Abstract
Background
Dysregulation of coagulation is considered a major barrier against successful pig organ xenotransplantation in nonhuman primates. Inflammation is known to promote activation of coagulation. The role of pro-inflammatory factors as well as the relationship between inflammation and activation of coagulation in xenograft recipients is poorly understood.
Methods
Baboons received kidney (n=3), heart (n=4) or artery patch (n=8) xenografts from α1,3-galactosyltransferase gene-knockout (GTKO) pigs or GTKO pigs additionally transgenic for human complement regulatory protein CD46 (GTKO/CD46). Immunosuppression (IS) was based on either CTLA4-Ig or anti-CD154 costimulation blockade. Three artery patch recipients did not receive IS. Pro-inflammatory cytokines, chemokines and coagulation parameters were evaluated in the circulation after transplantation. In artery patch recipients, monocytes and dendritic cells (DC) were monitored in peripheral blood. Expression of tissue factor (TF) and CD40 on monocytes and DC were assessed by flow cytometry. C-reactive protein (C-RP) levels in the blood and C-RP deposition in xenografts as well as native organs were evaluated. Baboon and pig C-RP mRNA in heart and kidney xenografts were evaluated.
Results
In heart and kidney xenograft recipients, the levels of INFγ, TNF-α, IL-12 and IL-8 were not significantly higher after transplantation. However, MCP-1 and IL-6 levels were significantly higher after transplantation, particularly in kidney recipients. Elevated C-RP levels preceded activation of coagulation in heart and kidney recipients, where high levels of C-RP were maintained until the time of euthanasia in both heart and kidney recipients. In artery patch recipients, INFγ, TNF-α, IL-12, IL-8 and MCP-1 were elevated with no IS, while IL-6 was not. With IS, INFγ, TNF-α, IL-12, IL-8 and MCP-1 were reduced, but IL-6 was elevated. Elevated IL-6 levels were observed as early as 2 weeks in artery patch recipients. While IS was associated with reduced thrombin activation, fibrinogen and C-RP levels were increased when IS was given. There was a significant positive-correlation between C-RP, IL-6, and fibrinogen levels. Additionally, absolute numbers of monocytes were significantly increased when IS was given, but not without IS. This was associated with increased CD40 and TF expression on CD14+ monocytes and lineageneg CD11c+ DC, with increased differentiation of the pro-inflammatory CD14+ CD11c+ monocyte population. At the time of euthanasia, C-RP deposition in kidney and heart xenografts, C-RP positive cells in artery patch xenograft and native lungs were detected. Finally, high levels of both pig and baboon C-RP mRNA were detected in heart and kidney xenografts.
Conclusions
Inflammatory responses precede activation of coagulation after organ xenotransplantation. Early upregulation of C-RP and IL-6 levels may amplify activation of coagulation through upregulation of TF on innate immune cells. Prevention of systemic inflammation in xenograft recipients (SIXR) may be required to prevent dysregulation of coagulation and avoid excessive IS after xenotransplantation.
Keywords: C-reactive protein, Coagulation, Dendritic cells, Interleukin-6, Inflammation, Monocytes, Pig, Primate, Xenotransplantation
INTRODUCTION
The introduction of α1,3-galactosyltransferase gene-knockout (GTKO) pigs transgenic for human complement-regulatory proteins, markedly improved pig xenograft survival in non-human primates (NHP) (1). However, dysregulation of coagulation in the form of thrombotic microangiopathy in the graft and/or consumptive coagulopathy (CC) in the recipient remains a major barrier (2, 3). It has been reported that increased immunosuppressive therapy (IS) significantly prolongs organ xenograft survival, but not systemic anticoagulation (4, 5). However, the underlying mechanisms of prevention of dysregulation of coagulation by IS in xenograft recipients is not fully understood.
We have previously reported high levels of recipient (primate) tissue factor (TF) mRNA in heart (3) and kidney (2) xenografts, and TF-positive macrophages in heart xenografts (3). Furthermore, upregulated TF expression on peripheral blood mononuclear cells and platelets in kidney xenografts recipients coincides with the onset of CC (2). It is known that, TF exposure activates the coagulation system following vascular injury (6). In addition, TF has been recently considered a promoter as well as a marker of inflammation (6, 7). The interaction between inflammation and coagulation can initiate an amplification circuit (8) that results in uncontrolled production of inflammatory mediators and coagulation factors. While pro-inflammatory cytokines protect against pathogens and limit tissue damage, their overproduction can disrupt immune regulation and promote excessive inflammation (9).
Innate immune cells, which are a major source of pro-inflammatory cytokines, have been considered a barrier to successful pig-to-primate xenotransplantation (10). Pro-inflammatory cytokines, tumor necrosis factor-alpha (TNF-α) (11) and interleukin (IL)-6 (12), and C-reactive protein (C-RP) (13) are known to promote TF expression. Although recognized following pig islet xenotransplantation (14-17), the possible role of pro-inflammatory factors in activation of coagulation after xenotransplantation is poorly understood.
We evaluated inflammatory factors in pig organ and artery patch xenograft recipients. We provide evidence of inflammatory responses associated with pro-coagulant and inflammatory innate immune cells after xenotransplantation. Our data suggest that, in the presence of immunosuppressive therapy (IS), systemic inflammatory responses precede and most likely promote activation of coagulation in xenograft recipients.
MATERIALS AND METHODS
Animals
Baboons (Papio species, n=15: Division of Animal Resources, Oklahoma University Health Sciences Center, Oklahoma City, OK) weighing 6-10kg, were recipients. GTKO or GTKO/CD46 pigs weighing 10-20kg (Revivicor, Blacksburg, VA), provided organs and artery patch grafts (18, 19). Animal care was in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by Institute of Laboratory Animal Resources and published by National Institutes of Health (NIH publication No. 86-23, revised 1985). Protocols were approved by University of Pittsburgh institutional Animal Care and Use Committee.
Pig-to-baboon xenotransplantation models (Table 1)
Table 1.
Xenograft recipients and immunosuppressive therapy
| Group | Recipients | ID | Graft | Survival (days) | Immunosuppressive Therapy(a) |
|---|---|---|---|---|---|
| Organ xenotransplantation (n=7) | Heart (n=3) |
B27104 B26704 B16107 |
GTKO GTKO GTKO/CD46 |
56 35 26 |
|
| Kidney (n=4) | B4207 B4307 B26904 B16307 |
GTKO/CD46 GTKO/CD46 GTKO GTKO/CD46 |
9 10 6 10 |
Anti-CD154mAb + ATG + MMF | |
| Pig artery patch xenotransplantation (n=8) | n=3 |
B26504 B7506 B4007 |
GTKO GTKO GTKO |
95 14* 29 |
None (b) |
| n=2 | B7906 B3707 |
GTKO GTKO |
32 29 |
Anti-CD154mAb + ATG + MMF | |
| n=3 |
B18408 B18608 B18208 |
GTKO GTKO GTKO |
33 29 29 |
CTLA4-Ig + ATG + MMF |
For details of dosages, see Supplementary Table 1.
Recipients stated to have no IS received cobra venom factor and methylprednisolone only on days −1, 0, and 1
ATG = Anti-thymocyte globulin / MMF = Mycophenolate mofetil
Euthanasia on day 14 due to rupture of patch graft.
Organ xenotransplantation (n=7)
Surgical techniques of heart (n=3) and kidney (n=4) transplantation have been described (2, 3). Tissue and blood samples from two heart xenografts (survived 35 and 56 days (3)) and three kidney xenograft recipients (survived 6, 9 and 10 days (2), respectively) were evaluated at euthanasia.
Artery patch xenotransplantation (n=8)
Surgical procedure has been described (20). Recipients were electively euthanized 1-3 month after transplantation.
Immunosuppressive therapy (IS)
IS was based on costimulation blockade, with thymoglobulin induction and mycophenolate mofetil maintenance in all kidney and heart recipients receiving IS (Supplementary Table1). For heart and kidney xenograft recipients, anti-CD154mAb-based regimen was administered (2, 3). Artery patch xenografts recipients received either no IS (n=3), or anti-CD154mAb- or CTLA4Ig-based IS (n=5) (20).
White blood cell numbers
Whole blood samples were analyzed before and after transplantation. Complete and differential white blood cell counts were obtained from the UPMC Central Laboratory. Absolute numbers of lymphocytes, monocytes, and neutrophils, as well as CD3+T cells were calculated based on white blood cell counts and percentages obtained by flow cytometry.
Flow cytometry
White blood cells were stained after ACK lysis of red blood cells. Cells were incubated at 4°C in the dark with fluorochrome-conjugated anti-human antibodies:- TF (FITC-conjugated-Cat# MATF-FITC; Affinity Biologicals, Ancaster, ON, Canada), CD14 (APC-conjugated IM2580U; Beckman Coulter; Brea, CA), CD11c (PE-conjugated Cat# 340713), CD11c (APC-conjugated Cat# 340714), CD56 (PE-conjugated Cat# 556647), CD20 (PE-conjugated Cat# 556633), CD3 (PE-conjugated Cat# 552127), and CD14 (PE-conjugated Cat# 557154) antibodies (all from BD Biosciences, San Jose, CA). Appropriate isotype control antibodies were used. Data acquisition was performed with BD™ LSR II flow cytometer (Becton Dickinson, San Diego, CA), and analyzed using Flowjo software (Flowjo, Ashland, OR).
Luminex multiplex immunoassays
Serum samples from naïve baboons and xenograft recipients were tested for interferon-gamma (IFN-γ), TNF-α, IL-12, monocyte chemoattractant protein-1 (MCP-1), IL-8, and IL-6 levels using LEGENDplex Luminex kits (BioLegend, San Diego, CA).
Measurement of C-reactive protein (C-RP) levels
C-RP levels were measured in serum samples by the UPMC laboratory.
Measurement of coagulation parameters
Plasma samples collected from naïve baboons and xenograft recipients were stored at −80°C. Thombin-antithrombin (TAT) complexes, fibrinogen, D-dimer, and fibrin degradation products (FDP) levels were measured by enzyme-linked immunosorbent assay (21)
Histopathology and Immunohistochemistry of organ and artery patch xenografts
Biopsies were obtained at euthanasia. Tissues were fixed in formalin and embedded in paraffin. Four-micron (4μm) sections were stained. Primary rabbit anti-C-RP (Cat#Ab3241, Abcam, Cambridge, MA) was used (1:50), and secondary goat anti-rabbit antibodies (Cat#BA-1000, Vector laboratories, Burlingame, CA) were used (1:200).
Evaluation of pig and baboon C-RP in xenografts by real time RT-PCR
Tissue specimens were snap-frozen in liquid nitrogen and stored at –80°C. Total RNA was isolated using RNeasy Mini Kit from QIAGEN (Valencia, CA). Purified RNA was quantified and assessed for purity and integrity by capillary electrophoresis using the Agilent Bioanalyzer (Santa Clara CA). cDNA was generated from 3-6μg of RNA using II-RNase H–reverse transcriptase (Invitrogen; Grand Island, NY). Sixty nanograms (60ng) of resultant cDNA were used in each PCR reaction. Species-specific primers and TaqMan probes were designed using baboon and pig sequences ((Supplementary Table 2). Primers and TET-labeled probes with BHQ (Black Hole QuencherR) quenchers were obtained from Integrated DNA Technologies (Coralville, IA). Real-time PCR assay was performed on an ABI Prism 7900 (Applied Biosystems). The expression of pig or baboon C-RP was normalized to pig or baboon house-keeping gene, ribosomal protein L32 (RPL32), and mRNA levels were expressed as relative fold increase over a normal pig or baboon kidney sample using ΔΔCT calculation. Species-specificity of C-RP assays was confirmed using baboon and pig liver samples.
Statistical analysis
Statistical significance was determined by paired, two-tailed Student's ‘t’ test and one-way ANOVA. Linear regression and correlation analyses were carried out using GraphPad Prism version-4 (GraphPad Software, San Diego, CA); (p<0.05 was considered significant).
RESULTS
Cytokine and chemokine levels in organ xenograft recipients
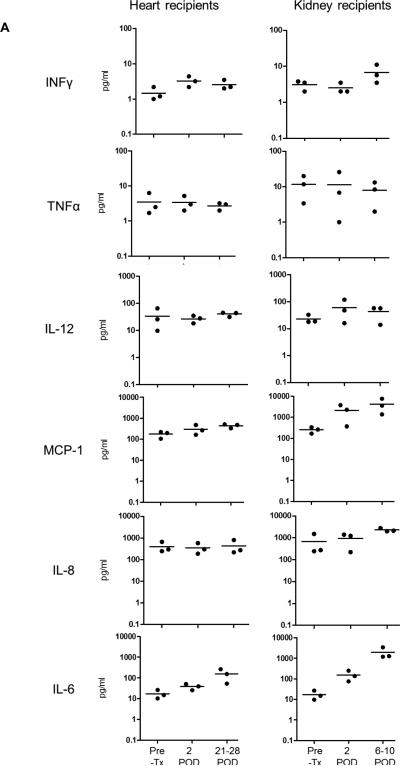
Pro-inflammatory cytokines are known to promote TF expression and activation of coagulation (11, 22). Despite being previously reported in pig-islet xenograft recipients (14-17), their role in the activation of coagulation after xenotransplantation is not well understood. We evaluated the levels of IFN-γ, TNF-α, IL-12, MCP-1, IL-8 and IL-6 in three heart recipients at 2 days and then 21, 26 and 28 days, and in four kidney recipients at 2 days and then 6, 9 and 10 days after transplantation (Figure 1A). In heart recipients, IFN-γ, TNF-α, IL-12 and IL-8 were not elevated after transplantation, while MCP-1 and IL-6 levels were elevated. In kidney recipients, the levels of IFN-γ and TNF-α were not elevated. IL-12 and IL-8 levels were elevated but not significantly, while MCP-1, and IL-6 levels were significantly elevated (p<0.05).
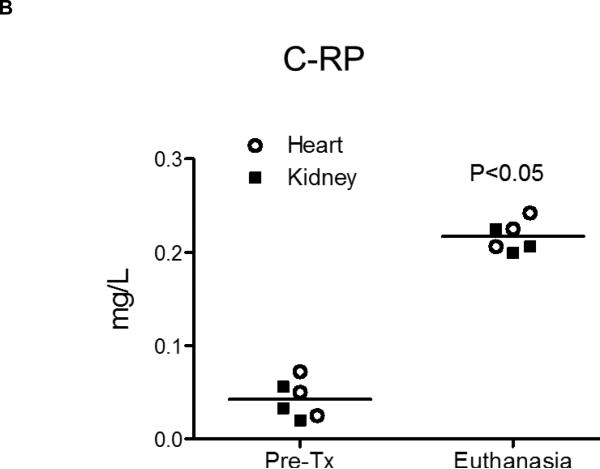
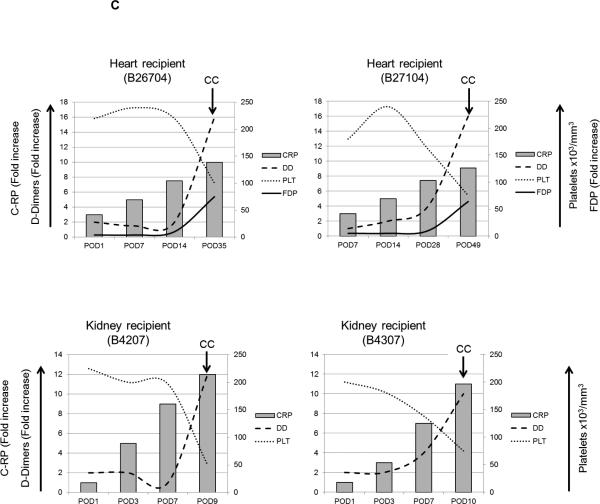
Figure 1. Pro-inflammatory cytokines, chemokines and C-RP levels in relation to activation of coagulation.
(A) Blood cytokine and chemokine levels were measured in three heart recipients on days 21, 26 and 28 after transplantation and in three kidney recipients at euthanasia (days 6, 9 and 10). Significantly higher levels of MCP-1 and IL-6 were detected in kidney recipients (*p<0.05).
(B) Significantly higher C-RP levels were detected in heart and kidney recipients at the time of euthanasia in comparison to before transplantation.
(C) Platelet counts, and fold increases in C-RP, D-Dimer, and FDP were calculated after heart xenotransplantation in heart and kidney xenograft recipients. High levels of C-RP were detected as early as 14 days after heart xenotransplantation and 3 days after kidney xenotransplantation, prior to the development of consumptive coagulopathy (CC) as indicated by reduced platelet counts, and elevated D-Dimer and FDP levels (FDP levels were not available from kidney recipients).
Elevated C-RP levels precede consumptive coagulopathy in heart and kidney xenograft recipients
C-RP is an acute-phase protein, which increases in the blood in response to inflammation. At the time of euthanasia, significantly high levels of C-RP were detected in both heart and kidney recipients (p<0.05) in comparison to before transplantation (Figure 1B).
Following organ xenotransplantation, activation of coagulation eventually leads to CC, which is based on laboratory tests (low platelet numbers, low fibrinogen levels, elevated D-dimer levels and/or fibrin degradation products [FDP]). In the current study, elevated C-RP levels at the time of euthanasia in pig and heart xenograft recipients suggest an inflammatory response. We measured C-RP levels in correlation with parameters of activation of coagulation in two heart and two kidney xenograft recipients.
In two heart recipients (survived 35 and 56 days), C-RP levels were elevated as early as 7 days after transplantation, before the development of CC, i.e., reduced platelet numbers, associated with elevated D-dimer and FDP levels. Similarly, in two kidney recipients, elevated C-RP levels were detected by day 3, before reduced platelets, and elevated D-Dimer and FDP were observed (Figure 1C).
These data indicate that inflammatory responses in organ xenograft recipients preceded activation of coagulation and the development of CC.
IS reduces pro-inflammatory cytokine and chemokine levels except IL-6 in pig artery patch xenograft recipients
Since organ xenograft recipients routinely receive IS, it is not possible to evaluate activation of coagulation and inflammatory responses in the absence of IS after xenotransplantation. Pig artery patch xenograft recipients can be maintained without IS until electively euthanized (20). We measured circulating cytokine and chemokine levels, at 2 weeks and one month after transplantation (Figure 2A).
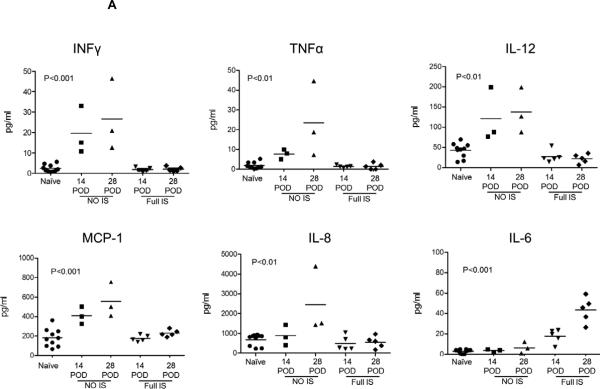
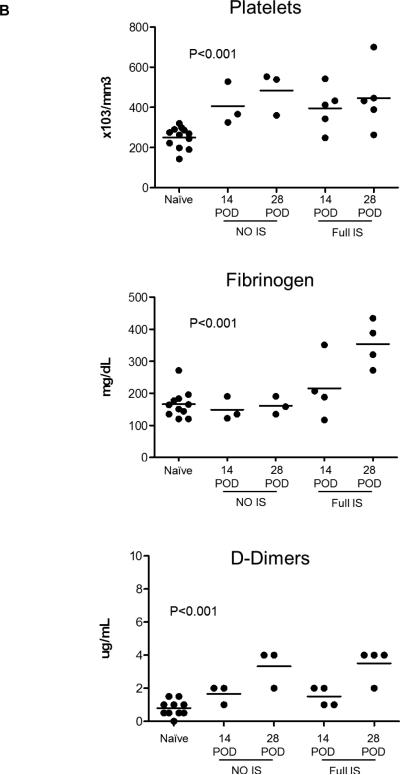
Figure 2. Effect of immunosuppressive therapy (IS) on pro-inflammatory cytokines, chemokines and C-RP levels in relation to activation of coagulation in pig artery patch recipients.
(A) Blood cytokine and chemokine levels after pig artery patch transplantation in comparison to naïve baboons at 14 and 28 days after transplantation. With no IS, elevated levels of IL-12, IFNγ, TNFα, MCP-1 and IL-8 were observed, and was markedly reduced with IS (n=5), whereas IL-6 levels were elevated with IS in comparison to naïve baboons and when no IS was given.
(B) Platelet counts, D-dimers and fibrinogen were evaluated in pig artery patch recipients at 2 weeks and one month after transplantation in comparison to naïve baboons. In naïve baboons, platelet numbers were 250+/−52×103/mm3, D-dimer levels were 1.3+/−1.1μg/mL, and fibrinogen levels were 166+/−43mg/dL. In pig artery patch recipients, platelet numbers were significantly elevated at 2 weeks without IS (395+/−109×103/mm3) or with IS (406+/−107×103/mm3), and at 4 weeks without IS (446+/−159×103/mm3) or with IS (484+/−107×103/mm3) (p<0.001). Without IS, fibrinogen levels remained comparable to those in naïve baboons at 2 weeks (149+/−36mg/dL) and at 4 weeks (161+/−28mg/dL). With IS, fibrinogen levels were slightly elevated at 2 weeks (216+/−98mg/dL), and elevated significantly at 4 weeks (354+/−72mg/dL) (p<0.001). D-dimer levels were significantly elevated at 2 weeks without (1.7+/−0.6μg/mL) or with (1.5+/−0.6μg/mL) IS, and at 4 weeks without IS (3.3+/−1.2μg/mL) or with IS (3.5+/−1μg/mL IS (p<0.001).
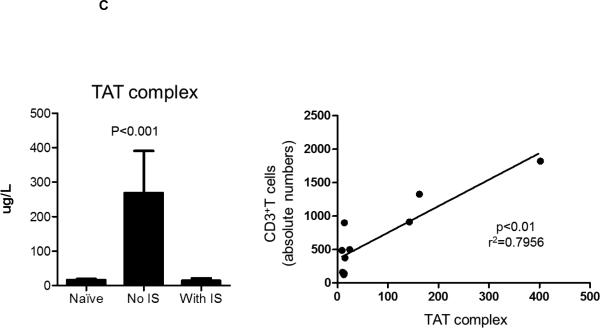
(C) Immunosuppression reduced TAT levels in pig artery patch recipients (left). In naïve baboons, TAT levels were 16.3+/−10μg/L. TAT levels were elevated after transplantation with no IS (235.3+/−144.4μg/L), but were reduced to normal levels when IS had been administered (14.2+/−7μg/L). There was a significant positive correlation (r2=0.7956; p<0.01) between TAT levels and absolute CD3+T cell numbers in pig artery patch recipients (right).
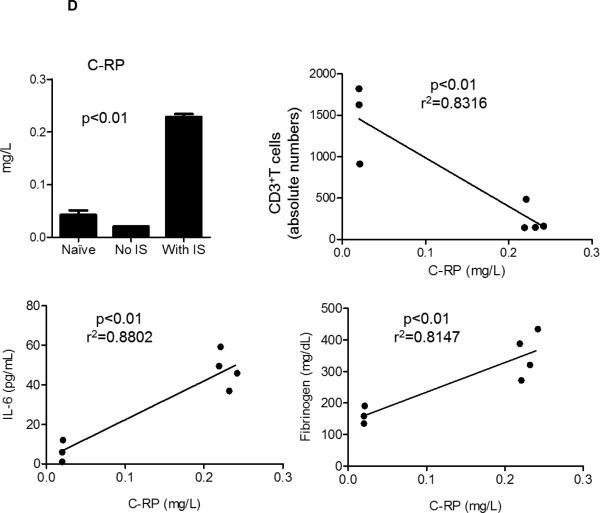
(D) C-RP levels in blood were measured before and after pig artery patch xenotransplantation and the fold increase in C-RP levels was determined. Without IS, there was no increase in C-RP level, whereas with IS there was a significant increase. There was a significant negative correlation between C-RP levels and the number of CD3+T cells, and a significant positive correlation between C-RP levels and IL-6 and fibrinogen levels.
After artery patch transplantation, the levels of IFN-γ, TNF-α, IL-8, IL-12, and MCP-1 were significantly elevated (p<0.001) when no IS was given. Four weeks after Tx. MCP-1 levels were significantly higher than at 2 weeks after Tx (p<0.05), with or without IS. Furthermore, IL-6 levels were not elevated. On the other hand, IS was associated with reduced levels of IFN-γ, TNF-α, IL-8, IL-12, and MCP-1, but not IL-6 which was significantly elevated (p<0.001). Four weeks after Tx, IL-6 levels were significantly higher than at 2 weeks after Tx (p<0.05). No difference in cytokine and chemokine levels were found between baboons receiving CTLA4-Ig-based or anti-CD154mAb-based IS (Table 1).
Effect of IS on activation of coagulation in pig artery patch xenografts
Activation of coagulation is a common feature in xenograft recipients. While platelet deposition has been detected in pig artery patch xenografts (20), features of activation of coagulation in pig artery recipients have not been reported. In parallel with the effect of IS on pro-inflammatory cytokines and chemokines, we measured platelet numbers, fibrinogen and D-dimer levels at 2 weeks and one month after transplantation, in the presence or absence of IS (Figure 2B). Significantly higher platelet numbers were observed with or without IS (p<0.001). Elevated D-Dimer levels were also observed with or without IS. Interestingly, significantly higher fibrinogen levels were only observed in immunosuppressed recipients (p<0.001). To further understand the influence of IS on activation of coagulation, we evaluated thrombin anti-thrombin complex (TAT) levels in pig artery patch recipients. Without IS, TAT levels were elevated, but were reduced to normal levels with IS (Figure 2C), with significant positive correlation between CD3+ T cell numbers and TAT levels (r2=0.7956; p<0.01).
These data indicate that activation of coagulation occurs in pig artery patch recipients but is not associated with CC. Furthermore, while IS reduces thrombin activation and delays activation of coagulation in xenograft recipients, as previously reported (4), it is associated with significantly high levels of fibrinogen and D-Dimer.
Immunosuppression is associated with high C-RP levels in artery patch xenograft recipients
C-RP and IL-6 are associated with inflammatory conditions in humans (23, 24). We measured C-RP levels in pig artery patch recipients before and after transplantation. With no IS, C-RP levels remained normal, but were markedly elevated with IS (Figure 2D). In correlation, we found significant negative correlation between elevated C-RP levels and reduced CD3+T cell numbers (p<0.01; r2=0.8316). Additionally, high levels of IL-6 and fibrinogen have been observed with IS (Figure 2A). We found a significant positive correlation between C-RP and IL-6 levels (p<0.01; r2=0.8802) and between C-RP and fibrinogen levels (p<0.01; r2=0.8147).
These data suggest that administration of T cell-directed IS in pig artery patch recipients delays activation of coagulation, but is associated with an inflammatory response associated with increased production of IL-6, C-RP and fibrinogen.
Monocyte numbers increase after artery patch and organ xenotransplantation
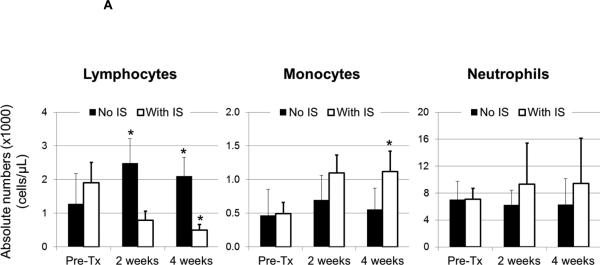
Absolute numbers of circulating leukocytes were calculated before and after artery patch transplantation (Supplementary Table 3 and Figure 3A). With no IS, lymphocyte numbers increased significantly at 2 and 4 weeks (p<0.05), whereas, with IS, they decreased significantly (p<0.05). Monocyte numbers increased without IS, although not significantly, but were significantly increased at 4 weeks (p<0.05) with IS. With no IS, mean neutrophil numbers were modestly reduced, and increased at 2 and 4 weeks, although not significantly. Similarly, decreased lymphocyte numbers in heart recipients were associated with increased monocyte and neutrophil numbers (Supplementary Figure 1). Comparable increases in monocyte or neutrophil numbers were not observed in kidney recipients, probably due to the short graft survival and follow-up (not shown).
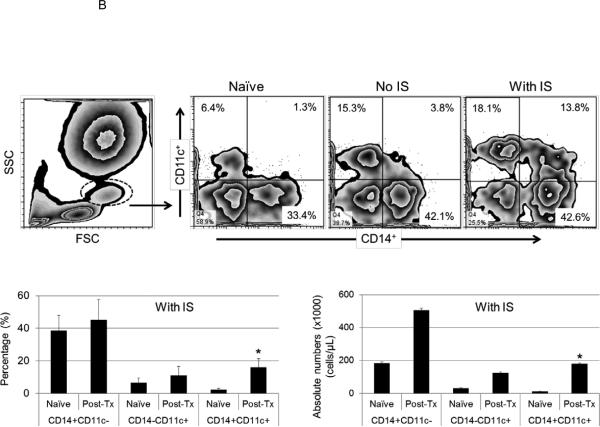
Figure 3. Immunosuppressive therapy (IS) is associated with increased pro-coagulant inflammatory innate immune cells after pig artery patch xenotransplantation.
(A) After pig artery patch xenotransplantation, absolute numbers of peripheral blood leukocytes were measured. With no IS, mean lymphocyte numbers at 2 and 4 weeks post-transplant increased by 49 % and 39%, respectively (*p<0.05), and were reduced with IS by 33 % and 37%, respectively (*p<0.05). With no IS, monocytes increased at 2 and 4 weeks by 32 % and 16%, respectively. With IS, monocytes increased more markedly at 2 and 4 weeks by 55 % and 56%, respectively (*p<0.05). Neutrophil numbers did not change significantly after transplantation with a modest reduction of 12 % and 11%, respectively. With IS, mean neutrophil numbers increased at 2 and 4 weeks after transplantation by 24 % and 25%, respectively.
(B) With IS, increased percentages of CD14+CD11c− (45%+/−12%) and CD14−CD11c+ (11%+/−5%) cells were detected in comparison to naïve baboons, where CD14+CD11c− and CD14−CD11c+ cells were 38%+/−9% and 6%+/−3%, respectively (bottom). A significant increase in the CD14+CD11c+ cells percentages (16%+/−5%) and absolute numbers (180+/−60×103/μL) was detected only in the presence of IS which was associated with a significantly higher numbers than in naïve baboons (2.4%+/−0.6%; (11+/−3×103/μL) (*p<0.05) (bottom).
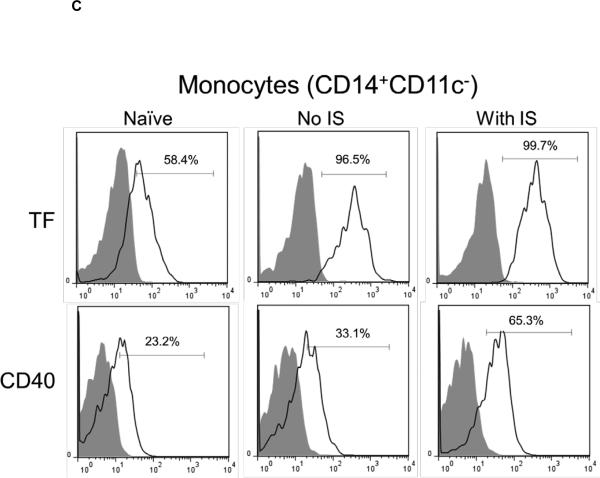
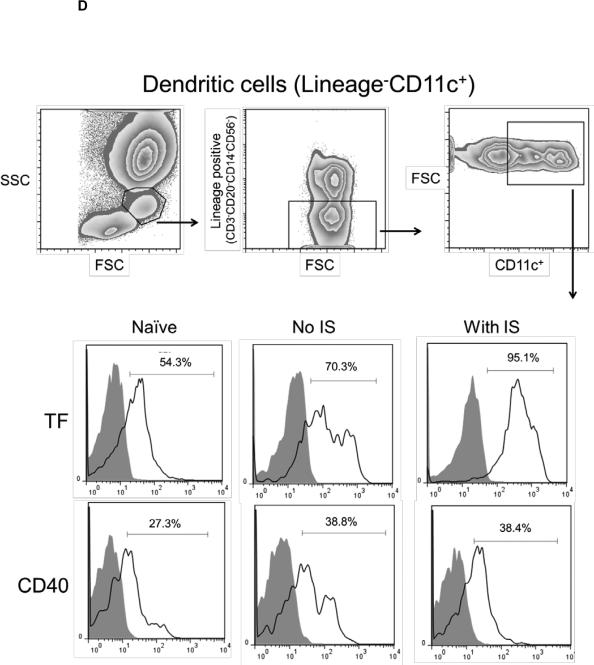
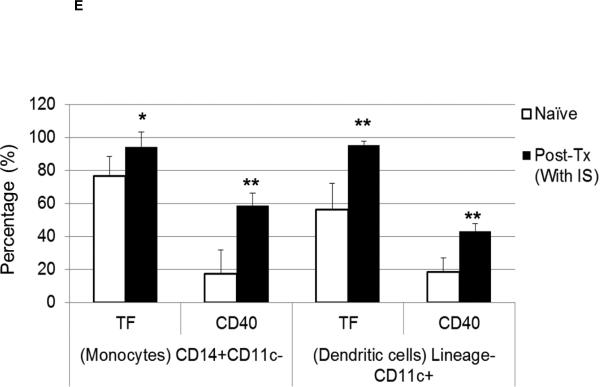
(C and D) With or without IS, there was increased TF and CD40 expression on monocytes (CD14+CD11c−) (C) and DC (lineageneg CD11c+) (D) in comparison to naïve baboons. With no IS, monocytes were 96.5% TF-positive and 33.1% CD40-positive, while DC were 70.3% TF-positive and 38.8% CD40-positive. (E) In naïve baboons, monocytes were 76.5%+/−11.8% TF-positive and 17.4%+/−14.4% CD40-positive, whereas DC were 56.2%+/−16% TF-positive and 18.3%+/−8.8% CD40-positive. With IS, monocytes were 94.4%+/−9.1% TF-positive and 58.6%+/−7.4% CD40-positive, while DC were 95.3%+/−2.3% TF-positive and 43%+/−4.6% CD40-positive In comparison to naïve baboons (* p<0.05 and ** p<0.01).
T cell-directed IS is associated with increased pro-inflammatory CD14+CD11c+ monocytes after artery patch xenotransplantation
Monocytes and dendritic cells (DC) are innate immune cells that play critical roles in inflammation and innate and adaptive immunity. Recently, inflammatory CD11c+DC have been shown to be monocyte-derived (25, 26). We evaluated CD11c expression on CD14+ monocytes in baboons after pig artery patch transplantation. With or without IS, CD14+CD11c− (monocyte) and CD14−CD11c+ (DC) populations were increased after transplantation. With no IS, only one baboon sample was available for analysis, and showed increased percentages of CD14+CD11c− (42.1%) and CD14−CD11c+ (15.3%) cells (Figure 3B, top). With IS, increased percentages of CD14+CD11c− and CD14−CD11c+ cells were also detected in comparison to naïve baboons (Figure 3B, bottom). Notably, a significant increase in the percentage of CD14+CD11c+ cells was detected only with IS, and was significantly higher than in naïve baboons (p<0.05).
These data suggest that, there was increased differentiation of CD14+ monocytes into inflammatory CD11c+DC after pig artery patch transplantation particularly with IS.
Upregulation of TF expression and activation of monocytes and DC after artery patch xenotransplantation
Upregulation of TF expression on innate immune cells can be related to inflammatory responses without dysregulation of coagulation (7). It is known that TNF-α (11), IL-6 (12), and C-RP (13) promote TF expression. We analyzed TF expression on CD14+CD11c− monocytes (Figure 3C) and lineagenegCD11c+DC (Figure 3D). As CD40 expression is known to be upregulated upon activation of antigen-presenting cells, we evaluated its expression in correlation with TF. One recipient with no IS was used as a control, in which TF and CD40 expression on monocytes and DC were higher after transplantation. Similarly, with IS, higher expression of TF and CD40 was detected on monocytes and DC. TF and CD40 expression on monocytes and DC (Figure 3E) was significantly higher (p<0.05, p<0.01, and p<0.01, p<0.01, respectively). TF expression was not measured on monocytes and DC in organ xenograft recipients (2, 3).
Collectively, these data suggest that in pig artery patch recipients, despite reduced TAT levels with IS (Figure 2C), there was increased differentiation of pro-inflammatory CD14+CD11c+ monocytes, associated with activation and upregulation of TF expression.
C-RP deposition in xenografts and native organs
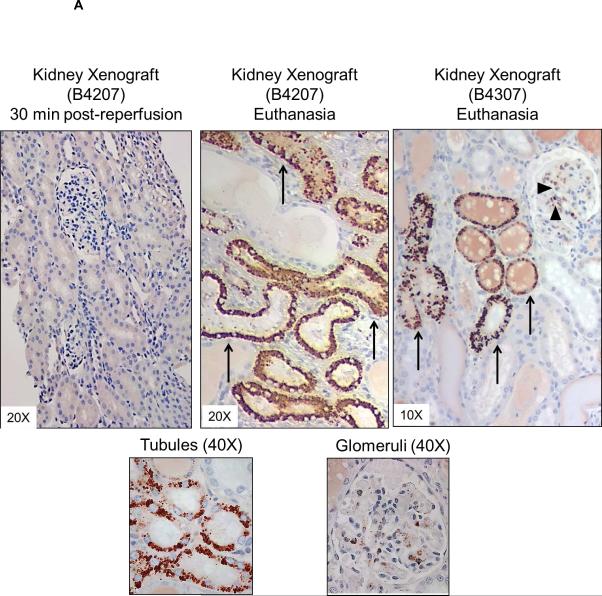
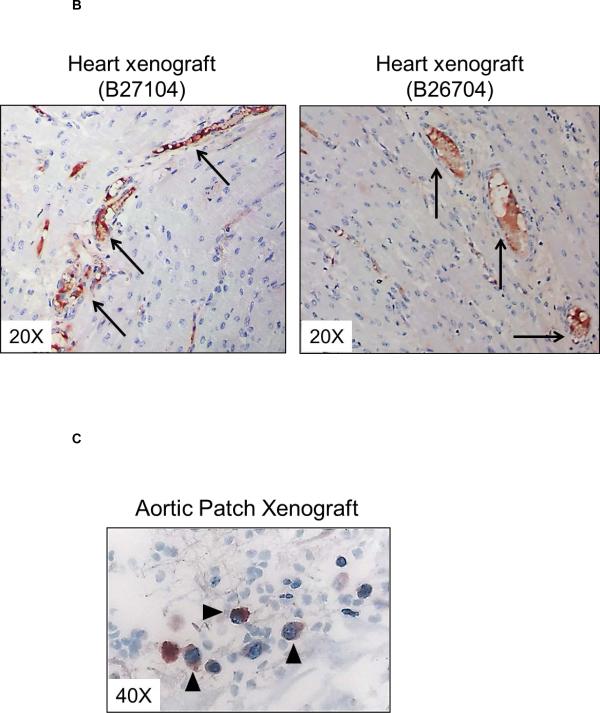
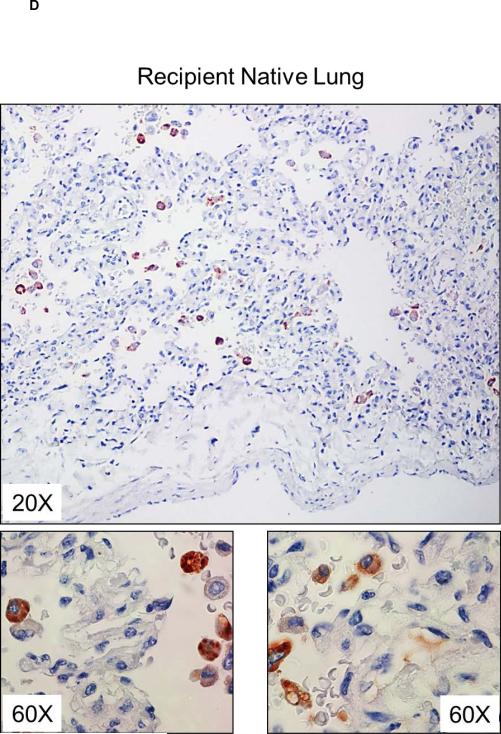
Deposition of C-RP in tissues is associated with inflammatory conditions (27). In kidney xenografts, no C-RP deposition was detected 30 minutes after graft reperfusion, but at euthanasia (at 9 and 10 days) there was strong deposition of C-RP in the tubules and glomeruli (Figure 4A). Less deposition of C-RP was evident in heart xenografts and was mainly in capillaries (Figure 4B). Few C-RP-positive cells were detected in artery patch xenografts (Figure 4C). In organ xenograft recipients, C-RP-positive cells were detected in the native lungs at euthanasia (Figure 4D).
Figure 4. C-RP deposition in xenografts and native organs.
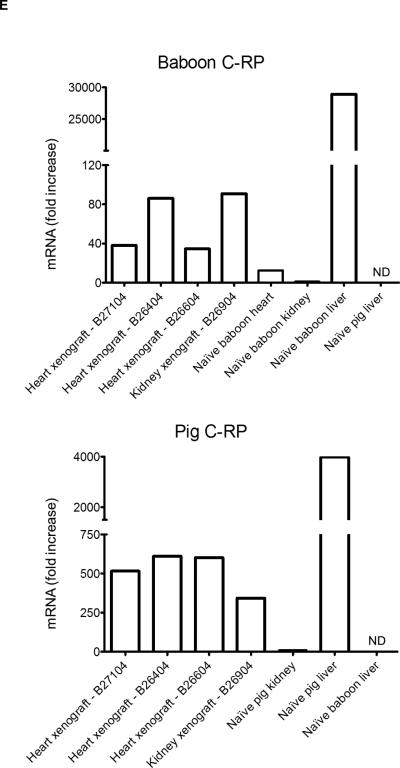
(A) C-RP deposition in kidney xenografts. At 30 min after reperfusion, no C-RP was detected. At the time of euthanasia, C-RP deposition was detected in glomeruli (arrow heads) and tubules (arrows). (B) Deposition of C-RP in heart xenografts (arrows) at the time of euthanasia was mainly in the capillaries, with minimal deposition in the interstitium. (C) A few C-RP-positive cells (arrow heads) were detected in artery patch xenografts. (D) Multiple C-RP-positive cells were detected in the native lungs of kidney xenograft recipients at euthanasia. (E) Relative expression of baboon (top) and pig (bottom) C-RP mRNA in kidney and heart xenografts at euthanasia. Tissues were obtained from a heart xenograft (B27104 - 56 days survival) and a kidney xenograft (B 4207 - 9 days survival). Additional samples were available from two heart xenografts (B26404 and B26604 - both 12 days survival). Baboon and pig livers were used as positive controls and to confirm species-specificity of the primers used. Data represent fold increases in comparison to mRNA in naïve baboon or pig kidney tissues, respectively. ND=not detected.
To determine whether C-RP detected in the organ xenografts is of baboon or pig origin, we evaluated baboon and pig C-RP mRNA levels in the organ xenografts at euthanasia. Samples were available from a heart xenograft (56-days) and a kidney xenograft (9-days). Additional samples were available from 2 heart xenograft recipients (B26604 and B26404; 12-days) (3). High levels of both baboon and pig C-RP mRNA were detected in organ xenografts at the time of euthanasia (Figure 4E), suggesting that both the xenograft and the recipient contribute to C-RP production after xenotransplantation.
DISCUSSION
After pig-to-primate xenotransplantation, thrombotic microangiopathy in the xenograft and/or CC in the recipient, are considered major barriers to prolonged xenograft survival (28). Increased IS has been advocated to prevent dysregulation of coagulation and no benefit was associated with conventional anticoagulation (4, 5). Meanwhile, intensive IS aimed at the induction of tolerance has prolonged kidney xenograft survival, yet survival though not beyond 83 days (5). Blocking elicited antibodies is thought to be critical for prevention of pig-endothelial cell activation and upregulation of pro-coagulant proteins in xenografts (29). Furthermore, reduction in white blood cell numbers in the blood can reduce prothrombinase complexes (30) and hence thrombin formation. In correlation, in the current study, IS was associated with delayed activation of coagulation in heart recipients (Figure 1C) (3), and reduced TAT levels in pig artery patch recipients (Figure 2C). However, same IS did not prolong kidney xenograft survival (2). This was associated with significantly higher pro-inflammatory cytokines and chemokines early after kidney xenotransplantation (Figure 1A), suggesting stronger innate and inflammatory responses to kidney xenografts in concurrence with upregulation of recipient TF expression on monocytes and platelets and CC development (2). More importantly, upregulation of pro-inflammatory factors and C-RP preceded the development of CC in both heart and kidney xenograft recipients.
Considering mutual amplification of inflammation and coagulation (8), escalating intensities of IS can regulate inflammatory responses and hence activation of coagulation after xenotransplantation. Also, Heparin has been administered routinely to prevent prothrombotic effects of anti-CD154mAb therapy. Due to its multiple anti-inflammatory activities, this may have delayed dysregulation of coagulation after organ xenotransplantation (2, 3).
The pig-to-baboon artery patch transplantation model (developed by our group) has proven useful in evaluating the efficacy of IS in preventing the adaptive immune responses to pig antigens (20). With no IS, a pig artery patch xenograft induces adaptive T cell response. Furthermore, platelet deposition was observed in the xenografts suggesting activation of coagulation in the recipient baboons, associated with elevated levels of pro-inflammatory CD154 (CD40 ligand) in the blood (31). In addition, data on coagulation dysregulation in the pig-to-baboon artery patch recipients correlated with some extent with data obtained from the pig-to-baboon heart transplantation model. In the current study, TAT levels were elevated in pig artery patch recipients when no IS was given, and were reduced with IS. Platelets have been recently considered as a crucial link between inflammation and thrombosis (32, 33). Interestingly, significantly elevated platelet numbers were observed with or without IS. Also, the role of fibrinogen as a critical factor in inflammation has been recognized (34, 35). In correlation, significantly higher fibrinogen levels were detected when IS was given. Meanwhile, IS reduced IFN-γ, TNF-α, IL-12, MCP-1, and IL-8 levels. Conversely, IL-6 and C-RP levels were only elevated when IS was given. These data indicate that IS in recipient baboons is associated with an inflammatory response as early as 2 weeks after pig artery patch transplantation.
In the early 1930s, C-RP was the first acute-phase protein recognized in humans and NHP during infection (36, 37). After allo-transplantation in humans, C-RP is considered a sensitive, but not specific, marker for graft-related complications (38-42). In the present study, elevated C-RP levels were observed early after kidney xenotransplantation and before CC development in heart recipients. We found significant positive correlation between IL-6 and C-RP levels in pig artery patch grafts recipients (Figure 2D). Both C-RP and fibrinogen are acute-phase reactant proteins, produced in response to acute inflammation. Furthermore, C-RP was identified in the organs as well as native lungs in xenograft recipients, indicating systemic inflammatory response. In our study, the role of each species C-RP in the development of systemic inflammation in the recipients requires further investigation. Furthermore, the variability in C-RP levels and deposition in organ xenografts may be related to the type of xenograft (43).
IL-6 is an inflammatory cytokine, which is generated by innate immune cells, including monocytes and macrophages (22, 44). IL-6 has been linked to inflammation and thrombotic complications (45), and is known to promote TF expression (46) on innate immune cells (22). IL-6 is known to induce C-RP production by liver hepatocytes (47) and smooth muscle cells (48), and high IL-6 levels are associated with high C-RP levels in patients with heart failure (49). These observations suggest that IS can be associated with an inflammatory response in xenograft recipients, as previously reported in humans (50). Furthermore, these observations suggest that blockade of IL-6 activity can be beneficial after xenotransplantation.
Elevation of pro-inflammatory factors can be related to the surgical procedure, i.e., to transplantation. Elevated C-RP and IL-6 levels were observed as early as 2-3 days after transplantation, and continued to rise until the day of euthanasia (6-10 days) in kidney recipients and for 4-6 weeks in heart recipients. This suggests that the rise in these pro-inflammatory factors was not related to the surgical procedure. In our nonhuman primate organ allo-transplantation studies, we evaluated C-RP levels after transplantation. In heart allograft recipient cynomolgus monkeys, mean C-RP level was 0.08+0.06 mg/L at 1-2 months after Tx, which increased to 0.14+0.07mg/L at the time of rejection, in comparison to C-RP levels in naïve animals (<0.02mg/L). In kidney allograft recipient rhesus monkeys, mean C-RP level was 0.06+0.04 mg/L at 4 weeks after Tx, which increased to 0.2+0.09mg/L at the time of rejection, in comparison to C-RP levels in naïve animals (<0.02mg/L). Accordingly, C-RP levels in organ allograft recipients are significantly lower than those observed in organ xenograft recipients. Additionally, fold increase in C-RP levels in response to a small pig artery patch (8-10 fold) was higher than that in organ allograft recipients (2-4 fold). Collectively, these observations suggest the inflammatory response in a xenograft recipient is much greater than to an allograft.
In organ and artery patch recipients, IS was associated with significantly increased monocytes. In pig artery patch recipients, pro-inflammatory CD14+CD11c+ monocytes were significantly increased with IS, together with significantly higher percentages of activated monocytes and DC and significant upregulation of TF expression (Figure 3). The pro-coagulant properties of innate immune cells are well characterized (51, 52). Activated monocytes and dendritic cells are known to upregulate TF expression, and to mediate inflammation and coagulation (53-55). Furthermore, the pro-inflammatory potential of TF has been well recognized (56-58). Recently, pigs transgenic for human coagulation-regulatory proteins; tissue factor pathway inhibitor, thrombomodulin, endothelial protein C receptor (EPCR), and CD39 have become available (59). The expression of these proteins in the xenograft should be beneficial to reduce dysregulation of coagulation and amplification of inflammation after xenotransplantation.
In humans, systemic inflammatory response syndrome (SIRS) is a well-described clinical entity that involves dysregulation of coagulation, and may progress to hemodynamic collapse. While it can occur with systemic infection, it is also seen after (sterile) injury and surgical procedures (60). On the other hand, dysregulation of coagulation and inflammation have been reported in NHP model of sepsis (61). A similar condition after xenotransplantation may share common mechanisms with SIRS, despite distinct triggering events.
Furthermore, inflammatory responses can influence tolerance induction in allograft recipients (62). Regulation of inflammatory responses may be required to reduce the intensity of IS used after xenotransplantation. Anti-inflammatory agents prevent TF expression and thrombosis (63). For example, we have shown that statins inhibit upregulation of TF expression on pig endothelial cells in response to primate xenoreactive antibodies (64) and regulate human and baboon T cell proliferation against pig endothelial cells (65).
In summary, successful inhibition of the adaptive immune response after xenotransplantation results in delayed activation of coagulation in xenograft recipients. Downregulation of pro-inflammatory cytokines by IS may contribute to delayed activation of coagulation. However, the development of inflammation as well as increased pro-inflammatory and pro-coagulant monocytes and DC, typically associated with innate immunity, may amplify dysregulation of activation and eventually lead to CC. Our data support the emerging paradigm that, despite prevention of adaptive immune responses, innate pro-inflammatory responses and activation of coagulation are likely interconnected and pathogenic after xenotransplantation. By implication, regulation of inflammation and coagulation in xenograft recipients may be mutually beneficial. This is of importance in relation to protocols pursuing long-term graft function and eventually tolerance induction in xenograft recipients.
Supplementary Material
ACKNOWLEDGEMENTS
MBE is supported, in part, by the Joseph A. Patrick Fellowship of the Thomas E. Starzl Transplantation Institute. BE was the recipient of an NIH T32 AI 074490 post-doctoral fellowship. Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute is, or has been, supported by NIH grants U19 AI090959, U01 AI068642, and R21 A1074844, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA. The baboons used in the study were from the Oklahoma University Health Sciences Center, Baboon Research Resources, which is supported by the Office of the Director, NIH, under Award #P40OD010431 and #P40OD010988.
ABBREVIATIONS
- CC
Consumptive coagulopathy
- C-RP
C-reactive protein
- DC
dendritic cells
- FDP
fibrin degradation products
- GTKO
α1,3-galactosyltransferase gene-knockout
- IS
immunosuppressive therapy
- SIRS
systemic inflammatory response syndrome
- TAT
thrombin-antithrombin complexes
- TF
tissue factor
Footnotes
DISCLOSURE
David Ayares is an employee of Revivicor Inc.
REFERENCES
- 1.Ekser B, Ezzelarab M, Hara H, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379(9816):672–83. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 2.Lin CC, Ezzelarab M, Shapiro R, et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010;10(7):1556–68. doi: 10.1111/j.1600-6143.2010.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87(6):805–12. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne GW, Davies WR, Oi K, et al. Increased immunosuppression, not anticoagulation, extends cardiac xenograft survival. Transplantation. 2006;82(12):1787–91. doi: 10.1097/01.tp.0000251387.40499.0f. [DOI] [PubMed] [Google Scholar]
- 5.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11(1):32–4. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 6.Chu AJ. Tissue factor, blood coagulation, and beyond: an overview. Int J Inflam. 2011;2011:367284. doi: 10.4061/2011/367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu AJ. Tissue factor mediates inflammation. Arch Biochem Biophys. 2005;440(2):123–32. doi: 10.1016/j.abb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Strukova S. Blood coagulation-dependent inflammation. Coagulation-dependent inflammation and inflammation-dependent thrombosis. Front Biosci. 2006;11:59–80. doi: 10.2741/1780. [DOI] [PubMed] [Google Scholar]
- 9.Ulloa L, Tracey KJ. The “cytokine profile”: a code for sepsis. Trends Mol Med. 2005;11(2):56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Schneider MK, Seebach JD. Current cellular innate immune hurdles in pig-to-primate xenotransplantation. Curr Opin Organ Transplant. 2008;13(2):171–7. doi: 10.1097/MOT.0b013e3282f88a30. [DOI] [PubMed] [Google Scholar]
- 11.Kambas K, Markiewski MM, Pneumatikos IA, et al. C5a and TNF-alpha up-regulate the expression of tissue factor in intra-alveolar neutrophils of patients with the acute respiratory distress syndrome. J Immunol. 2008;180(11):7368–75. doi: 10.4049/jimmunol.180.11.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruithof EK, Mestries JC, Gascon MP, Ythier A. The coagulation and fibrinolytic responses of baboons after in vivo thrombin generation--effect of interleukin 6. Thromb Haemost. 1997;77(5):905–10. [PubMed] [Google Scholar]
- 13.Wu J, Stevenson MJ, Brown JM, et al. C-reactive protein enhances tissue factor expression by vascular smooth muscle cells: mechanisms and in vivo significance. Arterioscler Thromb Vasc Biol. 2008;28(4):698–704. doi: 10.1161/ATVBAHA.107.160903. [DOI] [PubMed] [Google Scholar]
- 14.Solomon MF, Kuziel WA, Mann DA, Simeonovic CJ. The role of chemokines and their receptors in the rejection of pig islet tissue xenografts. Xenotransplantation. 2003;10(2):164–77. doi: 10.1034/j.1399-3089.2003.01146.x. [DOI] [PubMed] [Google Scholar]
- 15.Chandra AP, Ouyang L, Yi S, et al. Chemokine and toll-like receptor signaling in macrophage mediated islet xenograft rejection. Xenotransplantation. 2007;14(1):48–59. doi: 10.1111/j.1399-3089.2006.00363.x. [DOI] [PubMed] [Google Scholar]
- 16.Yi S, Ouyang L, Ha H, et al. Involvement of CCR5 signaling in macrophage recruitment to porcine islet xenografts. Transplantation. 2005;80(10):1468–75. doi: 10.1097/01.tp.0000183398.82878.47. [DOI] [PubMed] [Google Scholar]
- 17.Remy S, Canova C, Daguin-Nerriere V, et al. Different mechanisms mediate the rejection of porcine neurons and endothelial cells transplanted into the rat brain. Xenotransplantation. 2001;8(2):136–48. doi: 10.1034/j.1399-3089.2001.00076.x. [DOI] [PubMed] [Google Scholar]
- 18.Dai Y, Vaught TD, Boone J, et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002;20(3):251–5. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- 19.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605):411–4. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ezzelarab MB, Ekser B, Echeverri G, et al. Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation. 2012;19(4):221–32. doi: 10.1111/j.1399-3089.2012.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ezzelarab M, Cortese-Hassett A, Cooper DK, Yazer MH. Extended coagulation profiles of healthy baboons and of baboons rejecting GT-KO pig heart grafts. Xenotransplantation. 2006;13(6):522–8. doi: 10.1111/j.1399-3089.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 22.Kotloff RM, Little J, Elias JA. Human alveolar macrophage and blood monocyte interleukin-6 production. Am J Respir Cell Mol Biol. 1990;3(5):497–505. doi: 10.1165/ajrcmb/3.5.497. [DOI] [PubMed] [Google Scholar]
- 23.Il'yasova D, Ivanova A, Morrow JD, et al. Correlation between two markers of inflammation, serum C-reactive protein and interleukin 6, and indices of oxidative stress in patients with high risk of cardiovascular disease. Biomarkers. 2008;13(1):41–51. doi: 10.1080/13547500701617708. [DOI] [PubMed] [Google Scholar]
- 24.Maksimovic M, Vlajinac H, Radak D, et al. Relationship between high-sensitivity C-reactive protein and risk factors in patients with peripheral arterial disease--a cross-sectional study. Angiology. 2013;64(3):230–6. doi: 10.1177/0003319712440303. [DOI] [PubMed] [Google Scholar]
- 25.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 26.Serbina NV, Salazar-Mather TP, Biron CA, et al. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19(1):59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 27.Baidoshvili A, Nijmeijer R, Lagrand WK, et al. Localisation of C reactive protein in infarcted tissue sites of multiple organs during sepsis. J Clin Pathol. 2002;55(2):152–3. doi: 10.1136/jcp.55.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cowan PJ, Robson SC, d'Apice AJ. Controlling coagulation dysregulation in xenotransplantation. Curr Opin Organ Transplant. 2011;16(2):214–21. doi: 10.1097/MOT.0b013e3283446c65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowan PJ, d'Apice AJ. Complement activation and coagulation in xenotransplantation. Immunol Cell Biol. 2009;87(3):203–8. doi: 10.1038/icb.2008.107. [DOI] [PubMed] [Google Scholar]
- 30.Tracy PB, Rohrbach MS, Mann KG. Functional prothrombinase complex assembly on isolated monocytes and lymphocytes. J Biol Chem. 1983;258(12):7264–7. [PubMed] [Google Scholar]
- 31.Ezzelarab MB, Ekser B, Isse K, et al. Increased soluble CD154 (CD40 ligand) levels in xenograft recipients correlate with the development of de novo anti-pig IgG antibodies. Transplantation. 2014;97(5):502–8. doi: 10.1097/TP.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 32.Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2003;23(12):2131–7. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- 33.Stokes KY, Granger DN. Platelets: a critical link between inflammation and microvascular dysfunction. J Physiol. 2012;590(Pt 5):1023–34. doi: 10.1113/jphysiol.2011.225417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bickel C, Rupprecht HJ, Blankenberg S, et al. Relation of markers of inflammation (C-reactive protein, fibrinogen, von Willebrand factor, and leukocyte count) and statin therapy to long-term mortality in patients with angiographically proven coronary artery disease. Am J Cardiol. 2002;89(8):901–8. doi: 10.1016/s0002-9149(02)02236-1. [DOI] [PubMed] [Google Scholar]
- 35.Davalos D, Ryu JK, Merlini M, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227. doi: 10.1038/ncomms2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abernethy TJ, Avery OT. The Occurrence during Acute Infections of a Protein Not Normally Present in the Blood : I. Distribution of the Reactive Protein in Patients' Sera and the Effect of Calcium on the Flocculation Reaction with C Polysaccharide of Pneumococcus. J Exp Med. 1941;73(2):173–82. doi: 10.1084/jem.73.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tillett WS, Francis T. Serological Reactions in Pneumonia with a Non-Protein Somatic Fraction of Pneumococcus. J Exp Med. 1930;52(4):561–71. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wullstein C, Drognitz O, Woeste G, et al. High levels of C-reactive protein after simultaneous pancreas-kidney transplantation predict pancreas graft-related complications and graft survival. Transplantation. 2004;77(1):60–4. doi: 10.1097/01.TP.0000100683.92689.27. [DOI] [PubMed] [Google Scholar]
- 39.Their M, Ronnholm K, Sairanen H, et al. Serum C-reactive protein in pediatric kidney and liver transplant patients. Pediatr Transplant. 2002;6(2):153–60. doi: 10.1034/j.1399-3046.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- 40.Abedini S, Holme I, Marz W, et al. Inflammation in renal transplantation. Clin J Am Soc Nephrol. 2009;4(7):1246–54. doi: 10.2215/CJN.00930209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dahle DO, Mjoen G, Oqvist B, et al. Inflammation-associated graft loss in renal transplant recipients. Nephrol Dial Transplant. 2011;26(11):3756–61. doi: 10.1093/ndt/gfr163. [DOI] [PubMed] [Google Scholar]
- 42.van Ree RM, Oterdoom LH, de Vries AP, et al. Elevated levels of C-reactive protein independently predict accelerated deterioration of graft function in renal transplant recipients. Nephrol Dial Transplant. 2007;22(1):246–53. doi: 10.1093/ndt/gfl511. [DOI] [PubMed] [Google Scholar]
- 43.Knosalla C, Yazawa K, Behdad A, et al. Renal and cardiac endothelial heterogeneity impact acute vascular rejection in pig-to-baboon xenotransplantation. Am J Transplant. 2009;9(5):1006–16. doi: 10.1111/j.1600-6143.2009.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ancuta P, Wang J, Gabuzda D. CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J Leukoc Biol. 2006;80(5):1156–64. doi: 10.1189/jlb.0206125. [DOI] [PubMed] [Google Scholar]
- 45.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148(2):209–14. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 46.Levi M, van der Poll T, ten Cate H, van Deventer SJ. The cytokine-mediated imbalance between coagulant and anticoagulant mechanisms in sepsis and endotoxaemia. Eur J Clin Invest. 1997;27(1):3–9. doi: 10.1046/j.1365-2362.1997.570614.x. [DOI] [PubMed] [Google Scholar]
- 47.Moshage HJ, Roelofs HM, van Pelt JF, et al. The effect of interleukin-1, interleukin-6 and its interrelationship on the synthesis of serum amyloid A and C-reactive protein in primary cultures of adult human hepatocytes. Biochem Biophys Res Commun. 1988;155(1):112–7. doi: 10.1016/s0006-291x(88)81056-8. [DOI] [PubMed] [Google Scholar]
- 48.Calabro P, Willerson JT, Yeh ET. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108(16):1930–2. doi: 10.1161/01.CIR.0000096055.62724.C5. [DOI] [PubMed] [Google Scholar]
- 49.Marcucci R, Gori AM, Giannotti F, et al. Markers of hypercoagulability and inflammation predict mortality in patients with heart failure. J Thromb Haemost. 2006;4(5):1017–22. doi: 10.1111/j.1538-7836.2006.01916.x. [DOI] [PubMed] [Google Scholar]
- 50.Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34(1):43–62. doi: 10.1007/s00281-011-0290-8. [DOI] [PubMed] [Google Scholar]
- 51.Morrice LM, McIntosh LC, Webster LM, Thomson AW. Generation of procoagulant activity by mononuclear phagocytes: optimisation of an in vitro assay. J Immunol Methods. 1985;85(2):421–34. doi: 10.1016/0022-1759(85)90151-6. [DOI] [PubMed] [Google Scholar]
- 52.Hiller E, Saal JG, Riethmuller G. Procoagulant activity of activated monocytes. Haemostasis. 1977;6(6):347–50. doi: 10.1159/000214201. [DOI] [PubMed] [Google Scholar]
- 53.van den Eijnden MM, Steenhauer SI, Reitsma PH, Bertina RM. Tissue factor expression during monocyte-macrophage differentiation. Thromb Haemost. 1997;77(6):1129–36. [PubMed] [Google Scholar]
- 54.Baroni M, Pizzirani C, Pinotti M, et al. Stimulation of P2 (P2X7) receptors in human dendritic cells induces the release of tissue factor-bearing microparticles. FASEB J. 2007;21(8):1926–33. doi: 10.1096/fj.06-7238com. [DOI] [PubMed] [Google Scholar]
- 55.Niessen F, Schaffner F, Furlan-Freguia C, et al. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 2008;452(7187):654–8. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 56.Bokarewa MI, Morrissey JH, Tarkowski A. Tissue factor as a proinflammatory agent. Arthritis Res. 2002;4(3):190–5. doi: 10.1186/ar405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldin-Lang P, Tran QV, Fichtner I, et al. Tissue factor expression pattern in human non-small cell lung cancer tissues indicate increased blood thrombogenicity and tumor metastasis. Oncol Rep. 2008;20(1):123–8. [PubMed] [Google Scholar]
- 58.van der Poll T. Tissue factor as an initiator of coagulation and inflammation in the lung. Crit Care. 2008;12(Suppl 6):S3. doi: 10.1186/cc7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iwase H, Ekser B, Hara H, et al. Regulation of human platelet aggregation by genetically modified pig endothelial cells and thrombin inhibition. Xenotransplantation. 2013 doi: 10.1111/xen.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsuda N, Hattori Y. Systemic inflammatory response syndrome (SIRS): molecular pathophysiology and gene therapy. J Pharmacol Sci. 2006;101(3):189–98. doi: 10.1254/jphs.crj06010x. [DOI] [PubMed] [Google Scholar]
- 61.Stearns-Kurosawa DJ, Lupu F, Taylor FB, Jr., et al. Sepsis and pathophysiology of anthrax in a nonhuman primate model. Am J Pathol. 2006;169(2):433–44. doi: 10.2353/ajpath.2006.051330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldstein DR. Inflammation and transplantation tolerance. Semin Immunopathol. 2011;33(2):111–5. doi: 10.1007/s00281-011-0251-2. [DOI] [PubMed] [Google Scholar]
- 63.Gebhard C, Stampfli SF, Gebhard CE, et al. Guggulsterone, an anti-inflammatory phytosterol, inhibits tissue factor and arterial thrombosis. Basic Res Cardiol. 2009;104(3):285–94. doi: 10.1007/s00395-008-0757-5. [DOI] [PubMed] [Google Scholar]
- 64.Lin CC, Ezzelarab M, Hara H, et al. Atorvastatin or transgenic expression of TFPI inhibits coagulation initiated by anti-nonGal IgG binding to porcine aortic endothelial cells. J Thromb Haemost. 2010;8(9):2001–10. doi: 10.1111/j.1538-7836.2010.03950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ezzelarab M, Welchons D, Torres C, et al. Atorvastatin down-regulates the primate cellular response to porcine aortic endothelial cells in vitro. Transplantation. 2008;86(5):733–7. doi: 10.1097/TP.0b013e3181821cad. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.