Abstract
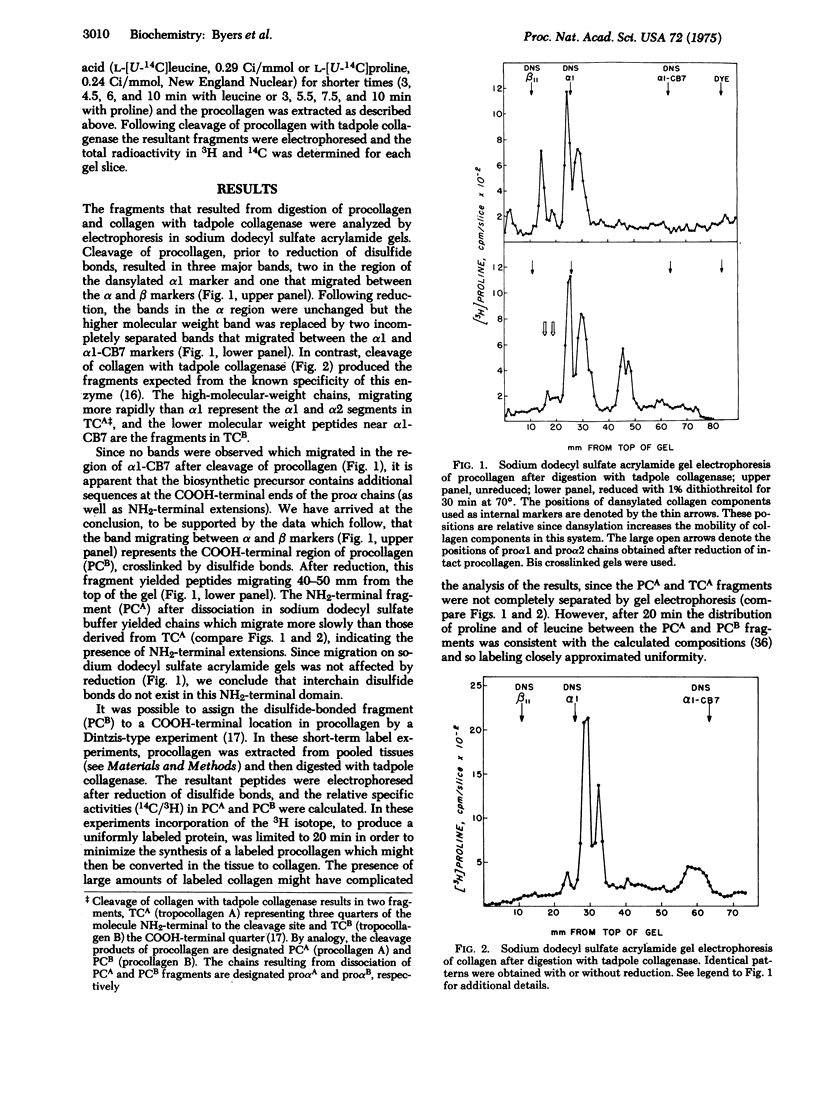
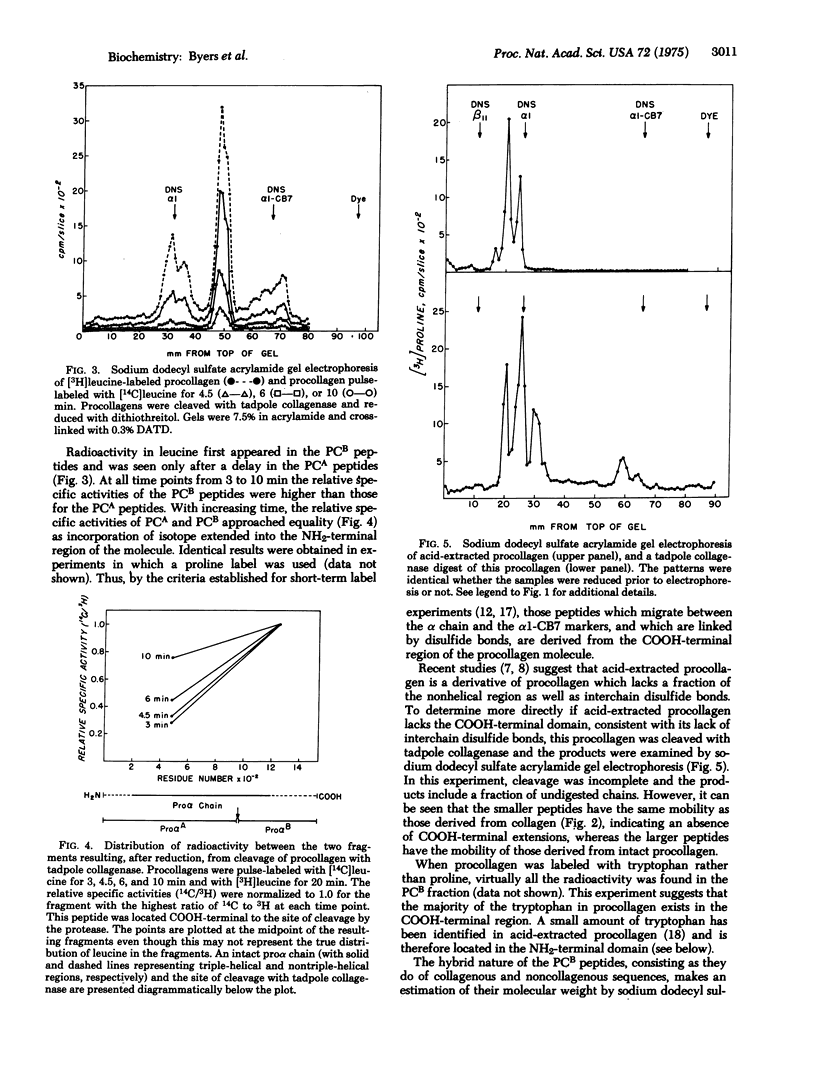
Tadpole collagenase (EC 3.4.24.3) cleaved chick cranial bone procollagen into two triple-stranded fragments, PCA and PCB. Only PCB, with an estimated molecular weight of about 60,000 for each component chain after reduction, was found to contain interchain disulfide bonds. The analogous cleavage of collagen is known to produce a large NH2-terminal fragment with a molecular weight of 70,000 for each chain and a small COOH-terminal fragment containing chains of about 25,000 molecular weight. Since PCB was too small to represent the product NH2-terminal to the site of collagenase cleavage, localization of interchain disulfide bonds to a COOH-terminal domain in procollagen was indicated. This assignment was conformed by Dintzis-type short-term labeling experiments. Procollagen obtained by acid extraction of bone lacked the COOH-terminal disulfide-bonded domain. The findings support a model for procollagen consisting of three proalpha chains each containing nonhelical NH2-terminal extensions of 20,000 molecular weight and COOH-terminal extensions of about 35,000 molecular weight, the latter linked by interchani disulfide bonds.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anker H. S. A solubilizable acrylamide gel for electrophoresis. FEBS Lett. 1970 Apr 16;7(3):293–293. doi: 10.1016/0014-5793(70)80185-5. [DOI] [PubMed] [Google Scholar]
- Bornstein P. The biosynthesis of collagen. Annu Rev Biochem. 1974;43(0):567–603. doi: 10.1146/annurev.bi.43.070174.003031. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Von der Mark K., Wyke A. W., Ehrlich H. P., Monson J. M. Characterization of the pro- 1 chain of procollagen. J Biol Chem. 1972 May 10;247(9):2808–2813. [PubMed] [Google Scholar]
- Byers P. H., McKenney K. H., Lichtenstein J. R., Martin G. R. Preparation of type III procollagen and collagen from rat skin. Biochemistry. 1974 Dec 3;13(25):5243–5248. doi: 10.1021/bi00722a030. [DOI] [PubMed] [Google Scholar]
- Chung E., Keele E. M., Miller E. J. Isolation and characterization of the cyanogen bromide peptides from the alpha 1(3) chain of human collagen. Biochemistry. 1974 Aug 13;13(17):3459–3464. doi: 10.1021/bi00714a006. [DOI] [PubMed] [Google Scholar]
- Dehm P., Olsen B. R., Prockop D. J. Antibodies to chick-tendon procollagen. Affinity purification with the isolated disulfide-linded NH2-terminal extensions and reactivity with a component in embryonic serum. Eur J Biochem. 1974 Jul 1;46(1):107–116. doi: 10.1111/j.1432-1033.1974.tb03602.x. [DOI] [PubMed] [Google Scholar]
- Fessler L. I., Burgeson R. E., Morris N. P., Fessler J. H. Collagen synthesis: a disulfide-linked collagen precursor in chick bone. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2993–2996. doi: 10.1073/pnas.70.10.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furthmayer H., Timpl R., Stark M., Lapière C. M., Kühn K. Chemical properties of the peptide extension in the palpha 1 chain of dermatosparactic skin procollagen. FEBS Lett. 1972 Dec 1;28(2):247–250. doi: 10.1016/0014-5793(72)80723-3. [DOI] [PubMed] [Google Scholar]
- Harper E., Bloch K. J., Gross J. The zymogen of tadpole collagenase. Biochemistry. 1971 Aug 3;10(16):3035–3041. doi: 10.1021/bi00792a008. [DOI] [PubMed] [Google Scholar]
- Harwood R., Grant M. E., Jackson D. S. The sub-cellular location of inter-chain disulfide bond formation during procollagen biosynthesis by embryonic chick tendon cells. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1188–1196. doi: 10.1016/s0006-291x(73)80020-8. [DOI] [PubMed] [Google Scholar]
- Jimenez S. A., Dehm P., Olsen B. R., Prokop D. J. Intracellular collagen and protocollagen from embryonic tendon cells. J Biol Chem. 1973 Jan 25;248(2):720–729. [PubMed] [Google Scholar]
- Kang A. H., Nagai Y., Piez K. A., Gross J. Studies on the structure of collagen utilizing a collagenolytic enzyme from tadpole. Biochemistry. 1966 Feb;5(2):509–515. doi: 10.1021/bi00866a016. [DOI] [PubMed] [Google Scholar]
- Kohn L. D., Isersky C., Zupnik J., Lenaers A., Lee G., Lapiére C. M. Calf tendon procollagen peptidase: its purification and endopeptidase mode of action. Proc Natl Acad Sci U S A. 1974 Jan;71(1):40–44. doi: 10.1073/pnas.71.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaers A., Ansay M., Nusgens B. V., Lapière C. M. Collagen made of extended -chains, procollagen, in genetically-defective dermatosparaxic calves. Eur J Biochem. 1971 Dec 10;23(3):533–543. doi: 10.1111/j.1432-1033.1971.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein J. R., Byers P. H., Smith B. D., Martin G. R. Identification of the collagenous proteins synthesized by cultured cells from human skin. Biochemistry. 1975 Apr 22;14(8):1589–1594. doi: 10.1021/bi00679a007. [DOI] [PubMed] [Google Scholar]
- Lichtenstein J. R., Martin G. R., Kohn L. D., Byers P. H., McKusick V. A. Defect in conversion of procollagen to collagen in a form of Ehlers-Danlos syndrome. Science. 1973 Oct 19;182(4109):298–300. doi: 10.1126/science.182.4109.298. [DOI] [PubMed] [Google Scholar]
- Monson J. M., Borstein P. Identification of a disulfide-linked procollagen as the biosynthetic precursor of chick-bone collagen. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3521–3525. doi: 10.1073/pnas.70.12.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAUGHTON M. A., DINTZIS H. M. Sequential biosynthesis of the peptide chains of hemoglobin. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1822–1830. doi: 10.1073/pnas.48.10.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nist C., Von Der Mark K., Hay E. D., Olsen B. R., Bornstein P., Ross R., Dehm P. Location of procollagen in chick corneal and tendon fibroblasts with ferritin-conjugated antibodies. J Cell Biol. 1975 Apr;65(1):75–87. doi: 10.1083/jcb.65.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E., Tanzer M. L., Church R. L. Procollagen synthesis in cell culture: nascent chain population consistent with polycistronic mRNA. Biochem Biophys Res Commun. 1975 Mar 3;63(1):1–10. doi: 10.1016/s0006-291x(75)80002-7. [DOI] [PubMed] [Google Scholar]
- Rauterberg J. The C-terminal non-helical portion of the collagen molecule. Clin Orthop Relat Res. 1973 Nov-Dec;(97):196–212. doi: 10.1097/00003086-197311000-00027. [DOI] [PubMed] [Google Scholar]
- Schofield J. D., Uitto J., Prockop D. J. Formation of interchain disulfide bonds and helical structure during biosynthesis of procollagen by embryonic tendon cells. Biochemistry. 1974 Apr 23;13(9):1801–1806. doi: 10.1021/bi00706a004. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Taubman M. B., Goldberg B. Isolation of a disulfide-stabilized, three-chain polypeptide fragment unique to the precursor of human collagen. J Biol Chem. 1973 Oct 25;248(20):7033–7038. [PubMed] [Google Scholar]
- Smith B. D., Byers P. H., Martin G. R. Production of procollagen by human fibroblasts in culture. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3260–3262. doi: 10.1073/pnas.69.11.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M., Lenaers A., Lapiere C., Kühn K. Electronoptical studies of procollagen from the skin of dermatosparaxic calves. FEBS Lett. 1971 Nov 1;18(2):225–227. doi: 10.1016/0014-5793(71)80450-7. [DOI] [PubMed] [Google Scholar]
- Stark M., Miller E. J., Kühn K. Comparative electron-microscope studies on the collagens extracted from cartilage, bone, and skin. Eur J Biochem. 1972 May;27(1):192–196. doi: 10.1111/j.1432-1033.1972.tb01825.x. [DOI] [PubMed] [Google Scholar]
- Tanzer M. L., Church R. L., Yaeger J. A., Wampler D. E., Park E. Procollagen: intermediate forms containing several types of peptide chains and non-collagen peptide extensions at NH2 and COOH ends. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3009–3013. doi: 10.1073/pnas.71.8.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veis A., Anesey J., Yuan L., Levy S. J. Evidence for an amino-terminal extension in high-molecular-weight collagens from mature bovine skin. Proc Natl Acad Sci U S A. 1973 May;70(5):1464–1467. doi: 10.1073/pnas.70.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von der Mark K., Bornstein P. Characterization of the pro 1 chain of procollagen. Isolation of a sequence unique to the precursor chain. J Biol Chem. 1973 Apr 10;248(7):2285–2289. [PubMed] [Google Scholar]
- Vuust J., Piez K. A. A kinetic study of collagen biosynthesis. J Biol Chem. 1972 Feb 10;247(3):856–862. [PubMed] [Google Scholar]
- Vuust J., Piez K. A. Biosynthesis of the alpha chains of collagen studied by pulse-labeling in culture. J Biol Chem. 1970 Nov 25;245(22):6201–6207. [PubMed] [Google Scholar]



