Abstract
Acute myeloid leukemia (AML) relapse after allogeneic hematopoietic cell transplantation (alloHCT) remains a major therapeutic challenge. We studied outcomes of 1788 AML patients relapsing after alloHCT (1990–2010) during first or second complete remission (CR) to identify factors associated with longer post-relapse survival. Median time of post HCT relapse was 7 months (mo; range, 1–177). At relapse, 1231 patients (69%) received intensive therapy, including chemotherapy (CT) alone (n=660), donor lymphocyte infusion (DLI)±CT (n=202; %), or 2nd alloHCT±CT ±DLI (n=369), with subsequent CR rates of 29%. Median follow-up after relapse was 39 mo (range, <1–193). Survival for all patients was 23% at 1 year post-relapse; however, 3-yr overall survival correlated with time from HCT to relapse (4% for relapse during 1–6 mo period, 12% during 6 mo-2 yr, 26% during 2–3 yr, and 38% for ≥3 yr). In multivariable analysis, lower mortality was significantly associated with longer time from alloHCT to relapse (RR 0.55 for 6 mo-2 yr, RR 0.39 for 2–3 yr, and RR 0.28 for ≥3 yr; p<0.0001) and a 1st HCT using reduced-intensity conditioning (RR=0.77; 95% CI 0.66–0.88, p=0.0002). In contrast, inferior survival was associated with age >40 yr (RR=1.42, 95% CI 1.24–1.64; p<0.0001), active GVHD at relapse (RR=1.25, 95% CI 1.13–1.39; p<0.0001), adverse cytogenetics (RR=1.37, 95% CI 1.09–1.71; p=0.0062), mismatched URD (RR=1.61, 95% CI 1.22–2.13; p=0.0008), and use of cord blood for 1st HCT (RR=1.23, 95% CI 1.06–1.42; p=0.0078). AML relapse after alloHCT predicted poor survival; however, patients who relapsed ≥6 mo after their initial alloHCT had better survival and may benefit from intensive therapy such as 2nd alloHCT±DLI.
Keywords: AML, relapse, allogeneic transplant, DLI, second transplant
INTRODUCTION
Allogeneic hematopoietic cell transplantation (alloHCT) is a potentially curative treatment option for patients with acute myeloid leukemia (AML); however, relapse accounts for approximately 40% of alloHCT treatment failures. Among relapsed patients the 2-year postrelapse survival rate is reported at less than 20%. (1–7) Unfortunately, sustainable remissions are rare in patients with post-transplant AML relapse, especially for those relapsing soon after alloHCT. (8, 9) Commonly used treatment options for relapsed patients include intensive chemotherapy with or without donor lymphocyte infusion (DLI), second alloHCT, withdrawal of immunosuppression, or supportive care.(4, 7, 8, 10–13) Treatment decisions for management of relapsed AML could be improved by identifying prognostic factors associated with post-relapse survival and developing a risk stratification model.
A recent study by the European Blood and Marrow Transplantation (EBMT) group identified several prognostic factors associated with improved survival among AML patients who relapsed after reduced-intensity conditioning (RIC) alloHCT: longer interval from transplant to relapse, low bone marrow tumor burden at relapse, and absence of acute graft versus host disease (GVHD). Longer survival was seen primarily among patients who achieved complete remission (CR) with chemotherapy followed by either DLI or a second alloHCT.(1) These findings are consistent with other single-institution reports of alloHCT outcomes among patients treated for relapsed AML. These reports suggested that intensive therapy resulted in better survival than withdrawal of immunosuppression alone (5, 7, 11), independent of donor source or intensity of initial conditioning;(7) however, a detailed analysis of prognostic factors associated with survival was limited by the relatively small sample sizes of these previous reports. We therefore used the Center for International Blood and Marrow Transplant Research (CIBMTR) database to compare clinical outcomes and factors associated with survival among a large cohort of AML patients whose leukemia relapsed following alloHCT.
METHODS
Data Source
We used the CIBMTR observational registry to compare clinical outcomes and factors associated with survival among AML patients whose leukemia relapsed following alloHCT between 1990 – 2010. The CIBMTR is a research organization combined with the National Marrow Donor Program (NMDP) and collects information from over 500 transplantation centers worldwide that prospectively report detailed information on consecutive transplants. To ensure data quality, a computerized system and scheduled data audits independently check all collected data based on specific disease forms provided by participating transplant centers. Privacy protections for patients participating in observational studies conducted by the CIBMTR are in compliance with all applicable federal regulations. Additionally, the CIBMTR ensures protected health information for all participants under the Health Insurance Portability and Accountability Act (HIPPA) Privacy Rule.
Patient Selection and Definitions
Adult and pediatric patients with AML relapsing after alloHCT were included in the study if they were in first or second complete remission (CR) when they received myeloablative or RIC alloHCT. Patients with de novo or secondary AML and patients receiving related donor (RD), unrelated donor (URD), or umbilical cord blood (UCB) donor grafts were included. Patients whose AML relapsed within the first 30 days of transplantation (n=64) or whose relapse date or conditioning regimens were unavailable for analysis (n=106) were excluded.
CR was defined as <5% bone marrow blasts with no morphological evidence of leukemia in the marrow or peripheral blood. Secondary AML was defined as leukemia arising from underlying myelodysplastic syndrome (MDS) or treatment-related AML (t-AML) due to previous chemotherapy or radiation. The Southwest Oncology Group cytogenetic classification was used for cytogenetic risk stratification as previously reported. (14) Intensive therapy was defined as induction-type cytoreductive chemotherapy with or without DLI and/or second allograft. HLA-typing for URD recipients was classified using published CIBMTR criteria.(15) Intensity of conditioning regimens were classified according to established CIBMTR definitions. (16, 17)
Study Endpoints and Statistical Analysis
The primary study endpoint was overall survival (OS) of AML patients relapsing after alloHCT. OS was defined as the time from relapse to death or last follow up for surviving patients. Secondary endpoints included clinical and disease prognostic factors of OS after post-transplantation relapse. Long-term survival was defined as survival ≥1 year after alloHCT relapse.
The Kaplan-Meier method was used to estimate OS probability. (18) Cox proportional hazards regression model was used to identify factors predictive of survival. The assumption of proportional hazards for each factor was tested by adding a time-dependent covariate. When the test indicated differential effects over time (non-proportional hazards), models were constructed breaking the post-transplantation time course into two periods, using the maximized partial likelihood method to find the most appropriate breakpoint. A stepwise model selection approach was used to identify all significant risk factors predictive of survival. All statistical analysis was performed with SAS software (SAS Institute, Cary, NC, Version 9.2).
RESULTS
Patient Characteristics
We identified 1788 patients with AML relapsing after alloHCT from 286 CIBMTR centers and 43 countries. Of these, 413 patients survived ≥1 year after relapse (Table 1). Median time from transplantation to relapse was 7 months (range, 1–177 months), and median follow-up of survivors after post-transplantation relapse was 39 months (range, <1–193 months). Seventy percent of the patients underwent alloHCT in CR1. Median age of patients was 32 years (range, <1–76); 37% of patients were children (0–18 years old) and 39% were > 40 years old. Fifteen percent of patients had secondary AML, and 19% had unfavorable cytogenetics. A myeloablative conditioning regimen was used on over three-quarters of cases, and 52% of patients received a bone marrow graft. Donor types included HLA-identical RD (52%), well-matched URD (25%), UCB (13%), and mismatched URD (3%). Relapse within 6 months of transplantation occurred in 43% of patients, and isolated extramedullary relapse was rare (4%). AML relapse beyond 2 years of alloHCT occurred in only 18% of cases, and active GVHD prior to relapse was present in 41% of patients. The majority (n=1231, 69% of total) of patients received treatment for relapse, which included chemotherapy alone (37%), 2nd HCT with or without chemotherapy and/or DLI (21%), or DLI with or without chemotherapy (11%). However, only 15% of all patients achieved a subsequent CR. While 2nd HCT were rarely administered to those relapsing within 6 months, we found no association between use of intensive therapy and the time from HCT to relapse or the conditioning intensity of the 1st HCT.
Table 1.
Patient characteristics
| Variable | Total N (%) |
Survival ≥ 1 year post relapse N (%) |
|---|---|---|
| Number of patients | 1788 | 413 |
| Year of HCT | ||
| 1990–2000 | 745 (42) | 203 (49) |
| 2001–2010 | 1043 (59) | 210 (51) |
| HCT during CR1 | 1249 (70) | 312 (76) |
| CR2 | 539 (30) | 101 (24) |
| Age | ||
| Median (range) | 32 (<1–76) | 30 (1–75) |
| 0–18y | 613 (34) | 136 (33) |
| 19–40y | 439 (25) | 138 (33) |
| 41–76y | 736 (41) | 139 (34) |
| AML type | ||
| De novo | 1450 (81) | 348 (84) |
| Secondary | 276 (15) | 47 (11) |
| Missing | 62 (3) | 18 (4) |
| Cytogenetics scoring | ||
| Favorable | 138 (8) | 45 (11) |
| Intermediate / normal | 805 (45) | 190 (46) |
| Unfavorable | 334 (19) | 52 (13) |
| Missing | 511 (29) | 126 (31) |
| Myeloablative | 1374 (77) | 337 (82) |
| RIC/NMA | 414 (23) | 76 (18) |
| Graft type | ||
| Bone marrow | 935 (52) | 240 (58) |
| Peripheral blood | 621 (35) | 138 (33) |
| Cord blood | 232 (13) | 35 (8) |
| Donor type | ||
| HLA-id sibling | 936 (52) | 245 (59) |
| URD well matched | 317 (18) | 69 (17) |
| URD partially matched | 134 (7) | 35 (8) |
| URD mismatched | 56 (3) | 7 (2) |
| URD unknown | 113 (6) | 22 (5) |
| Cord blood | 232 (13) | 35 (8) |
| GVHD prophylaxis | ||
| ATG/alemtuzumab | 406 (23) | 80 (19) |
| Ex-vivo T cell depletion | 48 (3) | 12 (3) |
| CSA/tac ± other | 1334 (75) | 321 (78) |
| Time from HCT to relapse | ||
| Median (range) | 7 (1–177) | 14 (1–177) |
| < 6m | 774 (43) | 88 (21) |
| 6m–2y | 702 (39) | 191 (46) |
| 2–3y | 138 (8) | 52 (13) |
| ≥3y | 174 (10) | 82 (20) |
| AML relapse site | ||
| Extramedullary only | 80 (4) | 25 (6) |
| Bone Marrow ± other sites | 1046 (59) | 200 (48) |
| Not reported/missing | 662 (37) | 188 (44) |
| Active GVHD prior to relapse | ||
| Yes | 727 (41) | 170 (41) |
| No | 1028 (57) | 234 (57) |
| Missing | 33 (2) | 9 (2) |
| Treatment for relapse | ||
| 2nd HCT±chemo±DLI | 369 (21) | 182 (44) |
| DLI±chemo | 202 (11) | 57 (14) |
| Chemo only | 660 (37) | 87 (21) |
| Supportive care/no therapy | 357 (20) | 35 (8) |
| Missing | 200 (11) | 52 (13) |
| Response to therapy | ||
| CR | 271 (15) | 165 (40) |
| No response | 704 (39) | 121 (29) |
| Missing | 813 (45) | 127 (31) |
| Surviving at last follow-up | 229 (13) | 173 (42) |
| Median follow-up after relapse, months | 39 (<1–193) | 59 (12–193) |
Median time from HCT to relapse was 14 months for long-term survivors (> 1 year post relapse). Survivors living longer often received active treatment for relapse (79%), most frequently a 2nd HCT (44%), and commonly achieved subsequent CR (40%).
Management of Post-transplantation Relapse
A total of 267 patients received DLI for AML relapse, and DLI plus chemotherapy was used in 81% of them (Table 2). DLI was followed by 2nd HCT in 24% of patients treated with DLI. Median time from relapse to DLI was 2 months (<1–12 months), with 85% of patients receiving DLI within 6 months of leukemia relapse. Among all patients receiving DLI, 87 (32.6%) survived more than a year after leukemia relapse. The source of DLI was an HLA identical sibling donor for 61% of patients. Patients who received DLI and survived beyond 1 year often received subsequent 2nd HCT (34%).
Table 2.
Characteristics of patients treated with DLI and/or 2nd HCT
| Variable | Total N (%) |
Survival ≥ 1 year post relapse N (%) |
|---|---|---|
| DLI±2nd HCT | 267 | 87 |
| DLI+chemotherapy | ||
| Yes | 216 (81) | 75 (86) |
| No | 51 (19) | 12 (14) |
| DLI‡+2nd HCT | ||
| +2nd HCT | 65 (24) | 30 (34) |
| No 2nd HCT | 202 (76) | 57 (66) |
| Type of donor | ||
| HLA-identical sibling | 162 (61) | 59 (68) |
| Unrelated | 102 (38) | 26 (30) |
| Missing | 3 (1) | 2 (2) |
| Donor Gender | ||
| Male | 144 (54) | 46 (53) |
| Female | 105 (39) | 38 (44) |
| Missing | 18 (7) | 3 (3) |
| Time from relapse to DLI | ||
| Median (range) | 2 (<1–12) | 2 (<1–12) |
| ≤6m | 226 (85) | 70 (80) |
| >6m | 11 (4) | 5 (6) |
| Missing | 25 (9) | 9 (10) |
| 2nd HCT | 369 | 182 |
| Conditioning | ||
| MA | 181 (49) | 99 (54) |
| RIC/NMA | 110 (30) | 57 (31) |
| Missing | 78 (21) | 26 (14) |
| Donor type of 2nd HCT | ||
| Related | 197 (53) | 94 (52) |
| Unrelated | 127 (34) | 70 (38) |
| Cord Blood | 20 (5) | 9 (5) |
| Missing | 25 (7) | 9 (5) |
| Donor gender | ||
| Male | 168 (46) | 78 (43) |
| Female | 126 (34) | 67 (37) |
| Missing | 75 (20) | 37 (20) |
| Same donor as 1st HCT | ||
| No | 81 (22) | 49 (27) |
| Yes | 166 (45) | 73 (40) |
| Missing | 122 (33) | 60 (33) |
| Time from relapse to 2nd HCT | ||
| Median (range) | 3 (<1–50) | 3 (<1–50) |
| ≤6m | 299 (81) | 135 (74) |
| >6m | 52 (14) | 40 (22) |
| Missing | 18 (5) | 7 (4) |
Reflects DLI with or without chemotherapy
A 2nd HCT was performed on 369 patients of whom 182 (49.3%) survived more than a year after relapse. The 2nd HCT conditioning regimens were myeloablative for 49%, RIC/or non-myeloablative (NMA) for 30%, and unknown for the rest of the patients. RD 2nd HCT was performed in about 1/2, URD in 1/3, UCB in 5% of the patients, and 2nd HCT donor source was unknown for the rest of the cases. A different donor for the 2nd HCT was chosen in 45% of patients, but data on the donor was unavailable for 1/3 of patients. Median time from posttransplant leukemia relapse to the 2nd HCT was 3 months (<1–50 months), with the majority (81%) of relapses occurring within 6 months.
Among patients with evaluable status for response to therapy (n=846), subsequent CR was achieved in 29% of patients. DLI with or without chemotherapy (n=139) resulted in CR for 37% of cases; 2nd HCT with or without chemotherapy and/or DLI (n=264) resulted in CR for 44%; while chemotherapy alone (n= 443) induced CR in only 16%. Only rare remissions (6%) were observed among patients managed supportively.
Survival after Post-transplant Relapse
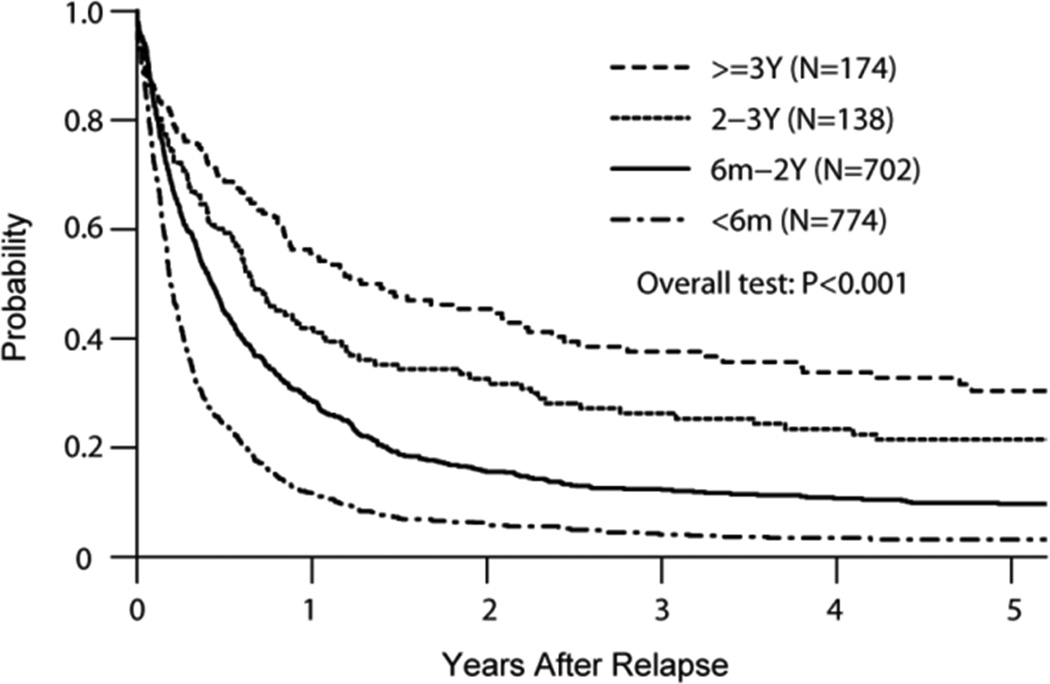
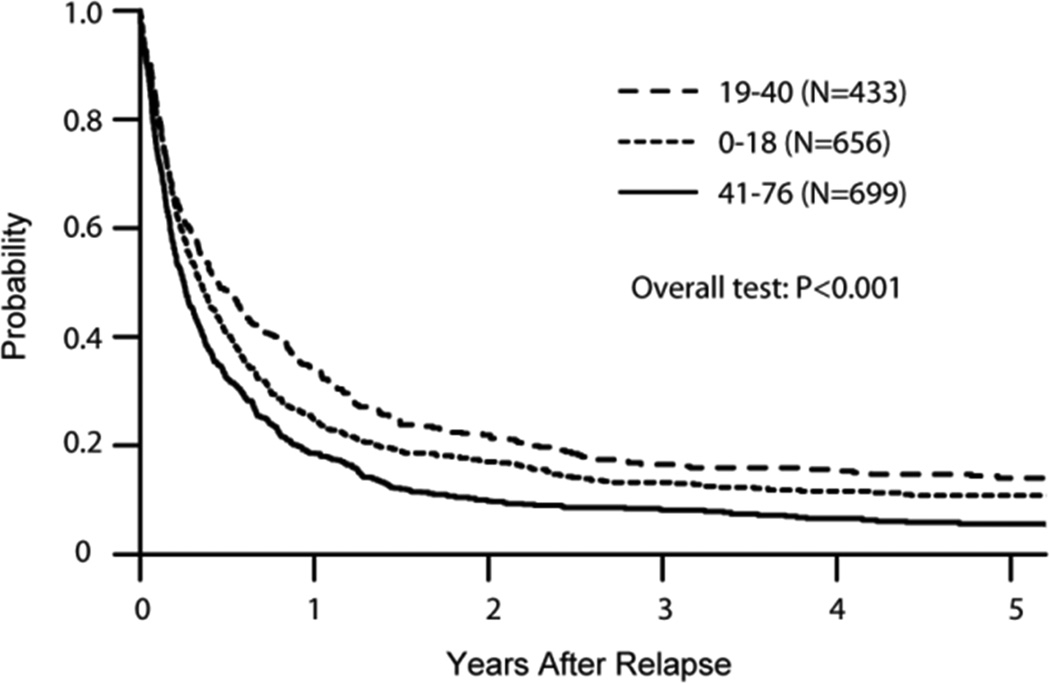
Median follow-up was 39 months after relapse (<1–193 months). Only 13% of all patients remained alive at the time of study analysis. Survival at 1 year after post-transplant relapse was 23%; however, survival probability at 3 years was only 4% for patients relapsing within 6 months of alloHCT, 12% for those relapsing within 6 month-2 years, 26% for 2–3 years, and 38% for 3+ years (Figure 1A). Adjusted probabilities of survival at 3 years were 13% for patients younger than 18 years, 17% for those 19–40 years old, and 8% for patients older than 41 years (Figure 1B). Median survival was 7 months (1–177 months) among patients receiving DLI and 12 months (1–150 months) among those receiving 2nd HCT. Cell-based therapy (DLI or 2nd HCT) resulted into significantly better ≥1 year post-relapse survival among patients relapsing 6 months and later post-HCT (Table 3). In multivariable analysis, a longer time from HCT to relapse (p<0.0001) and use of a RIC/NMA conditioning regimen (HR=0.77, p=0.0002) for the initial alloHCT were associated with better survival (Table 4). In contrast, age > 41 years (HR=1.42, p<0.0001), unfavorable cytogenetics (HR=1.37, p<0.0062), mismatched URD (HR=1.61, p<0.0008), UCB (HR=1.23, p<0.0078), and presence of active GVHD at relapse (HR=1.25, p<0.0001) were independent predictors of inferior survival.
Figure 1.
A: Adjusted Overall Survival by Time from HCT to Relapse
B: Adjusted Overall Survival by Age
Table 3.
Survival after DLI and 2nd HCT
| DLI | 2nd HCT | |||||
|---|---|---|---|---|---|---|
| Time from HCT to relapse | N | Survival ≥ 1 year post relapse N (%) |
p-value£ | N | Survival ≥ 1 year post relapse N (%) |
p-value£ |
| <6 months | 90 | 12 (13) | <0.001 | 110 | 35 (32) | <0.001 |
| 6 months–2 years | 81 | 28 (35) | 167 | 92 (55) | ||
| 2 – 3 years | 14 | 7 (50) | 37 | 23 (62) | ||
| ≥ 3 years | 17 | 10 (59) | 55 | 32 (58) | ||
| Median (range) | 7 (1–177)* | 13 (2–106) | 12 (1–150)† | 14 (2–78) | ||
p-value reflects the time from HCT to relapse
Median survival of all patients receiving DLI
Median survival of all patients receiving 2nd HCT
Table 4.
Factors influencing survival of AML patients relapsing after alloHCT
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | N | RR (95% CI) | P-value | RR (95% CI) | P-value | |
| Time from HCT to relapse | <0.0001 | <0.0001 | ||||
| < 6m | 774 | 1.00 | 1.00 | |||
| 6m-2y | 702 | 0.60 (0.53–0.66) | <0.0001 | 0.55 (0.50–0.62) | <0.0001 | |
| 2–3y | 138 | 0.40 (0.33–0.50) | <0.0001 | 0.39 (0.32–0.49) | <0.0001 | |
| ≥ 3y | 174 | 0.30 (0.24–0.37) | <0.0001 | 0.28 (0.23–0.35) | <0.0001 | |
| Year of HCT | ||||||
| 1990–2000 | 745 | 1.00 | NS | NS | ||
| 2001–2010 | 1043 | 1.19 (1.08–1.32) | 0.0006 | |||
| Age | <0.0001 | <0.0001 | ||||
| ≤ 18y | 656 | 1.00 | 1.00 | |||
| 19–40y | 433 | 0.86 (0.75–0.98) | 0.02 | 1.00 (0.87–1.15) | 0.10 | |
| ≥41y | 699 | 1.27 (1.13–1.42) | <0.0001 | 1.42 (1.24–1.64) | <0.0001 | |
| Gender | ||||||
| Male | 988 | 1.00 | NS | NS | ||
| Female | 800 | 0.98 (0.88–1.08) | 0.63 | |||
| Cytogenetics | <0.0001 | 0.02 | ||||
| Favorable | 138 | 1.00 | 1.00 | |||
| Intermediate/Norm | 805 | 1.27 (1.04–1.56) | 0.02 | 1.15 (0.94–1.41) | 0.18 | |
| Unfavorable | 334 | 1.64 (1.32–2.04) | <0.0001 | 1.37 (1.09–1.71) | 0.01 | |
| Unknown | 511 | 1.22 (0.99–1.51) | 0.06 | 1.13 (0.91–1.39) | 0.27 | |
| Conditioning | ||||||
| MA | 1374 | 1.00 | 1.00 | |||
| RIC/NMA | 414 | 1.18 (1.05–1.33) | 0.01 | 0.77 (0.66–0.88) | 0.0002 | |
| Donor Type | <0.0001 | 0.0007 | ||||
| RD/URD-Matched | 1387 | 1.00 | 1.00 | |||
| URD-Mismatched | 56 | 1.65 (1.25–2.17) | 0.0003 | 1.61 (1.22–2.13) | 0.0008 | |
| URD-Unknown | 113 | 1.10 (0.90–1.35) | 0.37 | 1.10 (0.89–1.36) | 0.37 | |
| Cord Blood | 232 | 1.38 (1.19–1.60) | <0.0001 | 1.23 (1.06–1.42) | 0.01 | |
| Active GVHD at relapse | 0.20 | 0.0002 | ||||
| No | 1028 | 1.00 | 1.00 | |||
| Yes | 727 | 1.10 (0.99–1.21) | 0.07 | 1.25 (1.13–1.39) | <0.0001 | |
| Unknown | 33 | 1.01 (0.70–1.47) | 0.95 | 1.05 (0.72–1.52) | 0.80 | |
Relapse or persistent leukemia was the primary cause of death in 71% of cases. While relapse was the cause of death in 80% of patients surviving less than a year, only 42% of longer surviving patients died of leukemia. Infection (4%) followed by GVHD (3%) and organ failure (3%) were the next most frequent causes of death and were similar in longer and shorter survivors.
DISCUSSION
In the 1788 AML patients relapsing after myeloablative or RIC/NMA alloHCT we found that survival after post-transplant relapse was significantly influenced by time from HCT to relapse, patients’ age, cytogenetic risk group, donor type, HLA matching and conditioning intensity. Although similar to prior reports we observed poor survival following AML relapse after alloHCT, (1, 2, 5, 7, 11, 19) longer remission after the initial alloHCT was an independent predictor of better survival. Patients who remained in remission ≥3 years after HCT had promising survival even after late relapse. In contrast, patients whose AML relapsed within 6 months of alloHCT had dismal survival, as observed in prior reports.(1, 5) We also observed favorable survival after relapse in those receiving a 2nd HCT with or without chemotherapy and/or DLI, particularly those achieving CR; this outcome is also consistent with prior reports. (1, 5, 19)
Other prognostic factors associated with survival after post-alloHCT relapse were patient age, active GVHD at the time of relapse, cytogenetic risk group, donor type/HLA matching, and conditioning intensity. Older patients are more likely to be unfit at the time of relapse, unable to tolerate further intensive therapy, and are more likely to be managed supportively. The 37% of our patients who were children were more likely to receive further intensive therapy and survive longer. Similarly, active GVHD at the time of relapse precludes the use of potentially valuable cell-based therapy and might increase the risk of infectious complications. Poor survival was seen with partially matched URD and UCB transplants. (7) Without the option of DLI for UCB patients, the only potential curative option for these patients remains a 2nd HCT, but this was rarely performed. Somewhat surprisingly, RIC/NMA conditioning at 1st HCT was associated with better survival independent of patient age and time from transplant to relapse. Previous reports of single-institution studies have made similar observations. (5, 7) We speculate that the lower risks of post-HCT morbidity after RIC/NMA conditioning may allow these patients to be better candidates for subsequent intensive therapy versus those treated with more intensive myeloablative alloHCT. It is also possible that leukemia relapse after RIC/NMA conditioning may remain more sensitive to chemotherapy than after myeloablative conditioning and subsequently contribute to a better clinical outcome.
We had no available data to assess the influence of tumor burden at the time of relapse on subsequent response to therapy and survival. In addition, we were unable to systematically analyze the impact of each therapeutic approach on subsequent clinical outcomes because these were intermediate events occurring after relapse and data reporting was incomplete. Additionally, we were unable to directly assess the effect of immunosuppression withdrawal on achievement of remission; however, all previous reports suggest that this approach alone has minimal, if any, therapeutic benefit. (7, 20)
In conclusion, relapse of AML after alloHCT predicted poor outcomes. We recommend that patients with longer remission after initial alloHCT be considered for 2nd HCT or chemotherapy plus DLI, as this approach was associated with prolonged survival in our cohort. Patients who relapse early, are elderly, or have active GVHD at the time of relapse are unlikely to benefit from intensive therapy and might best be managed supportively. Beyond compassionate supportive care, new approaches including prevention strategies (21) are needed for patients with early relapse after alloHCT.
Acknowledgements
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
Financial Disclosure Statement: The authors do not have any disclosures.
REFERENCES
- 1.Schmid C, Labopin M, Nagler A, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012 Feb 9;119(6):1599–1606. doi: 10.1182/blood-2011-08-375840. [DOI] [PubMed] [Google Scholar]
- 2.Devillier R, Crocchiolo R, Etienne A, et al. Outcome of relapse after allogeneic stem cell transplant in patients with acute myeloid leukemia. Leukemia & lymphoma. 2013 Jun;54(6):1228–1234. doi: 10.3109/10428194.2012.741230. [DOI] [PubMed] [Google Scholar]
- 3.Savani BN, Mielke S, Reddy N, Goodman S, Jagasia M, Rezvani K. Management of relapse after allo-SCT for AML and the role of second transplantation. Bone marrow transplantation. 2009 Dec;44(12):769–777. doi: 10.1038/bmt.2009.300. [DOI] [PubMed] [Google Scholar]
- 4.Schmid C, Labopin M, Nagler A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007 Nov 1;25(31):4938–4945. doi: 10.1200/JCO.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- 5.Thanarajasingam G, Kim HT, Cutler C, et al. Outcome and prognostic factors for patients who relapse after allogeneic hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013 Dec;19(12):1713–1718. doi: 10.1016/j.bbmt.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Brink MR, Porter DL, Giralt S, et al. Relapse after allogeneic hematopoietic cell therapy. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010 Jan;16(1 Suppl):S138–S145. doi: 10.1016/j.bbmt.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bejanyan N, Oran B, Shanley R, et al. Clinical outcomes of AML patients relapsing after matched-related donor and umbilical cord blood transplantation. Bone marrow transplantation. 2014 Jun 2; doi: 10.1038/bmt.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eapen M, Giralt SA, Horowitz MM, et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone marrow transplantation. 2004 Oct;34(8):721–727. doi: 10.1038/sj.bmt.1704645. [DOI] [PubMed] [Google Scholar]
- 9.Arellano ML, Langston A, Winton E, Flowers CR, Waller EK. Treatment of relapsed acute leukemia after allogeneic transplantation: a single center experience. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007 Jan;13(1):116–123. doi: 10.1016/j.bbmt.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Loren AW, Porter DL. Donor leukocyte infusions for the treatment of relapsed acute leukemia after allogeneic stem cell transplantation. Bone marrow transplantation. 2008 Mar;41(5):483–493. doi: 10.1038/sj.bmt.1705898. [DOI] [PubMed] [Google Scholar]
- 11.Oran B, Giralt S, Couriel D, et al. Treatment of AML and MDS relapsing after reduced-intensity conditioning and allogeneic hematopoietic stem cell transplantation. Leukemia. 2007 Dec;21(12):2540–2544. doi: 10.1038/sj.leu.2404828. [DOI] [PubMed] [Google Scholar]
- 12.Levine JE, Braun T, Penza SL, et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002 Jan 15;20(2):405–412. doi: 10.1200/JCO.2002.20.2.405. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Lee KH, Kim S, et al. Combination chemotherapy of intermediate-dose cytarabine, idarubicin, plus etoposide and subsequent mobilized donor leukocyte infusion for relapsed acute leukemia after allogeneic bone marrow transplantation. Leukemia research. 2001 Apr;25(4):305–312. doi: 10.1016/s0145-2126(00)00142-9. [DOI] [PubMed] [Google Scholar]
- 14.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000 Dec 15;96(13):4075–4083. [PubMed] [Google Scholar]
- 15.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008 Jul;14(7):748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009 Dec;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009 Mar;15(3):367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 19.Kedmi M, Resnick IB, Dray L, et al. A retrospective review of the outcome after second or subsequent allogeneic transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009 Apr;15(4):483–489. doi: 10.1016/j.bbmt.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Porter DL, Alyea EP, Antin JH, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010 Nov;16(11):1467–1503. doi: 10.1016/j.bbmt.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bishop MR, Alyea EP, 3rd, Cairo MS, et al. National Cancer Institute's First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: summary and recommendations from the organizing committee. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011 Apr;17(4):443–454. doi: 10.1016/j.bbmt.2010.12.713. [DOI] [PMC free article] [PubMed] [Google Scholar]