Abstract
The assessment of ventricular function, cardiac chamber dimensions and ventricular mass is fundamental for clinical diagnosis, risk assessment, therapeutic decisions, and prognosis in patients with cardiac disease. Although cardiac computed tomography (CT) is a noninvasive imaging technique often used for the assessment of coronary artery disease, it can also be utilized to obtain important data about left and right ventricular function and morphology. In this review, we will discuss the clinical indications for the use of cardiac CT for ventricular analysis, review the evidence on the assessment of ventricular function compared to existing imaging modalities such cardiac MRI and echocardiography, provide a typical cardiac CT protocol for image acquisition and post-processing for ventricular analysis, and provide step-by-step instructions to acquire multiplanar cardiac views for ventricular assessment from the standard axial, coronal, and sagittal planes. Furthermore, both qualitative and quantitative assessments of ventricular function as well as sample reporting are detailed.
Keywords: Ventricular function, computed tomography, ejection fraction, left ventricular mass, wall motion abnormality, arrhythmogenic right ventricular dysplasia
INTRODUCTION
Although the mainstay for cardiac computed tomography (CT) is the assessment of coronary artery disease, the isotropic sub-mm spatial resolution, high temporal resolution and good contrast between ventricular lumen and myocardium make CT very well suited to obtain valuable information on ventricular function.1–3 Currently, several noninvasive imaging techniques are available for the assessment of ventricular function – each with their own limitations. Cardiac magnetic resonance imaging (CMR) is considered the gold standard.4 However, CMR is costly, time-consuming, and has limited availability to predominantly tertiary medical centers. Furthermore, some patients may not be able to undergo a CMR examination, for example due to metallic devices or clinical conditions such as claustrophobia and the inability to lay flat.2 Transthoracic echocardiography (TTE) is the cheapest and most routinely used method for the measurement of ventricular function but may be limited by poor acoustic windows in patients with obesity, chronic obstructive pulmonary disease (COPD), narrow rib intercostal spaces, or prior cardiothoracic surgery.5–7 Furthermore, assessment of the right ventricle can be difficult especially when assessing structural abnormalities such as arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D).8 Cardiac CT, with some modifications to the acquisition protocol, can be utilized to obtain accurate ventricular assessments comparable to CMR.9 Tube current modulation with improved noise reduction strategies have allowed for reduction of radiation dose with preserved image quality.10–12 For patients that are difficult to image by echocardiography and are undergoing CT imaging for coronary artery disease (CAD) without previous ventricular assessment, especially with a history of acute coronary syndrome (ACS) or heart failure (HF), or for patients who need specific assessment of right ventricular (RV) function or morphology, evaluation of ventricular contractile function by cardiac CT should be considered.
MULTIMODALITY COMPARISON OF LV VOLUMES, FUNCTION, AND MASS
As temporal resolution has improved, cardiac CT can be utilized to obtain important information on ventricular function, regional wall motion, and LV mass and results are comparable to measurements obtained via CMR.13–16 Unlike the evaluation of the coronary arteries, cardiac function can be accurately assessed without the need to alter heart rate via administration of beta-blockers.17 In retrospective analysis of 40 patients with suspected CAD where cardiac CT (with 64-slice single source, 64-slice dual-source and 128-slice dual source CT) and 1.5 Tesla CMR were performed 7 days apart, excellent correlations were observed between CT and CMR for LV volumes, function, and mass when both modalities were measured with exclusion of the papillary muscles (LV end systolic volume [LVESV] r = 0.98; LV end diastolic volume [LVEDV] r = 0.96; LV ejection fraction (LV EF) r = 0.94; and LV mass r = 0.97).10 Additionally, applying high-strength noise reduction strategy on post-processed images of electrocardiographic (ECG) tube current modulated datasets improves correlation of EF to CMR and reduces the variability of function and mass measurements by increasing the contrast-to-noise ratio.10 In a prospective study of 79 patients who underwent 64-slice single source cardiac CT for CAD evaluation, 1.5 Tesla CMR was performed within 1 week and very good agreement between the two imaging modalities was reported (LV EF: CT 52±14% vs. CMR 52±14%; r = 0.73); LVEDV): CT 74±21 ml/m2 vs. CMR 76±25 ml/m2; r = 0.59); LVESV CT 37±19 ml/m2 vs. CMR 38±23 ml/m2; r = 0.76).18
When comparing 16-slice CT in 88 patients with other imaging modalities (TTE and biplane cine-ventriculography) to 1.5 Tesla CMR within 48 hours, global and regional LV function was more accurate with CT than cine-venticulography, and CT was superior to TTE for global function.9 A meta-analysis of 27 studies using at least 64-slice CT (15 studies comparing CT and TTE and 12 studies comparing CT to MRI) showed no difference in LVEF between CT and CMR nor was a difference seen in LVEF between CT and TTE.6 In direct comparison with CMR, good correlation has been demonstrated for cardiac CT to estimate LV size, volume and function at a wide range of EF from severely reduced to hyperdynamic function (30–72%), including patients with significant valvular disease or orthotopic heart transplant.6, 19–21
INDICATIONS FOR CARDIAC CT
Per the most recent guidelines, the appropriate use criteria for the assessment of left ventricular (LV) or RV function by cardiac CT include the following22:
Assessment of LV function following ACS or HF patients with inadequate images from other noninvasive methods.
Quantitative assessment of RV function.
Assessment of RV morphology in suspected ARVC/D.
Assessment of LV function in ACS
The assessment of LV function in the post-ACS setting has prognostic and treatment implications.23, 24 Previous studies have demonstrated good correlation and reproducibility with TTE in patients with CAD for the evaluation of global EF.25 The identification of regional wall motion abnormalities and perfusion defects have shown to have good sensitivity (94%) and specificity (97%) in patients with ACS with excellent interobserver reliability for EF quantification (r = 0.83).26 Furthermore, the concomitant assessment of ventricular function and wall motion abnormalities by cardiac CT provides considerable incremental value in low-intermediate risk patients with suspected ACS in addition to coronary artery anatomy and perfusion assessment.27 Resting regional LV function has incremental value beyond coronary stenosis for determining ACS, especially in those presenting with acute chest pain.28 Regional LV function assessment by CT showed an improvement of positive predictive value (PPV) from 35% to 89% in patients with significant stenosis for detecting ACS.28, 29 However, this incremental value of having functional datasets must be weighed with the risk-benefit ratio from the concerns of higher radiation exposure with retrospectively-gated scans,30 and practice pattern change within the cardiac CT community towards prospectively-triggered scans to achieve the lowest radiation dose scan possible in keeping with the “As Low As Reasonably Achievable” ALARA principle at the expense of loss in functional analysis.31
Assessment of ventricular function in heart failure (HF)
Assessment of left ventricular systolic function has well known prognostic implications as a predictor of mortality and congestive heart failure.32, 33 As discussed in previous sections, cardiac CT has been shown to provide accurate and reproducible assessment of LV size, volume and function in patients with HF reduced EF (HFrEF), significant valvular disease, and orthotopic heart transplants.19–21 For patients with technically difficult studies and/or conflicting assessments of function on multiple non-invasive modalities that have contraindications to CMR, cardiac CT has significant utility in providing accurate and definitive assessment of ventricular function. Some examples of patients include patients with difficult acoustic echocardiographic windows and patients with contraindication to CMR such as those with metal in the eye, intracardiac devices (pacemaker, defibrillators, cardiac resynchronization therapy [CRT]), claustrophobia, and inability to lay flat for a prolonged period of time. For patients with HFrEF with low probability for CAD (i.e. nonischemic cardiomyopathy such as viral etiology), cardiac CT can provide data on LV EF and more importantly be used to exclude the presence of obstructive CAD, making an invasive diagnostic cardiac catheterization unnecessary.34 With respect to HFrEF patients under evaluation for cardiac resynchronization therapy (CRT) where device implantation is indicated for LV EF of <35%35, 36 or <30%37, 38 cardiac CT may in addition be useful for coronary venous assessment before biventricular lead placement,34 and if a retrospectively ECG-gated scan was performed, CT provided additional information on global and also on regional LV function. One area of research in this cohort is the utility of cardiac CT for assessing intraventricular dyssynchrony to predict the response to CRT and potentially for pre-procedural planning regarding left ventricular lead placement.39, 40
Furthermore, cardiac CT may play an important role in the assessment of HFrEF patients with left ventricular assist devices (LVADs) and can detect LVAD dysfunction as well as device related complications.41 Although echocardiography is the primary imaging modality to monitor patients with LVADs, it has its own limitations as previously discussed. In addition to anatomic information, a retrospectively-gated scan can evaluate inflow and outflow cannula position and angulation, cannula thrombi, pericannula fluid collection, position of blood pump, pericardial effusion and driveline position. In a small study of 14 patients with LVAD and persistent HF symptoms, retrospectively-gated 64-slice CT was performed and found abnormalities in 8 patients, of which 6 patients had their medical management altered as a direct result of the CT findings and underwent surgical intervention (device exchange and heart transplant).41
While the clinical indication for CT in patients with HFrEF is better defined, the utility of CT with respect to patients with HF preserved EF (HFpEF) is less clear. Scant data is available for cardiac CT and its use to assess for diastolic function. A feasibility study of 70 patients who underwent 64-slice CT and 2D TTE with tissue Doppler imaging reported good correlation between cardiac CT transmitral velocity (r = 0.73) and CT mitral septal tissue velocity (r = 0.87) as compared to TTE.42 However, larger studies are needed to validate these initial findings.
Assessment of right ventricular function
RV function plays an important role in the pathogenesis of many cardiovascular diseases and serves as an integral diagnostic and prognostic marker in many cardiopulmonary conditions that include CHF, pulmonary hypertension and pulmonary embolism.43, 44 TTE is the first line imaging modality for the assessment of the RV but can be technically difficult for the same reasons as the LV. In addition, the complex geometry of the RV, its thin walls and heavy trabeculation may further complicate edge recognition.18, 45, 46 CMR is considered the reference standard but has the same contraindications as stated previously.18 For these patients, cardiac CT is an alternative option for RV function evaluation. Adequate contrast enhancement of the right ventricular lumen is required, but it can typically be achieved by extending the duration of contrast injection by 10 seconds as compared to standard contrast injection protocols for coronary artery imaging.22 To minimize the overall contrast load, a second phase injection using a reduced flow rate or a diluted mixture of contrast at the same flow rate can be administered.47 Assessment of RV function by cardiac CT has been validated in multiple studies with good accuracy and reproducibility utilizing CMR as the reference standard.19, 48 A cohort that may frequently benefit from the CT-based assessment of RV volumes and function are the complex adult congenital heart disease patients, such as those after Tetralogy of Fallot repair. These patients frequently have pacemakers or defibrillators, yet the decision for optimal timing of pulmonary valve replacement is heavily dependent on accurate assessment of RV size and function. Pulmonary valve replacement should be undertaken before the RV end-diastolic volume index (RVEDVI) reaches 170 mL/m2 or the RV end-systolic volume index (RVESVI) reaches 85 mL/m2.49 In patients with chronic pulmonary regurgitation, timing of pulmonary valve replacement should be considered before RVEDVI exceeds 163 mL/m2 or RVESVI exceeds 80 mL/m2.50
Assessment of ARVC/D
Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia (ARVC/D) is a rare inherited cardiomyopathy predominantly involving the RV with progressive fibrofatty tissue and presence of ventricular arrhythmias.51 TTE is the first line imaging modality but is limited in its ability to detect RV dilatation and RWMA.8 Cardiac CT can assess RV dilatation, reduction of RV ejection fraction, severe segmental dilatation, and regional hypokinesis – all part of the major or minor criteria per the original task force criteria of the diagnosis of ARVC/D (Table 1).47, 52–54 The 2010 ACC/AHA appropriate use criteria designates cardiac CT as “Appropriate” for the evaluation of suspected right ventricular anomalies such as ARVC/D.22 One study with the use of major or minor criteria showed moderate to excellent sensitivity and positive predictive value.55 Per the guidelines, RVEDVI ≥ 110 mL/m2 for males and ≥100 mL/m2 for females or RV EF ≤40% are part of the major criteria values, while RVEDVI ≥ 100 mL/m2 for males and ≥90 mL/m2 for females or RV EF ≤45% are considered part of the minor criteria (Table 1).56, 57 Additionally, the presence of fatty tissue, bulging appearance, and dilatation of the RV are suggested in a small study of 77 patients to aid in the differentiation of ARVC/D from ventricular tachyarrhythmias.55 Similar to the assessment of LV function, the role of cardiac CT for ARVC/D assessment should be reserved for patients with inadequate TTE images and contraindications for CMR.
Table 1.
Revised Task Force Criteria for the Diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia (ARVC/D)54
| I. Global or regional dysfunction and structural alterations |
| Major criteria |
| By 2D echo: |
|
|
|
|
|
| By MRI: |
|
|
|
|
|
| By RV angiography: |
|
|
|
|
|
| Minor criteria |
| By 2D echo: |
|
|
|
|
|
| By MRI: |
|
|
|
|
|
| II. Tissue characterization of wall |
| Major criteria |
|
|
|
|
|
| Minor criteria |
|
|
|
|
|
| III. Repolarization abnormalities |
| Major criteria |
|
|
|
|
|
| Minor criteria |
|
|
|
|
|
| IV. Depolarization/conduction abnormalities |
| Major criteria |
|
|
|
|
|
| Minor criteria |
|
|
|
|
|
| V. Arrhythmias |
| Major criteria |
|
|
|
|
|
| Minor criteria |
|
|
|
|
|
| VI. Family history |
| Major criteria |
|
|
|
|
|
| Minor criteria |
|
|
|
PLAX: parasternal long-axis view; RVOT: RV outflow tract; BSA: body surface area; PSAX: parasternal short-axis view; aVF: augmented voltage unipolar left foot lead; and aVL: augmented voltage unipolar left arm lead.
Diagnostic terminology for original criteria: The diagnosis of ARVC/D is fulfilled by the presence of 2 major, or 1 major plus 2 minor criteria or 4 minor criteria from different groups. Diagnostic terminology for revised criteria: Definite diagnosis: 2 major or 1 major and 2 minor criteria or 4 minor from different categories; Borderline: 1 major and 1 minor or 3 minor criteria from different categories; Possible: 1 major or 2 minor criteria from different categories.
DATA ACQUISITION PROTOCOLS
Cardiac CT imaging should be performed using at least 64-slice CT scanner technology according to the vendor specific protocol for the evaluation of ventricular function. General recommendations for cardiac CT typically include a minimal contrast flow rate of 5ml/sec, but for specific analysis of ventricular function, lower injection rates may be sufficient. Retrospectively ECG-gated image reconstruction is necessary to reconstruct images through all phases of the cardiac cycle. Tube current modulation techniques are often applied for coronary artery imaging. They limit tube output in systole, a time period that is typically not used for image reconstruction in coronary CT angiography. Even with tube modulation, reconstruction of data sets for functional assessment is usually possible.30, 58 Functional analysis can also be performed by prospectively ECG-triggered protocols if sufficient “padding” is added to cover most of the cardiac cycle.59 However, the benefit of using such protocols over the standard retrospectively ECG-gating protocols with tube current modulation is unclear. The timing of contrast administration is standardized for arterial phase imaging (typically, scan acquisition starts 4–6 seconds after time to peak aortic opacification). Contrast injection for left ventricular and coronary imaging is typically performed for a time period that is equal to the duration of data acquisition (but at least 10 seconds if data acquisition is less than 10 seconds). To obtain adequate RV opacification, an additional 10 seconds of contrast injection should be added but may be given at a slower flow rate (e.g., 2 ml/sec), if the contrast injector permits such injection protocols, to minimize the amount of contrast given to patients.
IMAGE PROCESSING AND EVALUATION
While there lacks data for how best to post-process functional datasets, in our experience the following pointers may be taken into consideration. For functional analysis, a multiphase reformatted dataset of maximally 1.5 mm thick axial images without overlap should be reconstructed at 10% increments (10 phases) for single source CT scanners or 5% increments (20 phases) for dual-source CT scanners throughout the cardiac cycle from the onset of the R-wave for the assessment of global and regional LV function in a cine mode. Thinner slices can be reconstructed with overlap to improve spatial resolution but such fine spatial resolution, while important for coronary artery assessment, is not necessary for ventricular function analysis and would result in unnecessary large Digital Imaging and Communications in Medicine (DICOM) files. The rationale for reconstructing at 10% increments (10 phases) for single source CT scanners is due to the temporal resolution of these scanners of ~165–175 milliseconds (ms). At a heart rate of 60 beats per minute (bpm), at 10 phases of the cardiac cycle, the difference between each phase is 100 ms. Given the temporal resolution of the CT scanner itself, no benefit can be expected from reconstructing more phases. With dual-source CT scanners, the temporal resolution may be as low as 65; therefore, at a heart rate of 60 bpm, reconstructing 10 phases would not make full use of temporal resolution and reconstructing at 5% increments (20 phases) may be recommended.
Global and Regional LV function using a 17-segment model
The 17-segment model (Figure 1) creates a distribution of 35%, 35%, and 30% for the basal, mid-cavity, and apical thirds of the heart respectively.60 Global and regional LV function can be assessed visually with the multiphase reformatted dataset in cine mode to correspond to those used in echocardiography, including LV horizontal long-axis (4-chamber view), vertical long-axis (2-chamber view), LV outflow tract long-axis (parasternal long axis view), and short-axis views. Global LV function is reported as hyperdynamic, normal or reduced (mild, moderate, or severe). Regional wall motion (RWM) of the myocardium is assessed to be normal or abnormal (hypokinesia, akinesia, dyskinesia, aneurysm) in short-axis segments of the LV corresponding to the AHA/ACC/ASE 17-segment model.60 Abnormalities have to be present in at least two contiguous myocardial segments or in one segment visualized in two different views. Figure 2 demonstrates an example of a significant segmental wall motion abnormality.
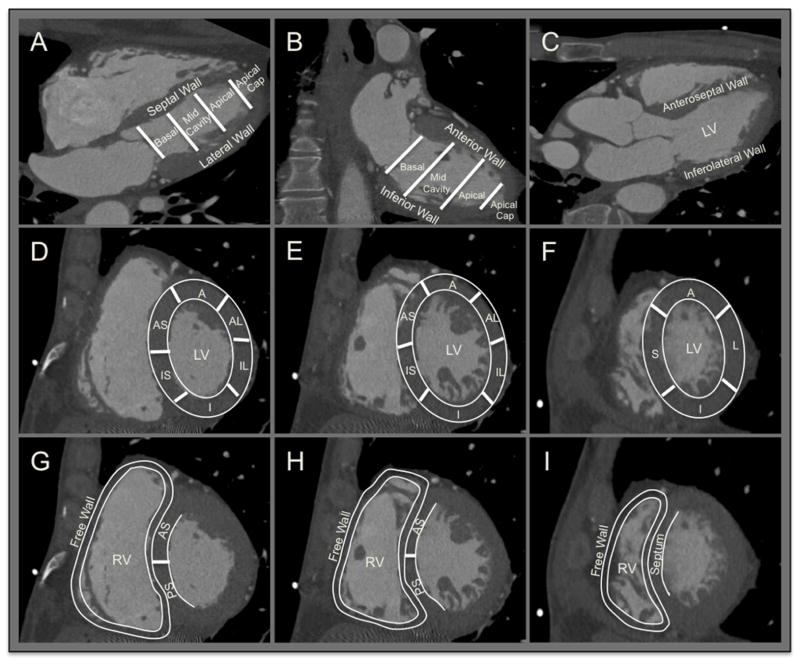
Figure 1. LV and RV Segmentation including the 17-segment Model.
A. Four-chamber view showing basal, mid-cavity and apical segments and the apical cap. B. Two-chamber view. C. Vertical long-axis 3-chamber (parasternal long axis) view. D, E and F. Short-axis view of the basal, mid-cavity and apical segments showing LV segmentation; anterior (A), anteroseptal (AS), anterolateral (AL), inferior (I), inferoseptal (IS), inferolateral (IL) and septal (S) segments. G, H and I. Short-axis view of the basal, mid-cavity and apical segments showing RV segmentation; free wall, anteroseptal (AS) and posteroseptal (PS) segments.
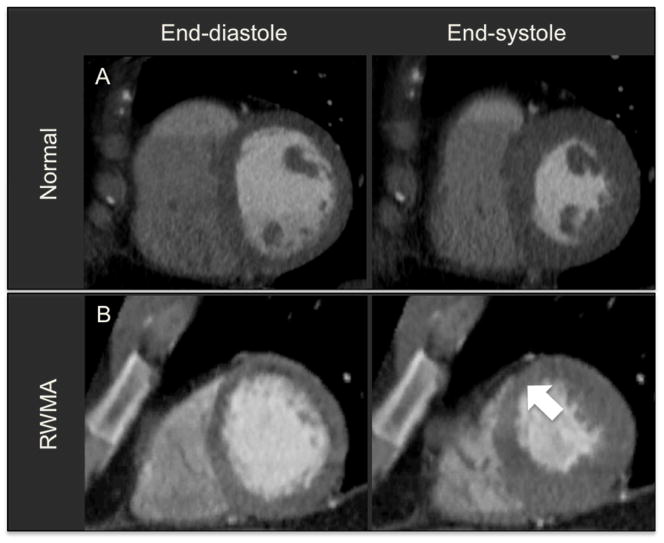
Figure 2. Example of Normal and Abnormal Regional Wall Motion.
A. Short-axis view in end-diastole and end-systole of a normal patient. B. Short-axis view in end-diastole and end-systole demonstrating regional wall motion abnormality in anteroseptal segment (white arrow).
Quantification of the left and right ventricles
To quantify cardiac chamber size and function, measurement of the LV internal boundaries are performed at end-diastole and end-systole.61 External boundaries have to be added to obtain information on myocardial thickening and mass. Figure 3 details a step-wise approach to obtain the correct planes that allow for left ventricular functional measurement. End-diastole can be defined at the onset of the QRS, but is preferably defined as the frame following mitral valve closure or the frame in the cardiac cycle in which the cardiac dimension is largest which is after atrial contraction in patients in sinus rhythm.61 End-systole is best defined as the frame preceding mitral valve opening or the time in the cardiac cycle in which the cardiac dimension is smallest.61 Not surprisingly, there is prognostic value in CT-based LV volumes and function. In 7758 patients from the Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter (CONFIRM) registry, worsening LV EF, and larger LV volumes predicted mortality and were associated with CAD.62 In this registry, LV EF was classified as normal (≥55%), mildly reduced (55% but ≥45%), moderately reduced (45% but ≥35%), and severely reduced (<35%). Abnormal LV end-systolic volume (LVESV) was defined as ≥90 mL and abnormal LV end-diastolic volume (LVEDV) as ≥200 mL.62
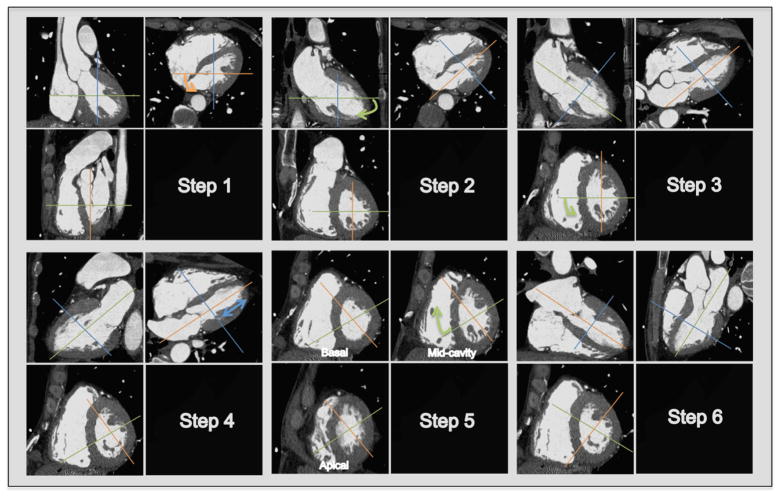
Figure 3. Stepwise Approach in Creating Planes for Ventricular Function Analysis.
Step 1. In the axial image, scroll to a modified 4-chamber level and rotate the reference line (orange) to be parallel to the LV cavity and positioned in the center of the mitral valve coursing through the apical cap. Step 2. In the 2-chamber view, rotate the reference line (green) to be parallel to the LV cavity and positioned in the center of the mitral valve coursing through the apical cap. These first 2 steps will get the double-oblique true short-axis. Step 3. In the short-axis view at the mid ventricular level, rotate the reference line (green) towards the RV crux to remove the LV outflow tract (LVOT) from the image to get the true 4-chamber view and 2-chamber view, which can be used for image interpretation. Step 4. In the 4-chamber view, move the reference line (blue) up and down to get the basal, mid-cavity and apical short-axis views for image interpretation. Step 5. In the mid-cavity short-axis, rotate the reference line (green) towards the 10 o’clock position to bring out the vertical long-axis 3-chamber (parasternal) view (Step 6).
Scant data is available on normative values for RV volume and function with cardiac CT. A small study of 103 normotensive, nonobese adults undergoing 64-slice CT reported the lower 95% confidence interval (CI) of RV EF to be 42.2%.63 A larger Multi-Ethnic Study of Atherosclerosis (MESA) of 487 patients undergoing CMR reported sex-differences in RV EF with lower limits of the 95% CI to be 51% in men and 58% in women.57 For most cardiac software package, automated LV function is readily available and more robust while that of RV function is limited and more frequently than not requires manual correction, which can be extremely time intensive.
MANUAL VERSUS AUTOMATED QUANTIFICATION
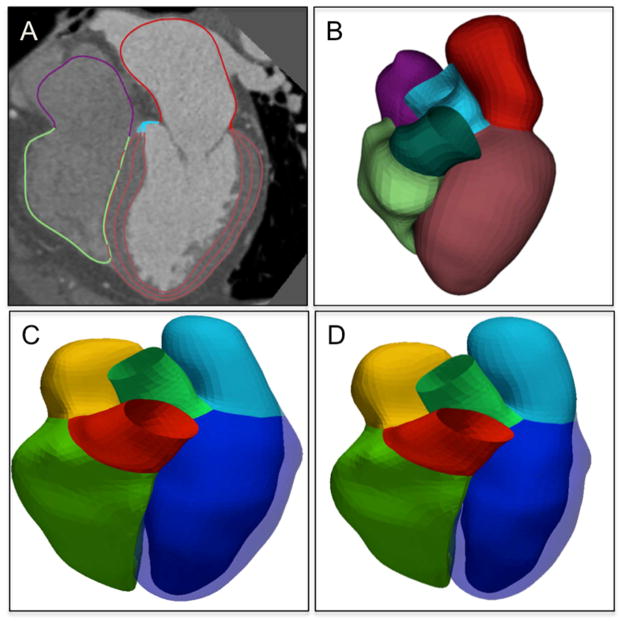
It remains a trade-off with automated post-processing software versus manual contours for delineating the endocardial and epicardial boundaries with respect to time required for quantification. The development of automated EF assessments has reduced the time needed for post-processing and has shown good agreement for the estimation of EF compared to semiautomated or manual assessments even if significant differences in LVEDV were observed.64 Automated post-processing software can automatically delineate the ventricular chamber throughout systole and diastole (Figure 4), and can provide an accurate and fully automated assessment of global LV function with significantly reduced processing time compared with manual or semi-automated methods.
Figure 4. Automated Heart Chamber Segmentation.
A. Four-chamber segmentation with multiplanar reformatting. B–D. Three-dimensional (3D) reconstruction of reformatted 4-chamber segmentation (B) at end-diastolic phase (C) and end-systolic phase (D). Courtesy of Guanglei Xiang PhD.
Depending on the software algorithm, papillary muscles may be included or excluded. The terminology of papillary muscle “inclusion” and “exclusion” is confusing. Most modern automatic software vendors use a blood pool extraction method for extracting volumes, whereby the papillary muscles, due to its different Hounsfield unit from contrast enhanced blood, are not included in determining the volumes and thus are “included” as part of the endocardial tracing, but are “excluded” from the volumetric measurements, yielding smaller volumes. Manual quantification of CT volumes and function, which values have traditionally been the ones compared to CMR, “excludes” the papillary muscles and are typically performed by drawing circular endocardial contours in radial short-axis stacks and applying modified Simpson’s summation of discs to calculate volumes. The exclusion of papillary muscles from the endocardial contours yields larger volumes.10, 13–16, 18 The inclusion or exclusion of papillary muscle can change the volumes measured, as well as sex differences for male and female for volumes, but not for EF.65, 66 In a study that included 179 subjects, significant difference was observed in LV parameters between both papillary muscles included and excluded groups. The difference ranges were 5.6% to 30.1% for LV volumes, 5.8% to 9.4% for LV mass, and 4.3% to 6.0% for LV EF.65 These findings emphasize the importance of including the papillary muscles in the measurement of LV cavity, which is essentially the traditional “exclusion” of the papillary muscle method from the LV volumetric contour.65, 67–69 There are now software vendors that have capabilities to “exclude” papillary muscles and delineate the endocardial contours similar to that which would be performed manually. These details may seem minutia, but if not aware of the differences may lead to inappropriate over- or under-estimation of reported volumes.
DATA ELEMENTS TO BE INCLUDED IN THE REPORT
Data elements suggested to be included in a report regarding ventricular function are as follows:
Cardiac morphology of the left and right atria and ventricles.
Qualitative global LV and RV function (if requested)
Regional wall motion abnormalities
Quantitative LV and RV (if requested) ejection fractions and volumes including indexing to body surface area
Table 2 depicts a sample report.
Table 2.
Sample Cardiac CT Report of Ventricular Morphology and Function
|
CARDIAC MORPHOLOGY AND FUNCTION: The right and left atria and ventricles are morphologically normal. Normal qualitative global LV function. No regional wall motion abnormality.
|
| Calculated BSA: m2 |
| LVEF: % |
| LV end diastolic volume: cc |
| LV end diastolic volume index: cc/m2 |
| LV end systolic volume: cc |
| LV end systolic volume index: cc/m2
|
| RVEF: % |
| RV end diastolic volume: cc |
| RV end diastolic volume index: cc/m2 |
| RV end systolic volume: cc |
| RV end systolic volume index: cc/m2 |
CARDIAC CT LIMITATIONS
Compared to other modalities, assessment of ventricular function by cardiac CT requires radiation exposure and administration of contrast dye. Patients with significant contrast dye allergies or renal insufficiency are not candidates for contrast-enhanced cardiac CT. Premature atrial and ventricular beats and atrial fibrillation can cause arrhythmia and misregistration artifacts, resulting in poor visualization of the endocardial and epicardial contours as well as high radiation dose due to the scanners’ built-in arrhythmia algorithms, which opens the temporal image acquisition window to full tube current when acquiring images in retrospectively-gated scan mode. Severe obesity can compromise image quality and thus reduce diagnostic accuracy or require higher doses of radiation.
CONCLUSION
In addition to the assessment of coronary artery disease, cardiac CT can be utilized to investigate LV function, RV function, and ventricular morphology It serves as alternative option for functional assessment particularly when other imaging modalities such as echocardiography yield inadequate images or in patients with contraindications to CMR.
Acknowledgments
Funding Sources: Dr. Truong is supported by the NIH (K23HL098370 and L30HL093896).
Footnotes
Disclosures: Dr. Min has served on the medical advisory boards of GE Healthcare, Arineta, Astra Zeneca, and Bristol-Myers Squibb; Speakers’ Bureau of GE Healthcare; and received research support from GE Healthcare, Vital Images, and Phillips Healthcare. Dr. Truong received grant support from St. Jude Medical, American College of Radiology Imaging Network, and Duke Clinical Research Institute.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stolzmann P, Scheffel H, Trindade PT, Plass AR, Husmann L, Leschka S, Genoni M, Marincek B, Kaufmann PA, Alkadhi H. Left ventricular and left atrial dimensions and volumes: comparison between dual-source CT and echocardiography. Invest Radiol. 2008;43:284–289. doi: 10.1097/RLI.0b013e3181626853. [DOI] [PubMed] [Google Scholar]
- 2.Takx RA, Moscariello A, Schoepf UJ, Barraza JM, Jr, Nance JW, Jr, Bastarrika G, Das M, Meyer M, Wildberger JE, Schoenberg SO, Fink C, Henzler T. Quantification of left and right ventricular function and myocardial mass: comparison of low-radiation dose 2nd generation dual-source CT and cardiac MRI. Eur J Radiol. 2012;81:e598–604. doi: 10.1016/j.ejrad.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Kim SS, Ko SM, Song MG, Kim JS. Assessment of global function of left ventricle with dual-source CT in patients with severe arrhythmia: a comparison with the use of two-dimensional transthoracic echocardiography. Int J Cardiovasc Imaging. 2010;26:213–221. doi: 10.1007/s10554-010-9692-2. [DOI] [PubMed] [Google Scholar]
- 4.Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJ, Cleland JG, Pennell DJ. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21:1387–1396. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 5.Gordon EP, Schnittger I, Fitzgerald PJ, Williams P, Popp RL. Reproducibility of left ventricular volumes by two-dimensional echocardiography. J Am Coll Cardiol. 1983;2:506–513. doi: 10.1016/s0735-1097(83)80278-2. [DOI] [PubMed] [Google Scholar]
- 6.Asferg C, Usinger L, Kristensen TS, Abdulla J. Accuracy of multi-slice computed tomography for measurement of left ventricular ejection fraction compared with cardiac magnetic resonance imaging and two-dimensional transthoracic echocardiography: a systematic review and meta-analysis. Eur J Radiol. 2012;81:e757–762. doi: 10.1016/j.ejrad.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Geleijnse ML, Fioretti PM, Roelandt JR. Methodology, feasibility, safety and diagnostic accuracy of dobutamine stress echocardiography. J Am Coll Cardiol. 1997;30:595–606. doi: 10.1016/s0735-1097(97)00206-4. [DOI] [PubMed] [Google Scholar]
- 8.Lindstrom L, Wilkenshoff UM, Larsson H, Wranne B. Echocardiographic assessment of arrhythmogenic right ventricular cardiomyopathy. Heart. 2001;86:31–38. doi: 10.1136/heart.86.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewey M, Muller M, Eddicks S, Schnapauff D, Teige F, Rutsch W, Borges AC, Hamm B. Evaluation of global and regional left ventricular function with 16-slice computed tomography, biplane cineventriculography, and two-dimensional transthoracic echocardiography: comparison with magnetic resonance imaging. J Am Coll Cardiol. 2006;48:2034–2044. doi: 10.1016/j.jacc.2006.04.104. [DOI] [PubMed] [Google Scholar]
- 10.Wai B, Thai WE, Brown H, Truong QA. Novel phase-based noise reduction strategy for quantification of left ventricular function and mass assessment by cardiac CT: comparison with cardiac magnetic resonance. Eur J Radiol. 2013;82:e337–341. doi: 10.1016/j.ejrad.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhave NM, Mor-Avi V, Kachenoura N, Freed BH, Vannier M, Dill K, Lang RM, Patel AR. Analysis of myocardial perfusion from vasodilator stress computed tomography: does improvement in image quality by iterative reconstruction lead to improved diagnostic accuracy? J Cardiovasc Comput Tomogr. 2014;8:238–245. doi: 10.1016/j.jcct.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Yuki H, Utsunomiya D, Funama Y, Tokuyasu S, Namimoto T, Hirai T, Itatani R, Katahira K, Oshima S, Yamashita Y. Value of knowledge-based iterative model reconstruction in low-kV 256-slice coronary CT angiography. J Cardiovasc Comput Tomogr. 2014;8:115–123. doi: 10.1016/j.jcct.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Brodoefel H, Kramer U, Reimann A, Burgstahler C, Schroeder S, Kopp A, Heuschmid M. Dual-source CT with improved temporal resolution in assessment of left ventricular function: a pilot study. AJR Am J Roentgenol. 2007;189:1064–1070. doi: 10.2214/AJR.07.2228. [DOI] [PubMed] [Google Scholar]
- 14.Nasir K, Katz R, Mao S, Takasu J, Bomma C, Lima JA, Bluemke DA, Kronmal R, Carr JJ, Budoff MJ. Comparison of left ventricular size by computed tomography with magnetic resonance imaging measures of left ventricle mass and volumes: the multi-ethnic study of atherosclerosis. J Cardiovasc Comput Tomogr. 2008;2:141–148. doi: 10.1016/j.jcct.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Schlosser T, Mohrs OK, Magedanz A, Voigtlander T, Schmermund A, Barkhausen J. Assessment of left ventricular function and mass in patients undergoing computed tomography (CT) coronary angiography using 64-detector-row CT: comparison to magnetic resonance imaging. Acta Radiol. 2007;48:30–35. doi: 10.1080/02841850601067611. [DOI] [PubMed] [Google Scholar]
- 16.Busch S, Johnson TR, Wintersperger BJ, Minaifar N, Bhargava A, Rist C, Reiser MF, Becker C, Nikolaou K. Quantitative assessment of left ventricular function with dual-source CT in comparison to cardiac magnetic resonance imaging: initial findings. Eur Radiol. 2008;18:570–575. doi: 10.1007/s00330-007-0767-y. [DOI] [PubMed] [Google Scholar]
- 17.Dewey M, Muller M, Teige F, Schnapauff D, Schink T, Hamm B, Lembcke A. Multisegment and halfscan reconstruction of 16-slice computed tomography for assessment of regional and global left ventricular myocardial function. Invest Radiol. 2006;41:400–409. doi: 10.1097/01.rli.0000201233.42994.9b. [DOI] [PubMed] [Google Scholar]
- 18.Maffei E, Messalli G, Martini C, Nieman K, Catalano O, Rossi A, Seitun S, Guaricci AI, Tedeschi C, Mollet NR, Cademartiri F. Left and right ventricle assessment with Cardiac CT: validation study vs. Cardiac MR. Eur Radiol. 2012;22:1041–1049. doi: 10.1007/s00330-011-2345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raman SV, Shah M, McCarthy B, Garcia A, Ferketich AK. Multi-detector row cardiac computed tomography accurately quantifies right and left ventricular size and function compared with cardiac magnetic resonance. Am Heart J. 2006;151:736–744. doi: 10.1016/j.ahj.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 20.Bak SH, Ko SM, Jeon HJ, Yang HS, Hwang HK, Song MG. Assessment of global left ventricular function with dual-source computed tomography in patients with valvular heart disease. Acta Radiol. 2012;53:270–277. doi: 10.1258/ar.2011.110247. [DOI] [PubMed] [Google Scholar]
- 21.Arraiza M, Azcarate PM, De Cecco CN, Viteri G, Simon-Yarza I, Hernandez-Estefania R, Rabago G, Bastarrika G. Assessment of left ventricular parameters in orthotopic heart transplant recipients using dual-source CT and contrast-enhanced echocardiography: comparison with MRI. Eur J Radiol. 2012;81:3282–3288. doi: 10.1016/j.ejrad.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O’Gara P, Rubin GD, Kramer CM, Berman D, Brown A, Chaudhry FA, Cury RC, Desai MY, Einstein AJ, Gomes AS, Harrington R, Hoffmann U, Khare R, Lesser J, McGann C, Rosenberg A, Schwartz R, Shelton M, Smetana GW, Smith SC, Jr American College of Cardiology Foundation Appropriate Use Criteria Task F, Society of Cardiovascular Computed T, American College of R, American Heart A, American Society of E, American Society of Nuclear C, North American Society for Cardiovascular I, Society for Cardiovascular A, Interventions, Society for Cardiovascular Magnetic R. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56:1864–1894. doi: 10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Bauters C, Deneve M, Tricot O, Meurice T, Lamblin N, Investigators C. Prognosis of patients with stable coronary artery disease (from the CORONOR study) Am J Cardiol. 2014;113:1142–1145. [Google Scholar]
- 24.Lewis EF, Moye LA, Rouleau JL, Sacks FM, Arnold JM, Warnica JW, Flaker GC, Braunwald E, Pfeffer MA, Study C. Predictors of late development of heart failure in stable survivors of myocardial infarction: the CARE study. J Am Coll Cardiol. 2003;42:1446–1453. doi: 10.1016/s0735-1097(03)01057-x. [DOI] [PubMed] [Google Scholar]
- 25.Palazzuoli A, Cademartiri F, Geleijnse ML, Meijboom B, Pugliese F, Soliman O, Calabro A, Nuti R, de Feyter P. Left ventricular remodelling and systolic function measurement with 64 multi-slice computed tomography versus second harmonic echocardiography in patients with coronary artery disease: a double blind study. Eur J Radiol. 2010;73:82–88. doi: 10.1016/j.ejrad.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Cury RC, Nieman K, Shapiro MD, Butler J, Nomura CH, Ferencik M, Hoffmann U, Abbara S, Jassal DS, Yasuda T, Gold HK, Jang IK, Brady TJ. Comprehensive assessment of myocardial perfusion defects, regional wall motion, and left ventricular function by using 64-section multidetector CT. Radiology. 2008;248:466–475. doi: 10.1148/radiol.2482071478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bezerra HG, Loureiro R, Irlbeck T, Bamberg F, Schlett CL, Rogers I, Blankstein R, Truong QA, Brady TJ, Cury RC, Hoffmann U. Incremental value of myocardial perfusion over regional left ventricular function and coronary stenosis by cardiac CT for the detection of acute coronary syndromes in high-risk patients: a subgroup analysis of the ROMICAT trial. J Cardiovasc Comput Tomogr. 2011;5:382–391. doi: 10.1016/j.jcct.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Seneviratne SK, Truong QA, Bamberg F, Rogers IS, Shapiro MD, Schlett CL, Chae CU, Cury R, Abbara S, Brady TJ, Nagurney JT, Hoffmann U. Incremental diagnostic value of regional left ventricular function over coronary assessment by cardiac computed tomography for the detection of acute coronary syndrome in patients with acute chest pain: from the ROMICAT trial. Circ Cardiovasc Imaging. 2010;3:375–383. doi: 10.1161/CIRCIMAGING.109.892638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, Truong QA, Cury RC, Abbara S, Shapiro MD, Moloo J, Butler J, Ferencik M, Lee H, Jang IK, Parry BA, Brown DF, Udelson JE, Achenbach S, Brady TJ, Nagurney JT. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53:1642–1650. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hausleiter J, Meyer T, Hermann F, Hadamitzky M, Krebs M, Gerber TC, McCollough C, Martinoff S, Kastrati A, Schomig A, Achenbach S. Estimated radiation dose associated with cardiac CT angiography. Jama. 2009;301:500–507. doi: 10.1001/jama.2009.54. [DOI] [PubMed] [Google Scholar]
- 31.Hausleiter J, Meyer TS, Martuscelli E, Spagnolo P, Yamamoto H, Carrascosa P, Anger T, Lehmkuhl L, Alkadhi H, Martinoff S, Hadamitzky M, Hein F, Bischoff B, Kuse M, Schomig A, Achenbach S. Image quality and radiation exposure with prospectively ECG-triggered axial scanning for coronary CT angiography: the multicenter, multivendor, randomized PROTECTION-III study. JACC Cardiovasc Imaging. 2012;5:484–493. doi: 10.1016/j.jcmg.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Cohn JN, Johnson GR, Shabetai R, Loeb H, Tristani F, Rector T, Smith R, Fletcher R. Ejection fraction, peak exercise oxygen consumption, cardiothoracic ratio, ventricular arrhythmias, and plasma norepinephrine as determinants of prognosis in heart failure. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI5–16. [PubMed] [Google Scholar]
- 33.Nicolosi GL, Latini R, Marino P, Maggioni AP, Barlera S, Franzosi MG, Geraci E, Santoro L, Tavazzi L, Tognoni G, Vecchio C, Volpi A. The prognostic value of predischarge quantitative two-dimensional echocardiographic measurements and the effects of early lisinopril treatment on left ventricular structure and function after acute myocardial infarction in the GISSI-3 Trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico. Eur Heart J. 1996;17:1646–1656. doi: 10.1093/oxfordjournals.eurheartj.a014747. [DOI] [PubMed] [Google Scholar]
- 34.Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O’Gara P, Rubin GD. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2010;122:e525–555. doi: 10.1161/CIR.0b013e3181fcae66. [DOI] [PubMed] [Google Scholar]
- 35.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L Cardiac Resynchronization-Heart Failure Study I. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 36.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM Comparison of Medical Therapy P, Defibrillation in Heart Failure I. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 37.Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, Yee R, Healey JS, Rouleau JL Resynchronization-Defibrillation for Ambulatory Heart Failure Trial I. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 38.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA, 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W, Investigators M-CT. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 39.Truong QA, Singh JP, Cannon CP, Sarwar A, Nasir K, Auricchio A, Faletra FF, Sorgente A, Conca C, Moccetti T, Handschumacher M, Brady TJ, Hoffmann U. Quantitative analysis of intraventricular dyssynchrony using wall thickness by multidetector computed tomography. JACC Cardiovasc Imaging. 2008;1:772–781. doi: 10.1016/j.jcmg.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Institute of Health. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Massachusetts General Hospital. Dual-Source Computed Tomography to Improve Prediction of Response to Cardiac Resynchronization Therapy (DIRECT) [cited 2014 July 31]. Available from: http://clinicaltrials.gov/show/NCT01097733 NLM Identifier: NCT01097733. [Google Scholar]
- 41.Acharya D, Singh S, Tallaj JA, Holman WL, George JF, Kirklin JK, Pamboukian SV. Use of gated cardiac computed tomography angiography in the assessment of left ventricular assist device dysfunction. ASAIO J. 2011;57:32–37. doi: 10.1097/MAT.0b013e3181fd3405. [DOI] [PubMed] [Google Scholar]
- 42.Boogers MJ, van Werkhoven JM, Schuijf JD, Delgado V, El-Naggar HM, Boersma E, Nucifora G, van der Geest RJ, Paelinck BP, Kroft LJ, Reiber JH, de Roos A, Bax JJ, Lamb HJ. Feasibility of diastolic function assessment with cardiac CT: feasibility study in comparison with tissue Doppler imaging. JACC Cardiovasc Imaging. 2011;4:246–256. doi: 10.1016/j.jcmg.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 43.van der Bijl N, Klok FA, Huisman MV, van Rooden JK, Mertens BJ, de Roos A, Kroft LJ. Measurement of right and left ventricular function by ECG-synchronized CT scanning in patients with acute pulmonary embolism: usefulness for predicting short-term outcome. Chest. 2011;140:1008–1015. doi: 10.1378/chest.10-3174. [DOI] [PubMed] [Google Scholar]
- 44.de Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, Lablanche JM. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32:948–954. doi: 10.1016/s0735-1097(98)00337-4. [DOI] [PubMed] [Google Scholar]
- 45.Sugeng L, Mor-Avi V, Weinert L, Niel J, Ebner C, Steringer-Mascherbauer R, Bartolles R, Baumann R, Schummers G, Lang RM, Nesser HJ. Multimodality comparison of quantitative volumetric analysis of the right ventricle. JACC Cardiovasc Imaging. 2010;3:10–18. doi: 10.1016/j.jcmg.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Ho SY, Nihoyannopoulos P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart. 2006;92 (Suppl 1):i2–13. doi: 10.1136/hrt.2005.077875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gopalan D. Right heart on multidetector CT. Br J Radiol. 2011;84(Spec No 3):S306–323. doi: 10.1259/bjr/59278996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo YK, Gao HL, Zhang XC, Wang QL, Yang ZG, Ma ES. Accuracy and reproducibility of assessing right ventricular function with 64-section multi-detector row CT: comparison with magnetic resonance imaging. Int J Cardiol. 2010;139:254–262. doi: 10.1016/j.ijcard.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 49.Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005;95:779–782. doi: 10.1016/j.amjcard.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 50.Lee C, Kim YM, Lee CH, Kwak JG, Park CS, Song JY, Shim WS, Choi EY, Lee SY, Baek JS. Outcomes of pulmonary valve replacement in 170 patients with chronic pulmonary regurgitation after relief of right ventricular outflow tract obstruction: implications for optimal timing of pulmonary valve replacement. J Am Coll Cardiol. 2012;60:1005–1014. doi: 10.1016/j.jacc.2012.03.077. [DOI] [PubMed] [Google Scholar]
- 51.Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289–1300. doi: 10.1016/S0140-6736(09)60256-7. [DOI] [PubMed] [Google Scholar]
- 52.Wu YW, Tadamura E, Kanao S, Yamamuro M, Nishiyama K, Kimura T, Kita T, Togashi K. Structural and functional assessment of arrhythmogenic right ventricular dysplasia/cardiomyopathy by multi-slice computed tomography: comparison with cardiovascular magnetic resonance. Int J Cardiol. 2007;115:e118–121. doi: 10.1016/j.ijcard.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 53.Bomma C, Dalal D, Tandri H, Prakasa K, Nasir K, Roguin A, Piccini J, Dong J, Mahadevappa M, Tichnell C, James C, Lima JA, Fishman E, Calkins H, Bluemke DA. Evolving role of multidetector computed tomography in evaluation of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Cardiol. 2007;100:99–105. doi: 10.1016/j.amjcard.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 54.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakajima T, Kimura F, Kajimoto K, Kasanuki H, Hagiwara N. Utility of ECG-gated MDCT to differentiate patients with ARVC/D from patients with ventricular tachyarrhythmias. J Cardiovasc Comput Tomogr. 2013;7:223–233. doi: 10.1016/j.jcct.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010;31:806–814. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tandri H, Daya SK, Nasir K, Bomma C, Lima JA, Calkins H, Bluemke DA. Normal reference values for the adult right ventricle by magnetic resonance imaging. Am J Cardiol. 2006;98:1660–1664. doi: 10.1016/j.amjcard.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 58.Pursnani A, Lee A, Mayrhofer T, Panagia M, Sharma U, Abbara S, Hoffmann U, Ghoshhajra BB. Feasibility of a radiation dose conserving CT protocol for myocardial function assessment. Br J Radiol. 2014;87:20130755. doi: 10.1259/bjr.20130755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feuchtner G, Goetti R, Plass A, Baumueller S, Stolzmann P, Scheffel H, Wieser M, Marincek B, Alkadhi H, Leschka S. Dual-step prospective ECG-triggered 128-slice dual-source CT for evaluation of coronary arteries and cardiac function without heart rate control: a technical note. Eur Radiol. 2010;20:2092–2099. doi: 10.1007/s00330-010-1794-7. [DOI] [PubMed] [Google Scholar]
- 60.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS American Heart Association Writing Group on Myocardial S, Registration for Cardiac I. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 61.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W American Society of Echocardiography’s N, Standards C Task Force on Chamber Q, American College of Cardiology Echocardiography C, American Heart A, European Association of Echocardiography ESoC. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 62.Arsanjani R, Berman DS, Gransar H, Cheng VY, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Callister TQ, Chang HJ, Cademartiri F, Chinnaiyan KM, Chow BJ, DeLago A, Hadamitzky M, Hausleiter J, Kaufmann P, LaBounty TM, Leipsic J, Raff G, Shaw LJ, Villines TC, Cury RC, Feuchtner G, Kim YJ, Min JK For the CI. Left Ventricular Function and Volume with Coronary CT Angiography Improves Risk Stratification and Identification of Patients at Risk for Incident Mortality: Results from 7758 Patients in the Prospective Multinational CONFIRM Observational Cohort Study. Radiology. 2014:122816. doi: 10.1148/radiol.14122816. [DOI] [PubMed] [Google Scholar]
- 63.Lin FY, Devereux RB, Roman MJ, Meng J, Jow VM, Jacobs A, Weinsaft JW, Shaw LJ, Berman DS, Callister TQ, Min JK. Cardiac chamber volumes, function, and mass as determined by 64-multidetector row computed tomography: mean values among healthy adults free of hypertension and obesity. JACC Cardiovasc Imaging. 2008;1:782–786. doi: 10.1016/j.jcmg.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 64.Greupner J, Zimmermann E, Hamm B, Dewey M. Automatic vs semi-automatic global cardiac function assessment using 64-row CT. Br J Radiol. 2012;85:e243–253. doi: 10.1259/bjr/65747000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mao SS, Li D, Rosenthal DG, Cerilles M, Zeb I, Wu H, Flores F, Gao Y, Budoff MJ. Dual-standard reference values of left ventricular volumetric parameters by multidetector CT angiography. J Cardiovasc Comput Tomogr. 2013;7:234–240. doi: 10.1016/j.jcct.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 66.Stojanovska J, Prasitdumrong H, Patel S, Sundaram B, Gross BH, Yilmaz ZN, Kazerooni EA. Reference absolute and indexed values for left and right ventricular volume, function and mass from cardiac computed tomography. J Med Imaging Radiat Oncol. 2014 doi: 10.1111/1754-9485.12186. [DOI] [PubMed] [Google Scholar]
- 67.Yamaoka O, Yabe T, Okada M, Endoh S, Nakamura Y, Mitsunami K, Kinoshita M, Mori M, Murata K, Morita R. Evaluation of left ventricular mass: comparison of ultrafast computed tomography, magnetic resonance imaging, and contrast left ventriculography. Am Heart J. 1993;126:1372–1379. doi: 10.1016/0002-8703(93)90536-i. [DOI] [PubMed] [Google Scholar]
- 68.Sievers B, Kirchberg S, Bakan A, Franken U, Trappe HJ. Impact of papillary muscles in ventricular volume and ejection fraction assessment by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2004;6:9–16. doi: 10.1081/jcmr-120027800. [DOI] [PubMed] [Google Scholar]
- 69.Papavassiliu T, Kuhl HP, Schroder M, Suselbeck T, Bondarenko O, Bohm CK, Beek A, Hofman MM, van Rossum AC. Effect of endocardial trabeculae on left ventricular measurements and measurement reproducibility at cardiovascular MR imaging. Radiology. 2005;236:57–64. doi: 10.1148/radiol.2353040601. [DOI] [PubMed] [Google Scholar]