Abstract
Innate recognition of fungi leads to strong adaptive immunity. Investigators are trying to exploit this observation in vaccine development by combining antigens with evolutionarily conserved fungal cell wall carbohydrates to induce protective responses. Best studied is β-1,3-glucan, a glycan that activates complement and is recognized by Dectin-1. Administration of antigens in association with β-1,3-glucan, either by direct conjugation or complexed in glucan particles, results in robust humoral and cellular immune responses. While the host has a host of mannose receptors, responses to fungal mannoproteins generally are amplified if cells are cooperatively stimulated with an additional danger signal such as a toll-like receptor agonist. Chitosan, a polycationic homopolymer of glucosamine manufactured by the deacetylation of chitin, is being studied as an adjuvant in DNA and protein-based vaccines. It appears particularly promising in mucosal vaccines. Finally, universal and organism-specific fungal vaccines have been formulated by conjugating fungal cell wall glycans to carrier proteins. A major challenge will be to advance these experimental findings so that at risk patients can be protected.
Keywords: β-glucan, mannan, chitosan, chitin, fungus, vaccine
Introduction
Vaccines are one of the greatest triumphs in the history of medicine (1). Despite these gains, effective vaccines remain elusive for many infectious diseases. Moreover, many existing vaccines use live, attenuated organisms. While many live vaccines confer long-lasting immunity, they also tend to have a higher frequency of adverse events, including the potential to cause the infectious disease they are designed to protect against (2). This is especially a problem for the ever growing population of immunocompromised individuals, in whom live vaccines are contraindicated because of the risk of disseminated infections.
Vaccines that contain killed whole organisms or purified antigens (“subunit vaccines”) are intrinsically safer. However, purified antigens tend to not elicit robust immune responses unless administered with an adjuvant to augment the adaptive immune response and generate immunological memory (3). One promising approach to improving subunit vaccines is to use vaccine platforms that function as delivery systems to efficiently target antigen to dendritic cells (DCs), the professional antigen-presenting cells of the immune system most responsible for initiating immune responses (1). Critical then for vaccine design is the delivery system and the choice of adjuvants. The adjuvant used in most licensed subunit vaccines, alum, generally biases towards Th2-type antibody responses (1, 3, 4). While antibody elicited by alum-adjuvanted vaccines is adequate to protect against many diseases, T cell defenses appear to be required to protect against many of the pathogens for which effective vaccines are lacking. In addition, vaccine-mediated T cell protection may work best if the response is skewed, as for example towards a Th1- or Th17-type response.
Innate defenses against fungi are robust and evolutionarily conserved in the animal kingdom. In mammals, if innate defenses fail, adaptive immunity gets triggered. The ongoing “epidemic” of fungal infections is primarily driven by the increased numbers of persons with defects in one or more host defenses mechanisms (5). For example, the vast majority of the over one million estimated annual cases of cryptococcosis have T cell immunocompromise due to AIDS (6) with a lesser contribution from the use of immunosuppressive medications to treat autoimmune diseases and to prevent transplant rejection. The lesson is that in when host defenses are intact, fungal infections are very rare. The question then arises whether this observation can be exploited in vaccine development. In other words, if innate recognition of fungi leads to strong adaptive immunity, can vaccines combine antigens with specific components of fungi to induce protective responses?
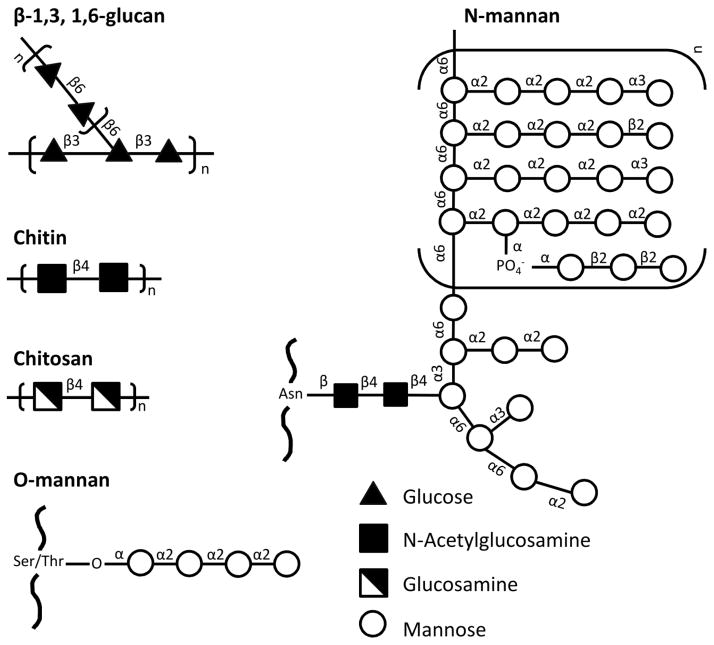
In this article, we review the studies examining this concept. Our focus is on the conserved carbohydrate components of the fungal cell wall that are recognized by receptors on dendritic cells (figure 1). These glycans include β-glucans (β-1,3-linked polymers of glucose with β-1,6 branches), chitin (homopolymer of N-acetylglucosamine); chitosan (deacetylated chitin) and mannans (mannose chains of varying lengths and configurations added to fungal proteins via N- or O-linkages) (7). Structurally, chitin and chitosan tends to be on the inner portion of the cell wall, β-glucans on the middle and mannans on the outside. While the extent varies depending on the fungal species, mannans “mask” β-glucans, chitin and chitosan, thereby impairing fungal recognition. Moreover, some fungi also have other masking glycans, including α-glucans and glucuronoxylomannan (8, 9).
Figure 1. Representative carbohydrate components of the fungal cell wall that have been exploited in vaccine design.
Note that there is considerable interspecies and intraspecies variability, particularly in the structures of O- and N-mannans.
β-glucans
Even primitive invertebrates such as the horseshoe crab are able to sense β-glucans and mount an innate immune response (7). In mammals, the major receptors that recognize β-glucans are Dectin-1 and complement receptor 3 (CR3). The β-glucan recognition site on CR3 is distinct from the complement recognition site. The scavenger receptors CD5, CD36 and SCARF1 have also been reported to recognize β-glucans. Both β-1,3- and β-1,6-linked glucans potently activate the alternative pathway of complement resulting in fungal opsonization and the generation of chemotaxins which can recruit inflammatory cells (10).
The immunostimulatory properties of β-glucans were recognized long before the discovery of Dectin-1 and other receptors for β-glucans. Notably, β-glucan preparations derived from fungi have a record of safety in both preclinical and human trials (11–13). Stimulation via Dectin-1 primes Th17, Th1, and cytotoxic T cell responses (14–16). Injection of antigen targeted to Dectin-1 induced CD4+ and CD8+ T cell responses at doses that antigen alone failed to produce a response (17). Thus, a natural extension of these findings has been to explore the use of β-glucans as vaccine adjuvants and delivery systems.
Our laboratories have extensively explored the use of glucan particles (GPs) as a vaccine delivery system. GPs are highly purified, hollow, porous cell wall shells derived from baker’s yeast following a series of hot alkali and organic extractions. GPs are composed primarily of β-1,3-glucan although do contain some chitin and β-1,6-glucans. Using KO mice and receptor antagonists, we demonstrated that GPs are recognized by Dectin-1(18), a C-type lectin receptor highly expressed on phagocytes including myeloid dendritic cells (mDCs) (19). If a source of complement is provided, GPs will activate complement and in vivo, phagocytosis of GPs is mediated by complement receptors and Dectin-1(10, 20). GPs stimulate mDCs to produce cytokines associated with beneficial responses in vaccine models(18). Moreover, synergistic DC cytokine responses are seen when GPs are combined with toll-like receptor (TLR) agonists(18).
Polymer-complexed cores can be constructed within the hollow GPs, enabling the delivery to DCs and macrophages of a wide variety of “payload” classes including proteins, siRNA, DNA, and other small molecules (20–25) (figure 2). As a proof of principle, we have constructed GPs containing complexed ovalbumin (GP-OVA), exploiting the plethora of immunological tools available to study this model antigen. Robust and long-lasting antigen-specific humoral (IgG1 and IgG2c) and T cell (Th1- and Th17-biased) responses were observed following subcutaneous immunization of mice with GPs “encapsulated” with antigen (20, 24). In contrast, consistent with alum being a poor stimulator of T cell responses, mice that received OVA admixed with alum predominantly exhibited IgG1 antibody responses. Interestingly, the Th17-skewing after GP-based immunization was diminished by about 50% in mice deficient in the third component of complement, but not in dectin-1−/− mice (20).
Figure 2. Glucan particles (GPs).
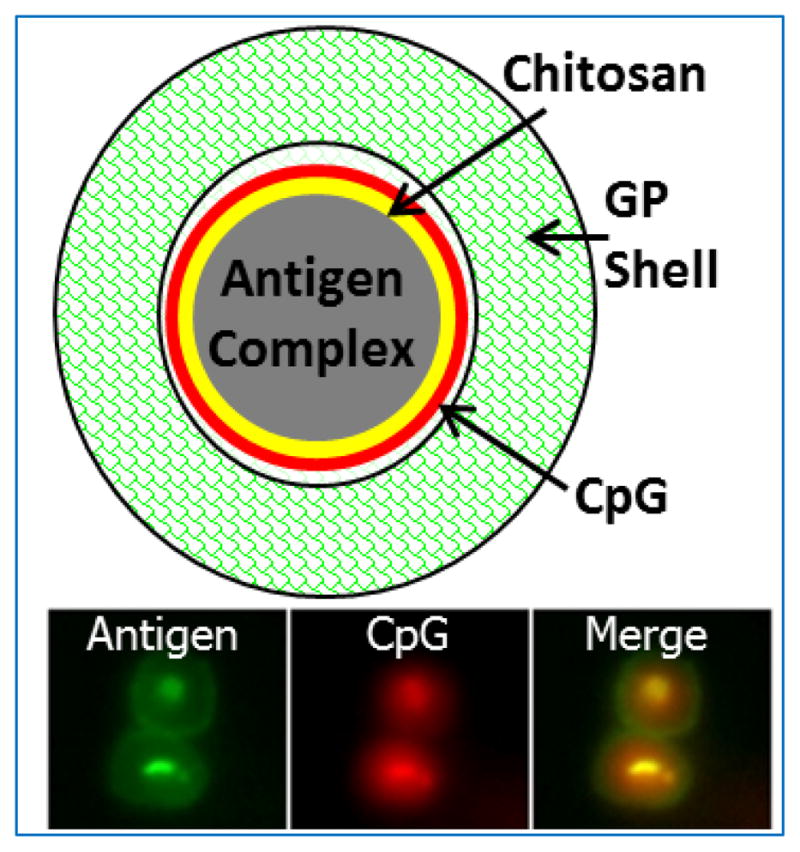
Top: Schematic showing the “layer-by-layer” design of a GP containing complexed antigen and CpG-rich DNA. Bottom: Epifluorescent photomicrographs of two abutting GPs loaded with antigen (fluorescently labeled green with FITC) and CpG (fluorescently labeled red with TRITC).
The above studies used yeast RNA to trap antigen within the GPs (20, 24). As the long-term goal is to develop human vaccines, we have begun to formulate GP-vaccines using pharmaceutical-grade materials generally regarded as safe. Mice were vaccinated with GPs containing OVA trapped with: 1) yeast RNA; 2) alginate calcium; and 3) alginate calcium plus chitosan. Robust antigen-specific CD4+ T cell and antibody responses were seen regardless of the trapping materials utilized (26). Note that alginate calcium and chitosan are approved by the United States Food and Drug Administration and generally regarded as safe (27, 28).
Ideal vaccines promote immune responses that are durable and of high quality (1). To examine this, mice received 3 vaccinations of GP-OVA and were euthanized 20 months later. Single cell suspensions of lymph node and spleens were then stimulated with OVA and analyzed by polychromatic flow cytometry for surface expression of CD3, CD4, and CD8 as well as intracellular staining for IFNγ, IL-17a and IL-4. Unvaccinated mice served as controls. As expected, the naïve mice had virtually no CD4+ T cells that stained for intracellular cytokines. However, 9.16%, 2.16% and 0.31% of the CD4+ cells from the vaccinated mice were positive for intracellular IFNγ, IL-17a and both cytokines, respectively (26). Staining for IL-4 was not observed. In addition to durability of response, antigen-sparing is desirable in vaccines (1). We found that mice immunized with sub-microgram doses of antigen still had brisk Th1- and Th17-biased CD4+ T cell and antibody responses (26). Finally, we demonstrated that GPs function as both a delivery system and an adjuvant ; antibody and T cell responses are seen when GPs and OVA are admixed but are substantially greater when OVA is encapsulated within the GPs (26). In unpublished work, we have demonstrated that vaccination of mice with GPs loaded with pathogen-specific antigens provides protection against certain lethal infections and validated the utility of GPs as a tool in vaccine antigen discovery.
Heat-killed S. cerevisiae genetically engineered to express antigens are undergoing clinical trials as immunotherapeutic vaccines for patients with certain cancers and chronic infections (29, 30). Importantly, in the human and animal studies, strong Ag-specific helper and cytotoxic T lymphocyte responses were elicited (29, 31–33). The latter is especially notable because it has been generally difficult to elicit CD8+ T cell responses using killed vaccines. The reported safety profile has thus far been favorable in phase I/II dose-escalation trials (29). However, because yeast proteins, lipids and nucleic acids are not eliminated, concerns regarding reactogenicity and autoimmunity could limit the appeal of this platform as a preventive vaccine given to predominantly healthy people.
Mannans
Mammalian and fungal cells share fundamental eukaryotic features of protein glycosylation; initial glycosylation occurs in the endoplasmic reticulum with further processing in the Golgi (34, 35). However, whereas fully processed mammalian glycoproteins rarely have terminal mannose groups, N-linked and O-linked glycans on yeasts generally are terminally mannosylated. N-linked glycans on yeast proteins can be hundreds of sugars long and have extensive branching, while O-linked glycans tend to be linear chains of two to six mannoses (34–37). Patterns of mannosylation can vary extensively when comparing different species of fungi. Strains and morphotypes within a species may also have heterogeneity of mannosylation.
Misfolded or incompletely processed mammalian proteins can have terminal mannosylation. Thus, a dilemma the host immune system faces is how to distinguish mannosylated self-proteins from foreign antigens. There is evidence that this is accomplished in two ways. First, responses are greatly amplified if cells are cooperatively stimulated with mannosylated ligands and toll-like receptor agonists (38). Presumably, having the second “danger” signal tells the host it is likely dealing with a pathogen. Indeed, some studies have demonstrated immunological non-responsiveness against processed mannosylated antigens (39, 40). Second, the host has multiple mannose receptors, with varying specificities with regards to the molecular configurations of the mannose chains that are recognized and the signaling pathways that are triggered. Myeloid C-type lectin receptors with reported affinity for mannose that have been implicated in recognition of fungi include the mannose receptor (CD206), DC-SIGN (CD209), Dectin-2 (CLEC6A), Mincle (CLEC4E) and Langerin (CD207) (41, 42).
C-type lectin receptors have cytoplasmic tails containing sorting motifs directing internalization into clathrin-coated vesicles. For example, mannosylated antigens taken up by the mannose receptor are endocytosed and released into the acidic environment of early endosomes. The mannose receptor then recycles to the cell surface while the released antigen is processed for subsequent presentation on MHC Class II molecules (43). This results in considerably more efficient intracellular degradation and antigen loading compared with macropinocytosis (44). DC-SIGN also contains internalization motifs which target antigen for presentation to T cells (45). Not surprisingly then, fungal mannosylation of antigens has been explored as a vaccination strategy.
For the encapsulated fungus, Cryptococcus neoformans, considerable evidence has accumulated that protein mannosylation drives T cell responses. Pioneering studies examined a crude culture supernatant which stimulated T cell responses in immunized mice. It was found that the mannoprotein fraction, defined by the ability to adhere to a Concanavalin A affinity column, was predominantly responsible for these responses (46). Subsequent work demonstrated that mannoproteins stimulate lymphoproliferation of CD4+ T cells obtained from HIV- and HIV+ cryptococcosis patients (47–50). Moreover, most cryptococcal proteins identified on the basis of immunoreactivity have been found to be mannoproteins. For example, our laboratory made two T cell hybridomas from mice immunized with disrupted C. neoformans cells. The antigens that stimulated the two hybridomas were both mannoproteins (designated MP98 and MP88) that shared structural features including a signal sequence, a functional domain, a serine/threonine-rich region (which features extensive O-mannosylation) and an omega site for attachment of a glycosylphosphatidylinositol (GPI) anchor (35, 51, 52). Moreover, we identified 53 other predicted mannoproteins that share these common features by in silico analysis (35).
To investigate the molecular basis of mannosylation in immune responses to cryptococcal proteins, MP98 was expressed recombinantly in a bacterium (Escherichia coli) and a yeast (Pichia pastoris), resulting in unglycosylated and mannosylated proteins, respectively (53). In addition, P. pastoris MP98 was chemically deglycosylated. The Pichia-derived antigen stimulated significantly more antigen-specific cytokine production compared with the E. coli–derived antigen. Moreover, responses were potently inhibited if the antigen was chemically deglycosylated or if MR were blocked with mannans. A similar strategy was utilized with the model antigen, OVA (54). Recombinant proteins containing immunoreactive portions of OVA were produced in P. pastoris with variations in the degree and type of glycosylation. The presence or absence of N-linked mannosylation was controlled by site-directed mutagenesis of potential N-linked glycosylation sites. Extensive O-linked mannosylation was accomplished by adding the S/T region of the cryptococcal mannoprotein, MP98. Completely unglycosylated counterparts were produced in E. coli. OVA containing N-linkages, extensive O-linkages, or both were more effective than unglycosylated Ags at inducing antigen-specific proliferation of CD4+ and CD8+ T cells in vitro (54–56). This effect was apparently dependent on mannose receptors as mannan inhibited the enhanced response.
Others too have incorporated mannosylation of antigens or delivery systems as part of an experimental vaccination strategy (57). While many of these systems effectively target antigen to the antigen-processing machinery of DCs, for reasons noted above a second adjuvant may be required to elicit sustained immunity (58). Finally, directing antigen to DCs via anti-DC-SIGN or anti-mannose receptor antibodies results in brisk antigen-specific T cell responses (59, 60).
Chitin and Chitosan
Chitin, a homopolymer consisting of β-1,4-linked repeating units of N-acetylglucosamine, is second only to cellulose as the most common natural polysaccharide on our planet (61). Deacetylation of chitin yields chitosan by conversion of N-acetylglucosamine to glucosamine and the polymer from a neutral polysaccharide to one that is polycationic. Some fungi (e.g., species of Mucor and Cryptococcus) have, in addition to chitin, relatively large portions of chitosan as part of their vegetative cell wall or in the case of Saccharomyces, as part of the spore wall. In these organisms, chitin deacetylase(s) enzymatically remove the acetyl group from N-acetylglucosamine.
Chitin is an integral component of all fungal cell walls and exoskeletons of crustacea and insects, which collectively are the principle sources for environmental exposure to chitin (62). Recent identification of chitin synthase genes in various other metazoans has reinforced the notion that chitin is an ancient polymer that has been integrated into a broad spectrum of eukaryotes (Zakrzewski et al., 2014). Thus, exposure to chitin is very common and can occur via a variety of routes including ingestion, inhalation and cutaneous contact. Mammals lack chitin but express multiple enzymes capable of degrading chitin and chitosan; their microbiomes also have this capability.
In fungi, chitin is buried in the cell wall and covalently linked to β-glucans. Its exposure to the environment (or mammalian host) is shielded by glucans and mannoproteins. Chitin in the exoskeleton of insects and crustaceans is likewise sequestered, in complex with numerous chitin binding proteins that organize chitin fibers into multiple layers. Insects also incorporate chitin into the peritrophic membrane that lines the gut. This chitin complexes with peritrophins and is periodically excreted. As chitin bearing organisms are degraded, chitin becomes more exposed.
Commercial preparations of chitin are typically made from crab and shrimp shell waste by a process that requires demineralization with acid and extraction with hot alkali. During this procedure the chitin is partially deacetylated. Chitosan is made by extended treatment in hot alkali to remove additional acetyl groups. Both “chitin” and “chitosan” are generic terms used to reflect the degree of deacetylation of the polymer. Chitin has been ascribed to polymers with <40% glucosamine and chitosan to ones having >60% glucosamine. Native chitin and possibly chitosan however approach being the respective homopolymers (61).
Chitin and chitosan have very different physiochemical properties. Chitin polymers complex into higher order crystalline-like structures and are insoluble in aqueous solutions; solubility is only gained upon hydrolysis to short oligosaccharides. Chitosan, regardless of polymer length, is soluble under mildly acidic conditions due to protonation of the amine group on glucosamine, but aggregates into an insoluble form as the pH rises to neutrality. The primary amine of glucosamine has made chitosan very versatile in biomedical applications as it enhances interaction with negatively charged biomolecules and its ease of being derivatized.
There may be multiple mammalian receptors for chitin and chitosan. One receptor of chitin is FlCBD1, which is an endocytic, membrane protein that binds the acetyl group of chitin through a extracellular, fibrinogen-like, recognition domain. It is expressed on enterocytes of the gut and on airway and salivary epithelial cells (63). Other proteins that bind chitin are secreted. These include the mammalian chitinases and chitinase-like proteins, which interact through chitin-binding domains. Their importance is in degradation, as well as in regulating immune responses to chitin (64). Another secreted protein RegIIIγ or HIP/PAP (its human counterpart) binds the peptidoglycan of gram-positive bacteria and is bactericidal. Chitin and the glycan portion of the peptidoglycan have similar structures (65). As chitin becomes deacetylated and converts to chitosan, the polymer becomes a polycation and interacts with negatively charged biomolecules, such as mucin, hyaluronan, nucleic acids, and fatty acids. These charge-based interactions could complicate efforts to identify a specific receptor for chitosan.
A variety of immunological effects have been attributed to chitin and/or chitosan, including stimulation of proinflammatory cytokines, contribution to asthma, and protection against infections and cancers (64). In recent studies, highly purified chitin too large to be phagocytosed was shown to induce in the lungs of mice release of Th2 cytokines IL-5 and Il-13, which directly led to accumulation of eosinophils and alternatively activated macrophages (66). Lung γδT cells were also activated to secrete IL-17 (Th-17 skewing) accompanied by increases in cytokines TNF-α, IL-1 and IL-23 (Th-1 skewing). Phagocytosis of pure chitin by mouse macrophages and dendritic cells, as well as human monocytes had little effect on cytokine secretion (67). However, phagocytosed chitosan activated the NLRP3 inflammasome with secretion of pro-inflammatory cytokines IL-1β and IL-18.
There is increasing use of chitin and chitosan in the biomedical industry. For example, chitosan nanofibrils of varying shapes have been studied as drug, nucleic acid, and protein delivery systems, as tissue engineering scaffolds to mimic a extracellular matrix, as promoters of wound healing, and as anti-microbials and vaccine adjuvants (68, 69). The only FDA approved use of chitosan however, is in hemostatic bandages under emergency conditions.
Chitosan has been extensively studied as a vaccine adjuvant, mainly for use under oral and nasal administration. Mucosal vaccines are designed to target and augment immunity at areas of the gut and respiratory and vaginal tracts that would first encounter a pathogen. Their reported advantage over vaccines delivered to non-mucosal tissues is the induction of antigen-specific, secreted IgA by B cells underlying the mucosal epithelium (70). FDA approved vaccines against influenza by nasal delivery and viruses and bacteria by oral delivery have used live, attenuated strains (influenza, polio, rotavirus, Salmonella typhi or heat-killed cells (Vibrio cholerae) to induce immunity. The challenge has been to develop subunit vaccines that effectively deliver antigens across mucus lined epithelium, bypassing hydrolytic and oxidative enzymes, to innate immune cells and lymphoid tissues. Chitosan has several properties that make it an attractive vaccine adjuvant (71). As a polycation, it is mucoadhesive through interaction with negatively charged sialic acid of mucin. Together with its ability to open tight junctions between epithelial cells, antigen is more effectively delivered to antigen-presenting cells. Chitosan is non-toxic, is slowly degraded and protects DNA plasmid- and protein-based vaccines from hydrolysis (28).
The effectiveness of chitosan as an adjuvant for intranasal delivery of influenza, pertussis and diphtheria vaccines was demonstrated in animal studies with protection to challenge, underlined by superior IgA responses and equivalent IgG responses to vaccination by injection (72). Studies continue to demonstrate that chitosan is an effective adjuvant for intranasal vaccines that promotes a protective cross-protective immune response against various strains of influenza (73).
While not typically studied by injection because its mucoadhesive properties become muted, the high viscosity of chitosan aids in a prolonged release of antigen. In mice, antigen mixed with chitosan augmented both antibody and cell-mediated responses over alum as adjuvant (74). When the amine groups on the polymer are partially derivatized by attachment of mannose, immune responses are superior to unmodified chitosan by intranasal vaccination. The addition of mannose improved targeting to antigen presenting cells expressing the mannose receptor (75).
Carbohydrate conjugate and mimeotope vaccines
Millions of cases of life-threatening fungal infections occur annually, mostly in immunosuppressed persons (5). Other mycoses, such as recurrent vulvovaginal candidiasis, can cause considerable morbidity in those afflicted. Thus, efforts have focused on developing vaccines that prevent development of fungal infections. Conserved cell wall glycans have appeal as “universal” fungal vaccines while glycans present on only some fungal species are candidate antigens for organism-specific vaccines. However, with rare exceptions, glycans do not elicit T cell responses and most fungal glycans are poor elicitors of antibody responses. To circumvent the latter problem, conjugate vaccines consisting of fungal glycans covalently linked to carrier proteins have been synthesized.
A “pan-fungal” conjugate vaccine consisting of laminarin (an algal derived β-1,3-D-glucan) linked to diphtheria toxoid (DT) was protective in models of candidiasis and aspergillosis and is in clinical trials(76, 77). Protection appears to be due to the development of a protective anti-β-glucan antibody response. While laminarin is recognized by β-glucan receptors, it generally acts as an antagonist (78) so it is not clear the contribution receptor-targeting plays to the efficacy of this vaccine. Less work has been performed examining the potential role of β-1,6-D-glucans. Neutrophils preferentially recognize β-1,6-D-glucans over β-1,3-D-glucans (79) but it remains speculative whether directed targeting of neutrophils would be of benefit in vaccine design.
Among medically important fungi, the C. neoformans species complex is unique in that it contains a capsule of which glucuronoxylomannan (GXM) is the major capsular component. A conjugate vaccine consisting of GXM linked to tetanus toxoid was immunogenic and partially protective in mice (80, 81). A peptide mimetic of GXM also induced protective responses against C. neoformans in mice (82). In contrast, a conjugate vaccine consisting of another cryptococcal capsular component, galactoxylomannan, elicited non-opsonic Abs and the vaccine was not protective (83). Other work has focused on conjugating β-1,2-mannotriose, which is found in the cell wall of Candida species, to a combination of tetanus toxoid and surface proteins of Candida albicans. Remarkably, immunization with some of these chimeric vaccines completely protected mice lethally challenged with C. albicans, even in the absence of adjuvants (84). An appeal of this system is it generates responses against both protein and carbohydrate components of the fungal cell wall.
Other promising vaccine approaches are focused on elicited antibody and/or T cell responses to fungal proteins (85–88) or employ live fungi with deletions of virulence factors (89). For example, a live, attenuated strain of Blastomyces dermatitidis null for the adhesion BAD1 was found to protect mice from three endemic mycoses; blastomycosis, histoplasmosis and coccidioidomycosis, by a mechanism dependent upon Th17 cells (90). When examining the contribution of the C-type lectin receptors Dectin-1, Dectin-2 and Mincle to vaccine immunity, the authors found that the specific receptors required for protective responses differed comparing the three causative fungal pathogens. However, the adapter protein Card9 was required for vaccine immunity against all three.
Conclusions
Persons with intact physical and immunological host defenses rarely get fungal infections due to strong innate defenses which link to adaptive immunity. Most of the innate responses are directed against three classes of glycans on the fungal cell wall; β-glucans, mannans and chitin/chitosan. These glycans serve as danger signals to the host that it might be under fungal attack and an immune response should be mounted. Our laboratory and others are exploring strategies that exploit this finding for use in vaccine development. In this regard, β-glucans appear to be the most promising of the glycans as strong T cell and antibody responses are seen when mice are injected with antigens associated with β-glucans. In contrast, mannans and chitin/chitosan are less immunostimulatory but still may have a role as part of vaccine delivery systems, particularly if administered with adjuvants such as ligands that stimulate TLRs. Finally, fungal glycans tend to be poorly immunogenic. Many studies have examined protection against experimental mycoses afforded by vaccines comprised of protein-carbohydrate conjugates or peptide mimeotopes. A major challenge will be to advance these experimental findings into the clinical arena so that at risk patients can be protected.
Acknowledgments
This work was supported in part by National Institutes of Health grants RO1AI026780, RO1AI102618 and HL112671. The authors apologize to those whose work could not be cited due to space limitations.
References
- 1.Levitz SM, Golenbock DT. Beyond empiricism: informing vaccine development through innate immunity research. Cell. 2012;148:1284–92. doi: 10.1016/j.cell.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coffman RL, Sher A, Seder RA. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKee AS, Munks MW, Marrack P. How Do Adjuvants Work? Important Considerations for New Generation Adjuvants. Immunity. 2007;27:687–90. doi: 10.1016/j.immuni.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–93. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Science translational medicine. 2012;4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 6.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 7.Levitz SM. Innate Recognition of Fungal Cell Walls. PLoS Pathog. 2010;6:e1000758. doi: 10.1371/journal.ppat.1000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rappleye CA, Eissenberg LG, Goldman WE. Histoplasma capsulatum α-(1,3)-glucan blocks innate immune recognition by the beta-glucan receptor. Proc Natl Acad Sci U S A. 2007;104:1366–70. doi: 10.1073/pnas.0609848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levitz SM, Tabuni A. Binding of Cryptococcus neoformans by human cultured macrophages. Requirements for multiple complement receptors and actin. Journal of Clinical Investigation. 1991;87:528–35. doi: 10.1172/JCI115027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal S, Specht CA, Huang H, Ostroff GR, Ram S, Rice PA, Levitz SM. Linkage Specificity and Role of Properdin in Activation of the Alternative Complement Pathway by Fungal Glycans. mBio. 2011;2 doi: 10.1128/mBio.00178-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novak M, Vetvicka V. Beta-glucans, history, and the present: immunomodulatory aspects and mechanisms of action. J Immunotoxicol. 2008;5:47–57. doi: 10.1080/15476910802019045. [DOI] [PubMed] [Google Scholar]
- 12.Weitberg AB. A phase I/II trial of beta-(1,3)/(1,6) D-glucan in the treatment of patients with advanced malignancies receiving chemotherapy. J Exp Clin Cancer Res. 2008;27:40. doi: 10.1186/1756-9966-27-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams DL, Sherwood ER, Browder IW, McNamee RB, Jones EL, Di Luzio NR. Pre-clinical safety evaluation of soluble glucan. Int J Immunopharmacol. 1988;10:405–14. doi: 10.1016/0192-0561(88)90127-0. [DOI] [PubMed] [Google Scholar]
- 14.Geijtenbeek TBH, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–79. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–8. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 16.LeibundGut-Landmann S, Osorio F, Brown GD, Reis e Sousa C. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood. 2008;112:4971–80. doi: 10.1182/blood-2008-05-158469. [DOI] [PubMed] [Google Scholar]
- 17.Carter RW, Thompson C, Reid DM, Wong SY, Tough DF. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J Immunol. 2006;177:2276–84. doi: 10.4049/jimmunol.177.4.2276. [DOI] [PubMed] [Google Scholar]
- 18.Huang H, Ostroff GR, Lee CK, Wang JP, Specht CA, Levitz SM. Distinct patterns of dendritic cell cytokine release stimulated by fungal beta-glucans and toll-like receptor agonists. Infect Immun. 2009;77:1774–81. doi: 10.1128/IAI.00086-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osorio F, Reis e Sousa C. Myeloid C-type Lectin Receptors in Pathogen Recognition and Host Defense. Immunity. 2011;34:651–64. doi: 10.1016/j.immuni.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Huang H, Ostroff GR, Lee CK, Agarwal S, Ram S, Rice PA, Specht CA, Levitz SM. Relative contributions of dectin-1 and complement to immune responses to particulate beta-glucans. Journal of immunology. 2012;189:312–7. doi: 10.4049/jimmunol.1200603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aouadi M, Tesz GJ, Nicoloro SM, Wang M, Chouinard M, Soto E, Ostroff GR, Czech MP. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009;458:1180–4. doi: 10.1038/nature07774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soto ER, Ostroff GR. Characterization of multilayered nanoparticles encapsulated in yeast cell wall particles for DNA delivery. Bioconjug Chem. 2008;19:840–8. doi: 10.1021/bc700329p. [DOI] [PubMed] [Google Scholar]
- 23.Soto ER, Caras AC, Kut LC, Castle MK, Ostroff GR. Glucan particles for macrophage targeted delivery of nanoparticles. J Drug Deliv. 2012;2012:143524. doi: 10.1155/2012/143524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang H, Ostroff GR, Lee CK, Specht CA, Levitz SM. Robust Stimulation of Humoral and Cellular Immune Responses following Vaccination with Antigen-Loaded beta-Glucan Particles. MBio. 2010;1:e00164–10. doi: 10.1128/mBio.00164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tesz GJ, Aouadi M, Prot M, Nicoloro SM, Boutet E, Amano SU, Goller A, Wang M, Guo CA, Salomon WE, Virbasius JV, Baum RA, O’Connor MJ, Jr, Soto E, Ostroff GR, Czech MP. Glucan particles for selective delivery of siRNA to phagocytic cells in mice. Biochem J. 2011;436:351–62. doi: 10.1042/BJ20110352. [DOI] [PubMed] [Google Scholar]
- 26.Huang H, Ostroff GR, Lee CK, Specht CA, Levitz SM. Characterization and optimization of the glucan particle-based vaccine platform. Clinical and vaccine immunology : CVI. 2013;20:1585–91. doi: 10.1128/CVI.00463-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Y, Luo Y, You J, Shen H, Hu S. Biochemical, sensory and microbiological attributes of bream (Megalobrama amblycephala) during partial freezing and chilled storage. Journal of the science of food and agriculture. 2012;92:197–202. doi: 10.1002/jsfa.4572. [DOI] [PubMed] [Google Scholar]
- 28.Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Advanced drug delivery reviews. 2010;62:3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Ardiani A, Higgins JP, Hodge JW. Vaccines based on whole recombinant Saccharomyces cerevisiae cells. FEMS Yeast Research. 2010;10:1060–9. doi: 10.1111/j.1567-1364.2010.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiff ER, Everson GT, Tsai N. HCV-specific cellular immunity, RNA reductions, and normalization of ALT in chronic HCV subjects after treatment with GI-5005, a yeastbased immunotherapy targeting NS3 and core: a randomized, double-blind, placebo controlled phase 1b study [abstract 1304] Hepatology international. 2007;46 (Suppl S1):816A. [Google Scholar]
- 31.Lu Y, Bellgrau D, Dwyer-Nield LD, Malkinson AM, Duke RC, Rodell TC, Franzusoff A. Mutation-selective tumor remission with Ras-targeted, whole yeast-based immunotherapy. Cancer research. 2004;64:5084–8. doi: 10.1158/0008-5472.CAN-04-1487. [DOI] [PubMed] [Google Scholar]
- 32.Stubbs AC, Martin KS, Coeshott C, Skaates SV, Kuritzkes DR, Bellgrau D, Franzusoff A, Duke RC, Wilson CC. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat Med. 2001;7:625–9. doi: 10.1038/87974. [DOI] [PubMed] [Google Scholar]
- 33.Wansley EK, Chakraborty M, Hance KW, Bernstein MB, Boehm AL, Guo Z, Quick D, Franzusoff A, Greiner JW, Schlom J, Hodge JW. Vaccination with a Recombinant Saccharomyces cerevisiae Expressing a Tumor Antigen Breaks Immune Tolerance and Elicits Therapeutic Antitumor Responses. Clinical Cancer Research. 2008;14:4316–25. doi: 10.1158/1078-0432.CCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gemmill TR, Trimble RB. Overview of N- and O-linked oligosaccharide structures found in various yeast species. Biochim Biophys Acta. 1999;1426:227–37. doi: 10.1016/s0304-4165(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 35.Levitz SM, Specht CA. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS Yeast Res. 2006;6:513–24. doi: 10.1111/j.1567-1364.2006.00071.x. [DOI] [PubMed] [Google Scholar]
- 36.Cutler JE. N-glycosylation of yeast, with emphasis on Candida albicans. Med Mycol. 2001;39(Suppl 1):75–86. [PubMed] [Google Scholar]
- 37.Ernst JF, Prill SK. O-glycosylation. Med Mycol. 2001;39(Suppl 1):67–74. [PubMed] [Google Scholar]
- 38.Dan JM, Wang JP, Lee CK, Levitz SM. Cooperative stimulation of dendritic cells by Cryptococcus neoformans mannoproteins and CpG oligodeoxynucleotides. PLoS ONE. 2008;3:e2046. doi: 10.1371/journal.pone.0002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geijtenbeek TB, van Vliet SJ, Engering A, t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 40.van Kooyk Y, Engering A, Lekkerkerker AN, Ludwig IS, Geijtenbeek TB. Pathogens use carbohydrates to escape immunity induced by dendritic cells. Curr Opin Immunol. 2004;16:488–93. doi: 10.1016/j.coi.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annual review of immunology. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nature immunology. 2012;13:817–22. doi: 10.1038/ni.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engering AJ, Cella M, Fluitsma DM, Hoefsmit EC, Lanzavecchia A, Pieters J. Mannose receptor mediated antigen uptake and presentation in human dendritic cells. Adv Exp Med Biol. 1997;417:183–7. doi: 10.1007/978-1-4757-9966-8_31. [DOI] [PubMed] [Google Scholar]
- 45.Engering A, Geijtenbeek TB, van Vliet SJ, Wijers M, van Liempt E, Demaurex N, Lanzavecchia A, Fransen J, Figdor CG, Piguet V, van Kooyk Y. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol. 2002;168:2118–26. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- 46.Murphy JW, Mosley RL, Cherniak R, Reyes GH, Kozel TR, Reiss E. Serological, electrophoretic, and biological properties of Cryptococcus neoformans antigens. Infect Immun. 1988;56:424–31. doi: 10.1128/iai.56.2.424-431.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoy JF, Murphy JW, Miller GG. T cell response to soluble cryptococcal antigens after recovery from cryptococcal infection. J Infect Dis. 1989;159:116–9. doi: 10.1093/infdis/159.1.116. [DOI] [PubMed] [Google Scholar]
- 48.Levitz SM, North EA. Lymphoproliferation and cytokines profiles in human peripheral blood mononuclear cells stimulated by Cryptococcus neoformans. J Med Vet Mycol. 1997;35:229–36. doi: 10.1080/02681219780001201. [DOI] [PubMed] [Google Scholar]
- 49.Jarvis JN, Casazza JP, Stone HH, Meintjes G, Lawn SD, Levitz SM, Harrison TS, Koup RA. The phenotype of the Cryptococcus-specific CD4+ memory T-cell response is associated with disease severity and outcome in HIV-associated cryptococcal meningitis. The Journal of infectious diseases. 2013;207:1817–28. doi: 10.1093/infdis/jit099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang CC, Lim A, Omarjee S, Levitz SM, Gosnell BI, Spelman T, Elliott JH, Carr WH, Moosa MY, Ndung’u T, Lewin SR, French MA. Cryptococcosis-IRIS is associated with lower cryptococcus-specific IFN-gamma responses before antiretroviral therapy but not higher T-cell responses during therapy. The Journal of infectious diseases. 2013;208:898–906. doi: 10.1093/infdis/jit271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levitz SM, Nong S, Mansour MK, Huang C, Specht CA. Molecular characterization of a mannoprotein with homology to chitin deacetylases that stimulates T cell responses to Cryptococcus neoformans. Proc Natl Acad Sci U S A. 2001;98:10422–7. doi: 10.1073/pnas.181331398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang C, Nong SH, Mansour MK, Specht CA, Levitz SM. Purification and characterization of a second immunoreactive mannoprotein from Cryptococcus neoformans that stimulates T-Cell responses. Infect Immun. 2002;70:5485–93. doi: 10.1128/IAI.70.10.5485-5493.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Specht CA, Nong S, Dan JM, Lee CK, Levitz SM. Contribution of Glycosylation to T Cell Responses Stimulated by Recombinant Cryptococcus neoformans Mannoprotein. J Infect Dis. 2007;196:796–800. doi: 10.1086/520536. [DOI] [PubMed] [Google Scholar]
- 54.Lam JS, Mansour MK, Specht CA, Levitz SM. A model vaccine exploiting fungal mannosylation to increase antigen immunogenicity. J Immunol. 2005;175:7496–503. doi: 10.4049/jimmunol.175.11.7496. [DOI] [PubMed] [Google Scholar]
- 55.Lam JS, Huang H, Levitz SM. Effect of differential N-linked and O-linked mannosylation on recognition of fungal antigens by dendritic cells. PLoS ONE. 2007;2:e1009. doi: 10.1371/journal.pone.0001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luong M, Lam JS, Chen J, Levitz SM. Effects of fungal N- and O-linked mannosylation on the immunogenicity of model vaccines. Vaccine. 2007;25:4340–4. doi: 10.1016/j.vaccine.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Irache JM, Salman HH, Gamazo C, Espuelas S. Mannose-targeted systems for the delivery of therapeutics. Expert Opinion on Drug Delivery. 2008;5:703–24. doi: 10.1517/17425247.5.6.703. [DOI] [PubMed] [Google Scholar]
- 58.Keler T, Ramakrishna V, Fanger MW. Mannose receptor-targeted vaccines. Expert Opinion on Biological Therapy. 2004;4:1953–62. doi: 10.1517/14712598.4.12.1953. [DOI] [PubMed] [Google Scholar]
- 59.Tacken PJ, de Vries IJ, Gijzen K, Joosten B, Wu D, Rother RP, Faas SJ, Punt CJ, Torensma R, Adema GJ, Figdor CG. Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood. 2005;106:1278–85. doi: 10.1182/blood-2005-01-0318. [DOI] [PubMed] [Google Scholar]
- 60.He L-Z, Crocker A, Lee J, Mendoza-Ramirez J, Wang X-T, Vitale LA, O’Neill T, Petromilli C, Zhang H-F, Lopez J, Rohrer D, Keler T, Clynes R. Antigenic Targeting of the Human Mannose Receptor Induces Tumor Immunity. The Journal of Immunology. 2007;178:6259–67. doi: 10.4049/jimmunol.178.10.6259. [DOI] [PubMed] [Google Scholar]
- 61.Bueter CL, Specht CA, Levitz SM. Innate sensing of chitin and chitosan. PLoS pathogens. 2013;9:e1003080. doi: 10.1371/journal.ppat.1003080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Merzendorfer H. The cellular basis of chitin synthesis in fungi and insects: common principles and differences. European journal of cell biology. 2011;90:759–69. doi: 10.1016/j.ejcb.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 63.Shrive AK, Moeller JB, Burns I, Paterson JM, Shaw AJ, Schlosser A, Sorensen GL, Greenhough TJ, Holmskov U. Crystal structure of the tetrameric fibrinogen-like recognition domain of fibrinogen C domain containing 1 (FIBCD1) protein. The Journal of biological chemistry. 2014;289:2880–7. doi: 10.1074/jbc.M113.520577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, He CH, Takyar S, Elias JA. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annual review of physiology. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–30. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Dyken SJ, Mohapatra A, Nussbaum JC, Molofsky AB, Thornton EE, Ziegler SF, McKenzie AN, Krummel MF, Liang HE, Locksley RM. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and gammadelta T cells. Immunity. 2014;40:414–24. doi: 10.1016/j.immuni.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bueter CL, Lee CK, Wang JP, Ostroff GR, Specht CA, Levitz SM. Spectrum and mechanisms of inflammasome activation by chitosan. Journal of immunology. 2014;192:5943–51. doi: 10.4049/jimmunol.1301695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balan V, Verestiuc L. Strategies to improve chitosan hemocompatibility: A review. European Polymer Journal. 2014;53:171–88. [Google Scholar]
- 69.Dash M, Chiellini F, Ottenbrite RM, Chiellini E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Progress in Polymer Science. 2011;36:981–1014. [Google Scholar]
- 70.Zaman M, Chandrudu S, Toth I. Strategies for intranasal delivery of vaccines. Drug Delivery and Translational Research. 2013;3:100–9. doi: 10.1007/s13346-012-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Advanced drug delivery reviews. 2010;62:59–82. doi: 10.1016/j.addr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. Chitosan as a novel nasal delivery system for vaccines. Advanced drug delivery reviews. 2001;51:81–96. doi: 10.1016/s0169-409x(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 73.Mann AJ, Noulin N, Catchpole A, Stittelaar KJ, de Waal L, Veldhuis Kroeze EJ, Hinchcliffe M, Smith A, Montomoli E, Piccirella S, Osterhaus AD, Knight A, Oxford JS, Lapini G, Cox R, Lambkin-Williams R. Intranasal H5N1 vaccines, adjuvanted with chitosan derivatives, protect ferrets against highly pathogenic influenza intranasal and intratracheal challenge. PloS one. 2014;9:e93761. doi: 10.1371/journal.pone.0093761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine. 2007;25:2085–94. doi: 10.1016/j.vaccine.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yao W, Peng Y, Du M, Luo J, Zong L. Preventative Vaccine-Loaded Mannosylated Chitosan Nanoparticles Intended for Nasal Mucosal Delivery Enhance Immune Responses and Potent Tumor Immunity. Molecular Pharmaceutics. 2013;10:2904–14. doi: 10.1021/mp4000053. [DOI] [PubMed] [Google Scholar]
- 76.Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Costantino P, Rappuoli R, Cassone A. A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med. 2005;202:597–606. doi: 10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cassone A. Development of vaccines for Candida albicans: fighting a skilled transformer. Nature reviews Microbiology. 2013;11:884–91. doi: 10.1038/nrmicro3156. [DOI] [PubMed] [Google Scholar]
- 78.Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, Bose N, Chan AS, Magee AS, Danielson ME, Weiss A, Vasilakos JP, Underhill DM. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature. 2011;472:471–5. doi: 10.1038/nature10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rubin-Bejerano I, Abeijon C, Magnelli P, Grisafi P, Fink GR. Phagocytosis by human neutrophils is stimulated by a unique fungal cell wall component. Cell Host Microbe. 2007;2:55–67. doi: 10.1016/j.chom.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Devi SJ. Preclinical efficacy of a glucuronoxylomannan-tetanus toxoid conjugate vaccine of Cryptococcus neoformans in a murine model. Vaccine. 1996;14:841–4. doi: 10.1016/0264-410x(95)00256-z. [DOI] [PubMed] [Google Scholar]
- 81.Devi SJN, Schneerson R, Egan W, Ulrich TJ, Bryla D, Robbins JB, Bennett JE. Cryptococcus neoformans serotype A glucuronoxylomannan-protein conjugate vaccines: Synthesis, characterization, and immunogenicity. Infect Immun. 1991;59:3700–7. doi: 10.1128/iai.59.10.3700-3707.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fleuridor R, Lees A, Pirofski L. A cryptococcal capsular polysaccharide mimotope prolongs the survival of mice with Cryptococcus neoformans infection. Journal of immunology. 2001;166:1087–96. doi: 10.4049/jimmunol.166.2.1087. [DOI] [PubMed] [Google Scholar]
- 83.Chow SK, Casadevall A. Evaluation of Cryptococcus neoformans galactoxylomannan-protein conjugate as vaccine candidate against murine cryptococcosis. Vaccine. 2011;29:1891–8. doi: 10.1016/j.vaccine.2010.12.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xin H, Cartmell J, Bailey JJ, Dziadek S, Bundle DR, Cutler JE. Self-adjuvanting glycopeptide conjugate vaccine against disseminated candidiasis. PLoS One. 2012;7:e35106. doi: 10.1371/journal.pone.0035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spellberg BJ, Ibrahim AS, Avanesian V, Fu Y, Myers C, Phan QT, Filler SG, Yeaman MR, Edwards JE., Jr Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J Infect Dis. 2006;194:256–60. doi: 10.1086/504691. [DOI] [PubMed] [Google Scholar]
- 86.Ibrahim AS, Luo G, Gebremariam T, Lee H, Schmidt CS, Hennessey JP, Jr, French SW, Yeaman MR, Filler SG, Edwards JE., Jr NDV-3 protects mice from vulvovaginal candidiasis through T- and B-cell immune response. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xue J, Chen X, Selby D, Hung CY, Yu JJ, Cole GT. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect Immun. 2009;77:3196–208. doi: 10.1128/IAI.00459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hurtgen BJ, Hung CY, Ostroff GR, Levitz SM, Cole GT. Construction and evaluation of a novel recombinant T cell epitope-based vaccine against Coccidioidomycosis. Infection and immunity. 2012;80:3960–74. doi: 10.1128/IAI.00566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wuthrich M, Filutowicz HI, Klein BS. Mutation of the WI-1 gene yields an attenuated blastomyces dermatitidis strain that induces host resistance. The Journal of clinical investigation. 2000;106:1381–9. doi: 10.1172/JCI11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang H, LeBert V, Hung CY, Galles K, Saijo S, Lin X, Cole GT, Klein BS, Wuthrich M. C-type lectin receptors differentially induce th17 cells and vaccine immunity to the endemic mycosis of North America. Journal of immunology. 2014;192:1107–19. doi: 10.4049/jimmunol.1302314. [DOI] [PMC free article] [PubMed] [Google Scholar]