Abstract
Objective
Crohn’s disease (CD) is a chronic idiopathic inflammatory intestinal disorder associated with fecal dysbiosis. Fecal Microbial Transplant (FMT) is a potential therapeutic option for individuals with CD based on the hypothesis that changing the fecal dysbiosis could promote less intestinal inflammation.
Design
Nine patients, ages 12–19 years, with mild to moderate symptoms defined by Pediatric Crohn’s disease activity index (PCDAI of 10–29) were enrolled into a prospective open label study of FMT in CD (FDA IND 14942). Patients received FMT by nasogastric tube with follow up evaluations at 2, 6, and 12 weeks. PCDAI, C-reactive protein (CRP), and fecal calprotectin were evaluated at each study visit.
Results
All reported adverse events (AE) were graded as mild except for one individual who reported moderate abdominal pain after FMT. All AE were self limiting. Metagenomic evaluation of stool microbiome indicated evidence of FMT engraftment in seven out of nine patients. The mean PCDAI score improved with patients having a baseline of 19.7 ± 7.2, with improvement at 2 weeks to 6.4 ± 6.6, and at 6 weeks to 8.6 ± 4.9. Based upon PCDAI, 7/9 patients were in remission at 2 weeks, and 5/9 patients who did not receive additional medical therapy were in remission at week 6 and 12 weeks. No or modest improvement were seen in the patients who did not engraft or whose microbiome was most similar to their donor.
Conclusion
This is the first study to demonstrate that FMT for CD may be a possible therapeutic option for Crohn’s disease. Further prospective studies are required to fully assess the safety and efficacy of the FMT in patients with Crohn’s disease.
Keywords: Fecal microbial transplant, FMT, Crohn’s disease, inflammatory bowel disease, Pediatrics, fecal microbiome
Introduction
A variety of human and animal data supports the hypothesis that Crohn’s disease is a result of immune responses to the fecal microbiota in genetically susceptible individuals. Such immune responses may alter the microbiota resulting in dysbiosis, which may further promote inflammatory responses. Such dysbiosis in Crohn’s patients has been better defined in recent years with the advent of non culturable techniques, such as 16s RNA sequencing for the identification of species within the fecal microbiota. The dysbiosis in Crohn’s disease has been characterized by depletion of commensal bacteria including members of Firmicutes and Bacteroidetes, and a decreased abundance of class Clostridia including F. prausnitzii as well as an increase in Proteobacteria, though the actual significance of these species based alteration is only correlative and therefore is functionally unknown [1, 2] Numerous clinical studies have attempted to modulate the fecal microbiome in order to decrease the inflammatory immune response using prebiotic, probiotic, and antimicrobial therapy. The results of these trials, however, have been mixed.
The possibility of modifying the human microbiome to alter dysbiosis via fecal microbial transplant (FMT) was first reported by Eiseman et al. in 1958 in the treatment of fulminant pseudomembranous enterocolitis.[3] Since then, many case reports and case series of fecal transplantation were noted in the literature for treatment for Clostridium difficile (C. difficile), constipation, irritable bowel syndrome, and inflammatory bowel disease, with efficacy only proven for C. difficile toxin induced recurrent colitis. Fecal transplantation has been described in patients as young as 2 years of age, to patients over 90 years of age. Given the efficacy of this therapy in C. difficile infections, the American Gastroenterology Association recently wrote a position paper on the use of fecal transplantation. In this article, preparation, dosage, patient and donor workups are all reviewed.[4] To confirm the safety and potential efficacy of FMT in Crohn’s patients we performed a prospective study of FMT in pediatric Crohn’s disease.
Materials and Methods
This is a single center, open-label study designed to determine tolerability, preliminary safety, and potential efficacy in pediatric patients with Crohn’s disease. Nine patients with Crohn’s disease with mild to moderate disease symptoms as defined by a Pediatric Crohn’s Disease Activity Index (PCDAI)[5] between 10 and 29, aged 12 to 21 years were enrolled into this study. Each participant was followed in the study for approximately 12 weeks.
The protocol was approved by the Institutional Review Board of Seattle Children’s hospital. All patients/participants provided written informed consent or assent. Approval from the FDA (investigational new drug number 14942) was obtained. The study was registered with ClinicalTrials.gov (number: NCT01757964). Study participants were recruited from Seattle Children’s Hospital outpatient gastroenterology clinics.
Patients
All patients had a diagnosis of Crohn’s disease made by a primary gastroenterologist based upon history, physical exam, laboratory/radiological studies and gastrointestinal histology. All patients had mild to moderate symptoms with a PCDAI score between 10 and 29. Parent/guardian and child consent or assent was obtained. Patient medication for inflammatory bowel disease could not have changed for at least one month prior to FMT. Patient exclusion criteria included active or history of intraabdominal abscess, intraabdominal fistula, stricturing Crohn’s disease or other serious systemic disease. None of the patients received TNF inhibitors prior to transplant. Patients were allowed to maintain the use of other IBD medications including immunomodulators during the study.
Initial evaluation
Study participants had laboratory tests including complete blood count (CBC) with differential and platelets, C - reactive protein (CRP), albumin, stool studies for C. difficile, bacterial culture, ova and parasite. The American Association of Blood Banks Donor History questionnaire was used to evaluate study participant donors. Study participant donor laboratory studies included Hepatitis A IgG and IgM, Hepatitis B serum antigen, antibody and core antibody, Hepatitis C IgG, Human Immunodeficiency Virus 1 and 2 IgG, Rapid Plasma Reagin and Ebstein Barr Viral IgG and IgM, Cytomegalovirus IgG and IgM, as well as stool testing for C. difficile, bacterial culture, and examination of stool for ova and parasites. Study participant donors were not allowed to have antibiotics 3 months prior to procedure. Each patient had a single donor for transplant. The donor for each patient was one of their parents.
Stool Transplantation
Study participant recipients received premedication prior to fecal transplant, which included rifaximin 200 mg 3 times daily for 3 days until the evening prior to procedure. Study participant recipients also received Omeprazole (1mg/kg orally) on the day before and morning of the procedure. Transplant recipients also received 1 capful of MiraLAX in 8oz of water 3 times a day for 2 days. A nasogastric (NG) tube was placed for transplant and location confirmed by X-ray. Approximately 30 grams of donor stool was mixed with 100–200 milliliters (ml) of normal saline and blended with a commercial blender (Hamilton Beach Personal Blender, North Carolina, USA) at low speed for 2 – 4 minutes until a homogenous texture was achieved. The stool was then filtered twice using 4×4 gauze. Infusion was slowly administered via NG tube over a 3 minute period. The NG tube was flushed with 15 ml of normal saline over 1 minute. After 15 minutes, the NG tube was removed.
Post Transplantation follow-up
Study participant FMT recipients were called 2 days after transplantation and had clinical follow-up at 2, 6, and 12 weeks. Standardized questionnaires and the Pediatric Crohn’s disease activity index (PCDAI) were completed during each study visit. The PCDAI uses the patient history, laboratory values and the physical exam to create a validated score of that individual’s disease activity. A PCDAI score of < 10 denotes remission, 10–29 mild disease, and >= 30 moderate to severe disease activity. Study participant recipients/patient’s families were provided diary cards to assess possible side effects.
Statistical analysis
Descriptive statistics were prepared for all data including frequencies and percentages for categorical variables (e.g., gender, disease location) and means, standard deviations, quartiles, and ranges for quantitative variables (e.g., CRP, PCDAI). When lab measurements were above or below the threshold for measurement, the threshold was imputed prior to computing descriptive statistics.
Method section for microbiome
DNA Extraction
Total genomic DNA was extracted from stool using the PowerSoil DNA Isolation Kit (MoBio, Carlsbad, CA). The protocol was customized to include two incubation steps (65°C and 95°C for 10 min each) after the addition of lysis buffer (Solution C1). Additionally, the provided garnet beads for mechanical disruption were substituted for 0.5 g of 0.1 mm diameter Zirconia/Silica beads (BioSpec Products, Bartlesville, OK). Final DNA yield was quantified using the Qubit 2.0 Fluorometer (Life Technologies, Carlsbad, CA) prior to NGS library construction.
Metagenomic Sequencing
Sequencing was performed on either the Illumina HiSeq 2000 or MiSeq platform. Sequencing libraries were constructed from genomic DNA using Illumina’s Nextera technology (Illumina, Inc., San Diego, CA). Briefly, DNA preparations were simultaneously fragmented and tagged with adapter oligomers. A limited-cycle PCR reaction amplified all tagged fragments and added: 1) index sequences (the dual indexing strategy uses two 8-base indices) to allow demultiplexing of sequence reads for pooled samples, and 2) sequencing primer sequences. Following PCR enrichment, libraries were denatured and hybridized via DNA/DNA binding of adaptors to existing features on a glass flow cell compatible with the Illumina sequencers. Sequencing was performed using well-established, ultra-high throughput methods. The HiSeq2000 produced approximately 200 million pairs of 93 bp reads per lane, and we generated 25–30 million raw read pairs per sample using 7-8-plex pools. The single lane of the MiSeq generated 15–20 million raw pairs of 150 bp reads per sample.
Bioinformatics Analysis
Human DNA sequence was identified and removed using BMTagger19 (Rotmistrovsky, K. and R. Agarwala, BMTagger: Best Match Tagger for removing human reads from metagenomics datasets. 2011: unpublished.) with the Hg-19 Homo sapiens reference genome. Duplicate reads were marked and removed using EstimateLibraryComplexity, part of the Picard tool package (http://picard.sourceforge.net/index.shtml). Sequence reads with ambiguous bases were trimmed from each end. Reads with Phred quality scores less than 6 over the first 80 (HiSeq) or 120 (MiSeq) bases of each read and reads shorter than 80 (HiSeq) or 120 (MiSeq) bases after trimming were removed. Similarity scores were calculated using Compareads using two kmers of length 30 nucleotides.[6] Sequences for all samples were limited to between 20 and 30 million reads to maintain comparable sample sizes. A minimum of 20 million reads was required obtain sufficient read depth coverage of the samples (see Brittnacher et al., “ An alignment-free method of mammalian microbiome comparative analysis,” manuscript submitted). Relative species abundance was calculated from the sequence reads using MetaPhlAn.[7]
Results
A total of fourteen families were screened for the study. Five families were deemed unsuitable for transplant because of exclusion criteria including no suitable donor. Nine Crohn’s patients received fecal transplantation. The stool donor for each patient was their mother except for patient 10 and 13 where the fathers were the stool donors. Age of participants was 16.2 ± 2.9 years (range 12 – 19 years). Five were male. Average disease duration prior to FMT was 3.9 ± 1.8 years (range 0 – 7 years). Macroscopic disease at time of diagnosis was in the stomach/duodenum in 6 patients, colon in 7 patients and terminal ileum in 6 patients. At the time of transplant, 4 individuals were on methotrexate, 1 individual was on azathioprine, 1 individual was on mercaptopurine, 3 individuals on mesalamine therapy, and one newly diagnosed individual was on no medication at the time of FMT. (Table 1)
Table 1.
Patient Demographics for FMT recipients
| Patient | Gender | Age (years) | Disease duration (years) | Modified Paris classification | Disease location | Concomitant IBD medications |
|---|---|---|---|---|---|---|
| 1 | male | 19 | 5 | A2, L3, B1, G0 | colon, terminal ileum | methotrexate, folic acid |
| 2 | male | 16 | 3 | A1b, L1/4a, B1, G0 | duodenum, terminal ileum | mesalamine |
| 6 | female | 18 | 5 | A2, L1/4a,B1, G0 | stomach, duodenum, terminal ileum | 6-mercaptopurine |
| 7 | female | 12 | 4 | A1b, L2/4a, B1, G0 | duodenum, colon | methotrexate, folic acid |
| 8 | male | 17 | 7 | A2, L3/4ab,B1,G0 | esophagus, dudenum, colon, terminal ileum | mesalamine |
| 10 | female | 19 | 2 | A2, L3, B1, G0 | colon, terminal ileum | methotrexate, folic acid, mesalamine |
| 13 | male | 13 | 4 | A1b,L3/4b,B1,G0 | duodenum, colon, terminal ileum | azathioprine |
| 15 | male | 19 | 4 | A2,L2/4a,B1,G0 | duodenum, colon | methotrexate, folic acid |
| 18 | female | 13 | <1 | A1b,L2,B1,G0 | colon | none |
Inflammatory Bowel Disease (IBD)
All reported adverse events were graded as mild except for one individual who reported moderate abdominal pain after FMT. After the FMT, 5 patients complained of abdominal pain likely related to FMT (4/5 mild abdominal pain), and 5 had mild bloating likely related to FMT, 4 had diarrhea likely related to the pretreatment for the FMT or the FMT. Abdominal pain, bloating and diarrhea returned to baseline or improved within 48 hours of FMT. One individual had mild post FMT flatulence lasting one day. One individual had a mild stuffy nose after FMT, which was not likely related to the procedure but more likely reflected allergic responses. Three individual had side effects likely related to the nasogastric tube, 1 with rhinorrhea lasting 3 days after FMT, and two individuals with sore throats lasting 1 day post FMT.
Two weeks after FMT, 7 of the 9 patients were in clinical remission based upon PCDAI scoring. At 6 and 12 weeks, 5 of 9 patients who did not receive additional therapy were still in remission. Two individuals received additional standard medical therapies prior to the end of the study. Patient 15 began metronidazole prior to the 6 week follow up and then Infliximab after the 6 week follow up. Patient 13 began prednisone and methotrexate after 6 week follow up (Table 2). Two other individuals began standard medical therapy after 12 week follow up because of a flare in symptoms (Table 2). Mean PCDAI at baseline was 19.7± 7.2, at 2 weeks post FMT 6.4 ± 6.6, and at 6 weeks post FMT 8.6 ± 4.9. The 6 week and 12 week mean PCDAI of individuals not receiving additional medical intervention were 8.8 ± 5.2 and 11.1 ±7.5, respectively. All but one patient had improvement/normalization in their CRP at the week 2 follow up. The mean CRP levels decreased from 2.4 ± 1.2 mg/dL at baseline to 1.5 ± 0.6 mg/dL at the 2 weeks post FMT visit. At 6 weeks and 12 weeks post FMT in those individuals who did not start on additional medical therapy the mean CRP still remained below baseline level at 2.0 ± 1.2 mg/dL and 2.3 ± 2.3, respectively. No significant changes were noted in albumin levels, or hematocrit with FMT. (Table 2)
Table 2.
Laboratory, clinical and microbiome results for FMT recipients
| Patient | Sample | PCDAI | C-reactive protein (mg/dL) | Calprotectin (μg/g) | Clinical remission at 2 weeks | Engraftment score at 2 weeks (%) | Engraftment type | Pre-FMT similarity to donor (%) |
|---|---|---|---|---|---|---|---|---|
| Baseline | 27.5 | 3.1 | 1380 | |||||
| 1 | 2 week | 7.5 | 1.1 | 1287 | Yes | 10 | Gradual | 13 |
| 6 week | 12.5 | 1.3 | 729 | |||||
| 12 week | 10 | <0.8 | 827 | |||||
|
| ||||||||
| Baseline | 10 | 4.5 | 544 | |||||
| 2 | 2 week | 2.5 | 1.7 | 353 | Yes | −15 | Failure | 50 |
| 6 week | 2.5 | 3.4 | 718 | |||||
| 12 week | 10 | 7.4 | 791 | |||||
|
| ||||||||
| Baseline | 15 | <0.8 | <16 | |||||
| 6 | 2 week | 10 | <0.8 | <16 | No | 41 | Immediate | 37 |
| 6 week | 10 | <0.8 | <16 | |||||
| 12 week | 5 | <0.8 | <16 | |||||
|
| ||||||||
| Baseline | 22.5 | 1.4 | 23 | |||||
| 7 | 2 week | 5 | <0.8 | <16 | Yes | 46 | Immediate | 42 |
| 6 week | 5 | <0.8 | <16 | |||||
| 12 week | 2.5 | 1.5 | <16 | |||||
|
| ||||||||
| Baseline | 12.5 | 1.9 | 1456 | |||||
| 8 | 2 week | 2.5 | 1.6 | 899 | Yes | 11 | Gradual | 55 |
| 6 week | 2.5 | 2.3 | 672 | |||||
| 12 week | 7.5 | 1.1 | 1186 | |||||
|
| ||||||||
| Baseline | 27.5 | 4 | 1074 | |||||
| 10 | 2 week | 2.5 | 2.7 | 799 | Yes | 12 | Gradual | 32 |
| 6 week | 7.5 | 2.4 | 1349 | |||||
| 12 week | 20 | 2.3 | 1320 | |||||
|
| ||||||||
| Baseline | 22.5 | 2 | 596 | |||||
| 13 | 2 week | 2.5 | 1.5 | 532 | Yes | 14 | Gradual | 46 |
| 6 week | 15 | 3.9 | 1318 | |||||
| 12 week* | 2.5* | <0.8* | 434* | |||||
|
| ||||||||
| Baseline | 12.5 | 1.5 | >2500 | |||||
| 15 | 2 week | 2.5 | 1.7 | 1231 | Yes | 22 | Immediate | 31 |
| 6 week* | 7.5* | 1* | >2500* | |||||
| 12 week* | 0* | <0.8* | 22* | |||||
|
| ||||||||
| Baseline | 27.5 | 2.6 | 839 | |||||
| 18 | 2 week | 22.5 | 1.5 | 906 | No | 0 | Failure | 69 |
| 6 week | 15 | 1.1 | 1224 | |||||
| 12 week | 22.5 | 2.1 | 1172 | |||||
received additional standard treatment prior to visit
Stool calprotectin decreased or remained unchanged for all patients except for one whose level increased slightly at the 2 week follow up. The mean baseline calprotectin for all patients enrolled was 936 ± 782 mg/L with a mean level of 671 ± 474 mg/L at two week follow up. Although initial improvement was seen for most patients in calprotectin, the levels rose for most patients by the 12 weeks follow up (Table 2).
Microbiome
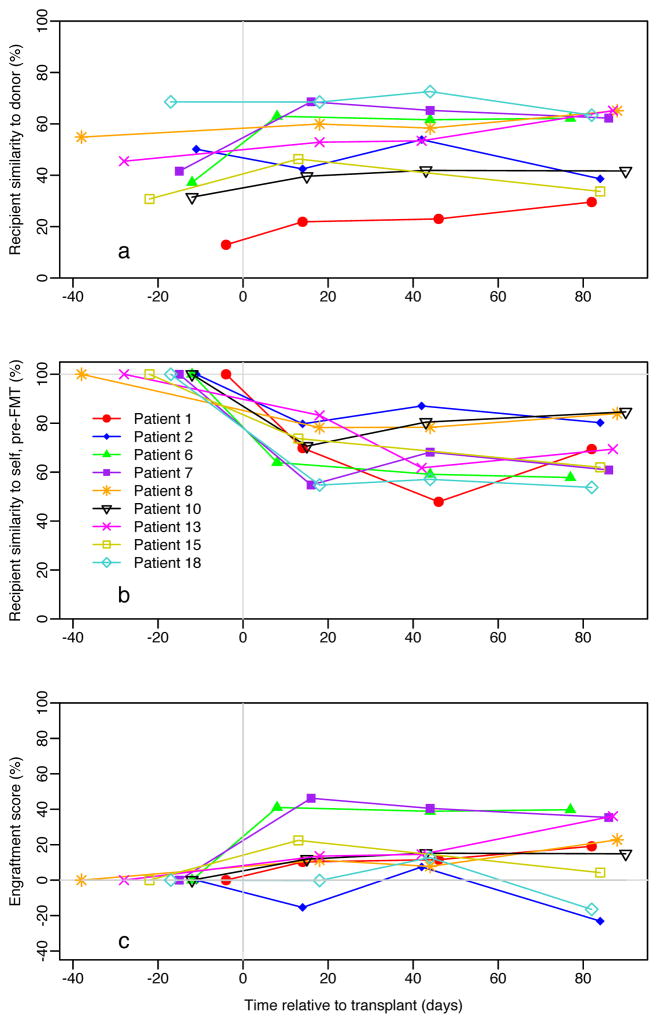
Fecal microbiome similarity to donor, pre-FMT had a mean of 41.7±16.1% (SD) and ranged from 13 to 69% (Figure 1a). Similarity to self, pre-FMT (Figure 1b) was also calculated for all samples. Similarity of donor stool was measured by the relative change in similarity to donor (Figure 1c; method paper currently submitted). The similarity score ranged from −15 to 46% (mean ± standard deviation (SD), 15.7 ± 18.9,) with individuals following one of three paths: no similarity (n=2), gradual similarity (n=4) and immediate similarity (n=3). Patients 2 and 18 did not appear to engraft while others (patients 1, 8, and 10) had a gradual increase in donor similarity over the 12 week period (Table 2). The third group (patients 6 and 7) had very quick similarity by the second week analysis. Similarity of donor stool was evident for the entire 12 week period for those who had initially engrafted. It is unclear at this stage if degree of similarity correlates with different clinical responses in the fecal microbiome to FMT, though of the two patients with no similarity one of these patients had no clinical response to FMT while the other had only a modest clinical response (Table 2).
Figure 1.
Fecal Microbiota Analysis of Fecal Microbial Transplant Recipient as a Function of Similarity to Donor, and Self.
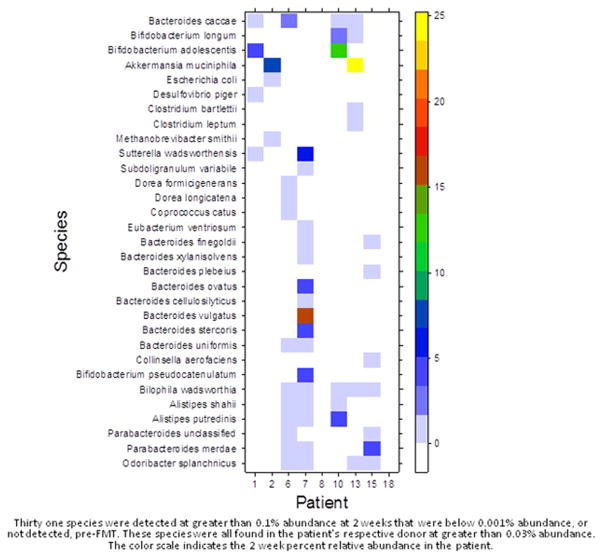
Using MetaPhlAN analysis of the metagenomics data, a total complement of 116 species were identified across all samples (Supplementary Table 1) of which 107 (92%) were found in the donors. About one third (11/30) of the thirty most abundant species in the donors were found in all nine donors (Supplementary Figure 1). In contrast, only three of the thirty most abundant species in the patients, pre-FMT were found in all nine patients (Supplementary Figure 2). Thus, greater homogeneity was observed for the donors in relation to the patients for the most abundant species. Only one species, Ruminococcus torques, was found in all donor and patient samples, pre-FMT. To determine which species were potentially transplanted during FMT we identified 31 species that were not detected in the patient baseline samples but were found following FMT at two weeks (Figure 2). These species were all detected in the patient’s respective donor. The species that were found in more than two of the nine patients were Bilophila wadsworthia (5 of 9), Odoribacter splanchnicus and Bacteroides caccae (4 of 9) and Alistipes shahii, A. putredinis and Parabacteroides merdae (3 of 9). Although there are many factors independent of transplant that could explain the presence of these newly detected species, we found that the percentage of these 31 species detected post-FMT for each patient was correlated with the similarity score at 2 weeks (Spearman r = 0.9). For example, the percentage of previously undetected species in patients 6, 7 and 15 at 2 weeks was 39, 45 and 26% and their similarity scores at 2 weeks were 41, 46 and 22%, respectively. In contrast, no new species were detected for patient 18 whose similarity score was 0%. High relative abundance of a species was not a determinant of which species were possibly transplanted. In patient 6, Ruminococcus bromii contributed toward a large fraction of the increase in similarity to donor at two weeks post-FMT (Supplementary Figure 3 [page 3]). However, R. bromii was not detected post-FMT in patients 1, 2, 10 and 18 even for donor abundances between 10 to 30% (Supplementary Figure 4 [page 8]).
Figure 2.
Microbial Species detected in Transplant Recipients at 2 weeks post Fecal Microbial Transplant that were below detection pre-FMT.
On evaluation of specific individual’s microbial similarity to donor prior to transplant, two patients stand apart from the overall group. The pre-FMT microbiome of Patient 1 was the least similar to donor parental microbiome at 13%. His clinical course was also one of the best with a decrease in PCDAI from 27.5 to 7.5 and a decrease in CRP from 3.1 mg/dL to 1.1 mg/dL. In contrast, patient 18 had the greatest similarity to donor, pre-FMT at 69%. This patient’s clinical course did not appear to be significantly altered: clinical remission with FMT was not achieved and additional medical therapy was required by the end of the study. The patient’s PCDAI went from 27.5 to 22.5 with CRP decreasing from 2.6 mg/dL to 1.5 mg/dL. Interestingly, two patients had significant clinical deterioration over the course of the study. The longitudinal analysis of fecal metagenomes during this period allowed us to observe a dramatic increase in the relative abundance of Escheria coli during a clinical disease flare. This data is highly correlative with a bloom of E. coli in response to inflammation or an expansion of E. coli could contribute to disease outcome. Therefore an examination of the relative abundance of E. coli in stool samples before and after FMT would suggest that a relative increase in the amount of E. coli in stool samples is associated with increased inflammation. For patient 18 who was a non-responder to FMT there was an increase from baseline after FMT. For those who responded initially but then had an increase in disease activity, E. coli abundance appears associated with increased calprotectin and thus worsening inflammation.
Discussion
The published experience of fecal microbial transplantation for active Crohn’s disease is limited. To date only two retrospective case reports exist in which fecal transplant has been used as a potential treatment for Crohn’s disease. Borody and Grehan each described one individual with Crohn’s Disease who had clinical benefit from FMT. [8, 9] The results of this prospective study show that FMT in this small cohort of pediatric patients with Crohn’s disease was safe, and well tolerated. Both clinical and laboratory improvements were seen in the majority of patients. This study provides further evidence that the fecal microbiota likely plays an important role in the pathogenesis of Crohn’s disease and indicates that FMT merits further study as a potentially therapy for Crohn’s disease.
Current therapies for Crohn’s disease focus on suppressing the immune system. In contrast, FMT focuses on a possible trigger of the immune dysregulation, the fecal microbiota. There have been many clinical observations implicating the fecal microbiota as a contributing agent in Crohn’s disease pathology. This includes the clinical efficacy of antibiotic therapy, clinical improvement with diversion of the fecal stream on distal disease activity, as well as serum reactivity towards microbial antigens including anti saccharomyces cerevisiae antibody (ASCA) and Outer Membrane Protein C of E. coli (Omp-C).[10–12] An additional factor known to impact the development of IBD and the fecal microbiota is antibiotic use. The development of pediatric IBD has been closely associated with antibiotic exposure early in life. Kronman et al. reported an 84% relative risk increase in development of IBD with antibiotic use. Exposure to antibiotics throughout childhood was associated with developing IBD but decreased with increasing age of exposure.[13] This suggests that immune dysregulation may come from a disruption of immune tolerance during development as a result of alteration of the microbiota by antibiotic use. [14, 15]
The goal of FMT in Crohn’s disease is to alter the fecal microbiome by decreasing potential “dysbiotic” bacterium that may be more pro-inflammatory. With that in mind, rifaximin, a non absorbed antibacterial agent was used prior to fecal microbial transplant to decrease the endogenous “dysbiotic” bacterial load in the transplant recipient with the goal of allowing a biologic niche for the transplanted stool to survive in. Although there is uncertainty regarding the specific clinical implications of dysbiosis, and whether the changes seen are a cause or effect of the disease itself, studies have noted differences within the fecal microbiota of patients with Crohn’s disease from healthy controls. This divergence from healthy individual’s fecal microbiota is characterized by a decrease in commensal bacteria including members of Firmicutes and Bacteroides as well as a relative increase in pro inflammatory bacteria such as Enterobacteriaceae. [1, 2]. In addition, a decrease in butyrate producing bacterium which are important in intestinal health have been seen in patients with Crohn’s.[16] Fluorescent in situ hybridization analysis has shown bacteria penetrating the mucus layer in 25% of colonic and 55% of ileal mucosal biopsies of patients with Crohn’s disease as compared to none in controls.[17] Although as a community, we often describe the microbial changes seen in Crohn’s disease as a dysbiosis, it is important to acknowledge our limited understanding of the fecal microbiome and that some of the changes seen may not represent a true functional dysbiosis. Our microbial analysis confirms the changes seen in other studies. One unique aspect of this study is our longitudinal analysis allowed us to observe a bloom of E. coli as associated with an increase in clinical symptoms and inflammatory markers. This indicates how rapidly a change in clinical status by an increase in intestinal inflammation can be associated with a bloom of bacteria that is both a commensal but well known as a pathogen from horizontally acquired virulence factors and in the setting of compromise of the intestinal barrier function. Though E. coli may not be the ultimate cause of Crohn’s disease it is interesting to speculate that its expansion and invasion could contribute to symptoms and disease.
There are many intriguing issues related to FMT which arise from this study. There was a significant difference in clinical outcome between the patient with the least and most microbial similarity between recipient and donor. This could indicate that the more divergent a Crohn’s patient is from his donor the more the potential benefit of transplantation. The diagnosis of dysbiosis by species analysis is speculative given the huge diversity in human microbiomes and the influence of diet and genetics on the microbiome content. Therefore, the non-species based analysis we performed coupled with the greater similarity in heritable factors and diet between parents and child may suggest who has a greater likelihood of benefit from FMT. Another possible predictor of disease activity and duration of efficacy appears to be the appearance or resurgence of E. coli. We notice a trend of increasing calprotectins with an increase in E. coli abundance. Although this finding may be a helpful predictor of efficacy of therapy there is no clear casual affect. However, in patients with significant dysbiosis with E. coli therapy targeted at its suppression followed by FMT could be another potential therapeutic trial in the future.
Although there was clinical and laboratory improvement in the majority of patients, there are number of significant limitations to this study. As an open labeled study, recruited patients and parents had a strong personal belief that FMT would improve symptoms. It cannot be excluded that participant bias could account for some of the effect seen in the PCDAI. In addition, the relative effect of pretreatment of patients with rifaximin and MiraLAX prior to FMT could have accounted for some of the benefits seen as well as the changes observed in the microbiome. Finally the small sample size for this study limits the precision of estimated effects of the FMT within our Crohn’s patients. Despite these limitations, this study suggests a further link between the fecal microbiota and Crohn’s disease and suggests that further study of FMT as a potential therapeutic option using controlled clinical trials with analysis of transplant similarity is warranted.
Supplementary Material
Supplementary Figure 1: Donor abundant species
Supplementary Figure 2: Patient abundant species
Supplementary Figure 3: Species patient stackplot
Supplementary Figure 4: Species sample barplot
Acknowledgments
This work was supported by grants from the Cuyamaca Foundation and Seattle Children’s Center for Clinical and Translational Research Academic Enrichment Fund. This publication was also supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000423.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
None of the authors have a conflict of interest.
References
- 1.Frank DN, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujimoto T, et al. Decreased abundance of Faecalibacterium prausnitzii in the gut microbiota of Crohn’s disease. J Gastroenterol Hepatol. 2013;28(4):613–9. doi: 10.1111/jgh.12073. [DOI] [PubMed] [Google Scholar]
- 3.Eiseman B, et al. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44(5):854–9. [PubMed] [Google Scholar]
- 4.Bakken JS, et al. Treating Clostridium difficile Infection With Fecal Microbiota Transplantation. Clin Gastroenterol Hepatol. 2011;9(12):1044–9. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyams J, et al. Evaluation of the pediatric crohn disease activity index: a prospective multicenter experience. J Pediatr Gastroenterol Nutr. 2005;41(4):416–21. doi: 10.1097/01.mpg.0000183350.46795.42. [DOI] [PubMed] [Google Scholar]
- 6.Maillet N, et al. Compareads: comparing huge metagenomic experiments. BMC Bioinformatics. 2012;13(Suppl 19):S10. doi: 10.1186/1471-2105-13-S19-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segata N, et al. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat Methods. 2012;9(8):811–4. doi: 10.1038/nmeth.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borody TJ, et al. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust. 1989;150(10):604. doi: 10.5694/j.1326-5377.1989.tb136704.x. [DOI] [PubMed] [Google Scholar]
- 9.Grehan MJ, et al. Durable alteration of the colonic microbiota by the administration of donor fecal flora. J Clin Gastroenterol. 44(8):551–61. doi: 10.1097/MCG.0b013e3181e5d06b. [DOI] [PubMed] [Google Scholar]
- 10.Zholudev A, et al. Serologic testing with ANCA, ASCA, and anti-OmpC in children and young adults with Crohn’s disease and ulcerative colitis: diagnostic value and correlation with disease phenotype. Am J Gastroenterol. 2004;99(11):2235–41. doi: 10.1111/j.1572-0241.2004.40369.x. [DOI] [PubMed] [Google Scholar]
- 11.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126(6):1620–33. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Winslet MC, et al. Fecal diversion in the management of Crohn’s disease of the colon. Dis Colon Rectum. 1993;36(8):757–62. doi: 10.1007/BF02048367. [DOI] [PubMed] [Google Scholar]
- 13.Kronman MP, et al. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics. 2012;130(4):e794–803. doi: 10.1542/peds.2011-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw SY, Blanchard JF, Bernstein CN. Association between the use of antibiotics and new diagnoses of Crohn’s disease and ulcerative colitis. Am J Gastroenterol. 2011;106(12):2133–42. doi: 10.1038/ajg.2011.304. [DOI] [PubMed] [Google Scholar]
- 15.Virta L, et al. Association of repeated exposure to antibiotics with the development of pediatric Crohn’s disease--a nationwide, register-based finnish case-control study. Am J Epidemiol. 2012;175(8):775–84. doi: 10.1093/aje/kwr400. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, et al. Increased proportions of bifidobacterium and the lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J Clin Microbiol. 2014;52(2):398–406. doi: 10.1128/JCM.01500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleessen B, et al. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand J Gastroenterol. 2002;37(9):1034–41. doi: 10.1080/003655202320378220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Donor abundant species
Supplementary Figure 2: Patient abundant species
Supplementary Figure 3: Species patient stackplot
Supplementary Figure 4: Species sample barplot