:238–250. doi: 10.1111/acps.12109. [DOI] [PubMed] [Google Scholar]
- 32.Sarrazin S, Poupon C, Linke J, et al. A Multicenter Tractography Study of Deep White Matter Tracts in Bipolar I Disorder: Psychotic Features and Interhemispheric Disconnectivity. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2013.4513. Published Online First: 12 February 2014. [DOI] [PubMed] [Google Scholar]
- 33.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 34.McDonald CR, Hagler DJ, Jr, Ahmadi ME, et al. Subcortical and cerebellar atrophy in mesial temporal lobe epilepsy revealed by automatic segmentation. Epilepsy Res. 2008;79:130–138. doi: 10.1016/j.eplepsyres.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerasa A, Messina D, Nicoletti G, et al. Cerebellar Atrophy in Essential Tremor Using an Automated Segmentation Method. Am J Neuroradiol. 2009;30:1240–1243. doi: 10.3174/ajnr.A1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rimol LM, Nesvåg R, Hagler DJ, Jr, et al. Cortical Volume, Surface Area, and Thickness in Schizophrenia and Bipolar Disorder. Biol Psychiatry. 2012;71:552–560. doi: 10.1016/j.biopsych.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 37.Lisdahl KM, Thayer R, Squeglia LM, McQueeny TM, Tapert SF. Recent binge drinking predicts smaller cerebellar volumes in adolescents. Psychiatry Res Neuroimaging. 2013;211:17–23. doi: 10.1016/j.pscychresns.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Javaid N, Kennedy JL, De Luca V. Ethnicity and age at onset in bipolar spectrum disorders. CNS Spectr. 2011;16:127–134. doi: 10.1017/S1092852912000296. [DOI] [PubMed] [Google Scholar]
- 39.Versace A, Almeida JRC, Hassel S, et al. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch Gen Psychiatry. 2008;65:1041–1052. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nordenskjöld R, Malmberg F, Larsson E-M, et al. Intracranial volume estimated with commonly used methods could introduce bias in studies including brain volume measurements. NeuroImage. 2013;83:355–360. doi: 10.1016/j.neuroimage.2013.06.068. [DOI] [PubMed] [Google Scholar]
- 41.Keller A, Castellanos FX, Vaituzis AC, Jeffries NO, Giedd JN, Rapoport JL. Progressive Loss of Cerebellar Volume in Childhood-Onset Schizophrenia. Am J Psychiatry. 2003;160:128–133. doi: 10.1176/appi.ajp.160.1.128. [DOI] [PubMed] [Google Scholar]
- 42.Edwards CR, Newman S, Bismark A, et al. Cerebellum volume and eyeblink conditioning in schizophrenia. Psychiatry Res Neuroimaging. 2008;162:185–194. doi: 10.1016/j.pscychresns.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reyes MG, Gordon A. Cerebellar vermis in schizophrenia. Lancet. 1981;2:700–701. doi: 10.1016/s0140-6736(81)91039-4. [DOI] [PubMed] [Google Scholar]
- 44.Tran KD, Smutzer GS, Doty RL, Arnold SE. Reduced Purkinje cell size in the cerebellar vermis of elderly patients with schizophrenia. Am J Psychiatry. 1998;155:1288–1290. doi: 10.1176/ajp.155.9.1288. [DOI] [PubMed] [Google Scholar]
- 45.Eastwood SL, Cotter D, Harrison PJ. Cerebellar synaptic protein expression in schizophrenia. Neuroscience. 2001;105:219–229. doi: 10.1016/s0306-4522(01)00141-5. [DOI] [PubMed] [Google Scholar]
- 46.Jacobsen LK, Giedd JN, Berquin PC, et al. Quantitative Morphology of the Cerebellum and Fourth Ventricle in Childhood-Onset Schizophrenia. Am J Psychiatry. 1997;154:1663–1669. doi: 10.1176/ajp.154.12.1663. [DOI] [PubMed] [Google Scholar]
- 47.Nopoulos PC, Ceilley JW, Gailis EA, Andreasen NC. An MRI study of cerebellar vermis morphology in patients with schizophrenia: evidence in support of the cognitive dysmetria concept. Biol Psychiatry. 1999;46:703–711. doi: 10.1016/s0006-3223(99)00093-1. [DOI] [PubMed] [Google Scholar]
- 48.Wassink TH, Andreasen NC, Nopoulos P, Flaum M. Cerebellar morphology as a predictor of symptom and psychosocial outcome in schizophrenia. Biol Psychiatry. 1999;45:41–48. doi: 10.1016/s0006-3223(98)00175-9. [DOI] [PubMed] [Google Scholar]
- 49.Ichimiya T, Okubo Y, Suhara T, Sudo Y. Reduced volume of the cerebellar vermis in neuroleptic-naive schizophrenia. Biol Psychiatry. 2001;49:20–27. doi: 10.1016/s0006-3223(00)01081-7. [DOI] [PubMed] [Google Scholar]
- 50.Loeber RT, Cintron CMB, Yurgelun-Todd DA. Morphometry of Individual Cerebellar Lobules in Schizophrenia. Am J Psychiatry. 2001;158:952–954. doi: 10.1176/appi.ajp.158.6.952. [DOI] [PubMed] [Google Scholar]
- 51.Okugawa G, Sedvall G, Nordström M, et al. Selective reduction of the posterior superior vermis in men with chronic schizophrenia. Schizophr Res. 2002;55:61–67. doi: 10.1016/s0920-9964(01)00248-1. [DOI] [PubMed] [Google Scholar]
- 52.Joyal CC, Pennanen C, Tiihonen E, Laakso MP, Tiihonen J, Aronen HJ. MRI volumetry of the vermis and the cerebellar hemispheres in men with schizophrenia. Psychiatry Res Neuroimaging. 2004;131:115–124. doi: 10.1016/j.pscychresns.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Bottmer C, Bachmann S, Pantel J, et al. Reduced cerebellar volume and neurological soft signs in first-episode schizophrenia. Psychiatry Res Neuroimaging. 2005;140:239–250. doi: 10.1016/j.pscychresns.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Sullivan EV, Deshmukh A, Desmond JE, et al. Contribution of alcohol abuse to cerebellar volume deficits in men with schizophrenia. Arch Gen Psychiatry. 2000;57:894–902. doi: 10.1001/archpsyc.57.9.894. [DOI] [PubMed] [Google Scholar]
- 55.Staal WG, Hulshoff Pol HE, Schnack HG, van Haren NE, Seifert N, Kahn RS. Structural brain abnormalities in chronic schizophrenia at the extremes of the outcome spectrum. Am J Psychiatry. 2001;158:1140–1142. doi: 10.1176/appi.ajp.158.7.1140. [DOI] [PubMed] [Google Scholar]
- 56.Cahn W, Hulshoff Pol HE, Bongers M, et al. Brain morphology in antipsychotic-naïve schizophrenia: a study of multiple brain structures. Br J Psychiatry Suppl. 2002;43:s66–s72. doi: 10.1192/bjp.181.43.s66. [DOI] [PubMed] [Google Scholar]
- 57.Hulshoff Pol HE, Schnack HG, Bertens MGBC, et al. Volume changes in gray matter in patients with schizophrenia. Am J Psychiatry. 2002;159:244–250. doi: 10.1176/appi.ajp.159.2.244. [DOI] [PubMed] [Google Scholar]
- 58.James AC, James S, Smith DM, Javaloyes A. Cerebellar, Prefrontal Cortex, and Thalamic Volumes Over Two Time Points in Adolescent-Onset Schizophrenia. Am J Psychiatry. 2004;161:1023–1029. doi: 10.1176/appi.ajp.161.6.1023. [DOI] [PubMed] [Google Scholar]
- 59.Levitt JJ, McCarley RW, Nestor PG, et al. Quantitative Volumetric MRI Study of the Cerebellum and Vermis in Schizophrenia: Clinical and Cognitive Correlates. Am J Psychiatry. 1999;156:1105–1107. doi: 10.1176/ajp.156.7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Button KS, Ioannidis JPA, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 61.Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. NeuroImage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McDonald C, Bullmore E, Sham P, et al. Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder Computational morphometry study. Br J Psychiatry. 2005;186:369–377. doi: 10.1192/bjp.186.5.369. [DOI] [PubMed] [Google Scholar]
- 63.Schaefer J, Giangrande E, Weinberger DR, Dickinson D. The global cognitive impairment in schizophrenia: Consistent over decades and around the world. Schizophr Res. 2013;150:42–50. doi: 10.1016/j.schres.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ancín I, Cabranes JA, Sántos JL, Sanchez-Morla E, Barabash A. Executive deficits: A continuum schizophrenia–bipolar disorder or specific to schizophrenia? J Psychiatr Res. 2013;47:1564–1571. doi: 10.1016/j.jpsychires.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 65.Moorhead TWJ, McKirdy J, Sussmann JED, et al. Progressive Gray Matter Loss in Patients with Bipolar Disorder. Biol Psychiatry. 2007;62:894–900. doi: 10.1016/j.biopsych.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Jissendi P, Baudry S, Balériaux D. Diffusion tensor imaging (DTI) and tractography of the cerebellar projections to prefrontal and posterior parietal cortices: A study at 3T. J Neuroradiol. 2008;35:42–50. doi: 10.1016/j.neurad.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord. 2012;14:375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- 68.Fanelli D, Ioannidis JPA. US studies may overestimate effect sizes in softer research. Proc Natl Acad Sci U S A. 2013;110:15031–15036. doi: 10.1073/pnas.1302997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: relation to ataxia. Neuropsychology. 2000;14:341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- 70.Shear PK, Sullivan EV, Lane B, Pfefferbaum A. Mammillary body and cerebellar shrinkage in chronic alcoholics with and without amnesia. Alcohol Clin Exp Res. 1996;20:1489–1495. doi: 10.1111/j.1530-0277.1996.tb01153.x. [DOI] [PubMed] [Google Scholar]