Abstract
We showed previously that garden cress constituent benzyl isothiocyanate (BITC) inhibits self-renewal of breast cancer stem cells (bCSC) in vitro and in vivo. The present study offers novel insights into the mechanism by which BITC inhibits bCSC. Flow cytometry and mammosphere assay was performed to quantify bCSC fraction. Protein expression was determined by western blotting. Apoptosis was assessed by flow cytometry using Annexin V-propidium iodide method. Cell migration was determined by Boyden chamber assay. BITC treatment resulted in a marked decrease in protein level of polycomb group protein B-lymphoma Moloney murine leukemia virus insertion region-1 (Bmi-1) in cultured human breast cancer cells (MCF-7, SUM159, MDAMB-231 and MDA-MB-361) and MDA-MB-231 xenografts in vivo. Overexpression (MCF-7) or knockdown (SUM159 and MDA-MB-231) of Bmi-1 protein had no meaningful impact on the BITC’s ability to inhibit cell viability and cell migration and/or induce apoptosis. On the other hand, inhibition of bCSC markers (aldehyde dehydrogenase 1 activity and mammosphere frequency) resulting from BITC exposure was significantly altered by Bmi-1 overexpression and knockdown. BITC was previously shown to cause activation of Notch1, Notch2 and Notch4 in association with induction of γ-secretase complex component Nicastrin, which are also implicated in maintenance of cancer stemness. BITC-mediated inhibition of bCSC was augmented by knockdown of Notch4 and Nicastrin, but not by RNA interference of Notch1 or Notch2. The present study highlights important roles for Bmi-1 and Notch4 in BITC-mediated suppression of bCSC.
Keywords: benzyl isothiocyanate, Bmi-1, Notch4, bCSC
Introduction
Search for non-toxic interventions to delay onset and/or progression of breast cancer continues mainly because most of the risk factors associated with this neoplasm are not easily modifiable and the currently available clinical options [e.g., selective estrogen receptor (ER) modulators] target only a subset of the disease [1, 2]. Despite a comprehensive understanding of the genomic landscape and the biology of the breast cancer [3, 4] this malignancy still accounts for > 40,000 deaths each year in the United States alone [5]. An emerging paradigm suggests that elimination of tumor initiating cells (also known as breast cancer stem cells; bCSC) as well as the transformed cells constituting bulk of the tumor mass may be necessary for successful prevention and treatment of breast cancer [6, 7]. The bCSC are implicated in initiation, maintenance, and metastatic spread of breast cancers [6, 7].
Phytochemicals present in our daily diet as well as in certain medicinal plants are attractive for cancer prevention [8, 9]. Benzyl isothiocyanate (BITC), an electrophilic compound in edible cruciferous vegetables, is one such phytochemical with compelling preclinical evidence for its efficacy against breast cancer [10]. Higher intake of broccoli, a member of the cruciferous vegetable family, is suggested to decrease the risk of breast cancer in premenopausal women [11]. BITC administration was associated with inhibition of chemically-induced breast cancer in experimental animals [12]. BITC has the ability to retard in vivo growth of mammary tumor xenografts in mice [13, 14]. For example, the primary tumor growth and pulmonary metastasis of 4T1 mammary carcinoma cells implanted into the mammary fat pads of female syngeneic BALB/c mice was significantly retarded by oral administration of 5 and 10 mg BITC/kg body weight/day for 32 days [14]. Moreover, the inhibitory effect was relatively more pronounced on lung metastasis than on primary tumors [14]. Work from our own laboratory has established mammary cancer chemopreventive efficacy of BITC (1 and 3 mmol BITC/kg diet for 25 weeks) in mouse mammary tumor virus-neu transgenic mice [15]. More recent work from our laboratory has demonstrated in vitro and in vivo suppression of bCSC fraction upon treatment with BITC [16]. However, the molecular mechanism underlying BITC-mediated inhibition of bCSC is still not fully understood.
The present study was designed to determine the role of B-lymphoma Moloney murine leukemia virus insertion region-1 (Bmi-1) and the Notch receptors, which have emerged as regulators of bCSC self-renewal and maintenance [17-19], in bCSC inhibition by BITC. For instance, the Notch4 activity was shown to be eight-fold higher in bCSC enriched populations compared with differentiated breast cancer cells [18]. A role for Notch1 in expansion of bCSC was also suggested very recently [20]. The effect of BITC on Bmi-1 expression is not known, but this compound was previously shown to cause activation of Notch1, Notch2, and Notch4 in a panel of human breast cancer cells [21].
Materials and methods
Ethics statement
Fresh-frozen MDA-MB-231 tumor xenograft specimens from control and BITC-treated mice from our previous study [13] were used to determine the in vivo effect of BITC administration on expression of Bmi-1 protein. Use of mice and their care was in accordance with the University of Pittsburgh Institutional Animal Care and Use Committee guidelines.
Reagents and cell lines
BITC (purity >98%) was purchased from LKT laboratories (St. Paul, MN) and dissolved in dimethyl sulfoxide (DMSO). Regents for cell culture were from Invitrogen-Life Technologies (Grand Island, NY). Antibodies against Bmi-1, cleaved Notch1, and Nicastrin were from Cell Signaling Technology (Danvers, MA); anti-β-actin and anti-Notch4 antibodies were from Sigma-Aldrich (St. Louis, MO); and anti-cleaved Notch 2 antibody was from EMD Millipore (Billerica, MA). Small interfering RNA (siRNA) targeted against Bmi-1, Notch1, Notch2, Notch4 and Nicastrin, were acquired from Santa Cruz Biotechnology (Dallas, TX), whereas a nonspecific control siRNA was purchased from Qiagen (Germantown, MD). Annexin V-FITC apoptosis detection kit was purchased from BD Biosciences (San Jose, CA). The Bmi-1 targeted small hairpin RNA (shRNA) and control shRNA were from Santa Cruz Biotechnology. The MCF-7, MDA-MB-231, and MDA-MB-361 cells were purchased from the American Type Culture collection (Manassas, VA). SUM159 cell line was purchased from Asterand (Detroit, MI). MCF-7 cells were stably transfected with empty pcDNA3.1 vector or the same vector encoding for Bmi-1 using FuGENE6. The pcDNA3.1-Bmi-1 plasmid was a generous gift form Dr. M. H. Yang (National Yang-Ming University, Taipei, Taiwan). Cells stably overexpressing Bmi-1 were generated by 8-week culture in medium containing 1 mg/mL of G418. SUM159 cells were stably transfected with 2 μg of control shRNA or Bmi-1-targeted shRNA using transfection medium and reagents from Santa Cruz Biotechnology. SUM159 clone with stable knockdown of Bmi-1 was selected with 1.5 μg/mL of Puromycin over a 4-week period.
Western blotting
Preparation of cell lysates and xenograft supernatants and the details of western blotting have been described by us previously [22, 23].
Cell viability assay
The effect of BITC on cell viability was ascertained after 72 h treatment by MTS method using reagents from Promega (Madison, WI) according to the supplier’s instructions or trypan blue dye exclusion assay as described previously [22].
Detection of apoptosis
Apoptotic fraction was quantified by flow cytometry after staining with Annexin V/propidium iodide as described by us previously [24].
Cell migration assay
Cell migration was determined as described by us previously [21] using Transwell Boyden chambers from Corning (New York, NY) containing 8 μm polycarbonate filter.
Aldehyde dehydrogenase 1 (ALDH1) activity determination
The ALDH1 activity was determined using ALDEFLUOR™ assay kit from STEMCELL Technologies (Vancouver, BC). Supplier’s protocol was followed for ALDH1 activity assay.
Mammosphere formation assay
Mammosphere formation assay was performed as described by us previously [16] with the exception that the culture medium was supplemented with 0.9% methylcellulose to reduce cell aggregation. Briefly, one thousand cells were plated in ultralow attachment plates from Corning in medium containing penicillin/streptomycin, B27, insulin, hydrocortisone, epidermal growth factor, basic fibroblast growth factor, 2-mercaptoethanol, and methylcellulose. Desired concentrations of BITC or DMSO (control) were then added to the plates. After a 5-day (MCF-7) or 7-day (SUM159) incubation, the mammospheres were scored under an inverted microscope.
Transfection with siRNA
Cells were transfected at 50-60% confluence with control (nonspecific siRNA) or desired siRNA (100-200 nM) for 24 h. The cells were then treated with DMSO or BITC and used for various assays.
Statistical analysis
For each experiment, statistical comparisons were performed to answer two specific questions: (a) whether overexpression or knockdown of a desired protein itself affected cellular phenotype (e.g., cell viability, apoptosis, migration or bCSC fraction), and (b) if BITC-mediated inhibition of a given phenotype was affected by overexpression or knockdown of the desired protein. Statistically significant differences addressing only these questions are shown in the figures.
Results
BITC treatment suppressed Bmi-1 protein level in human breast cancer cells and MDAMB-231 xenografts in vivo
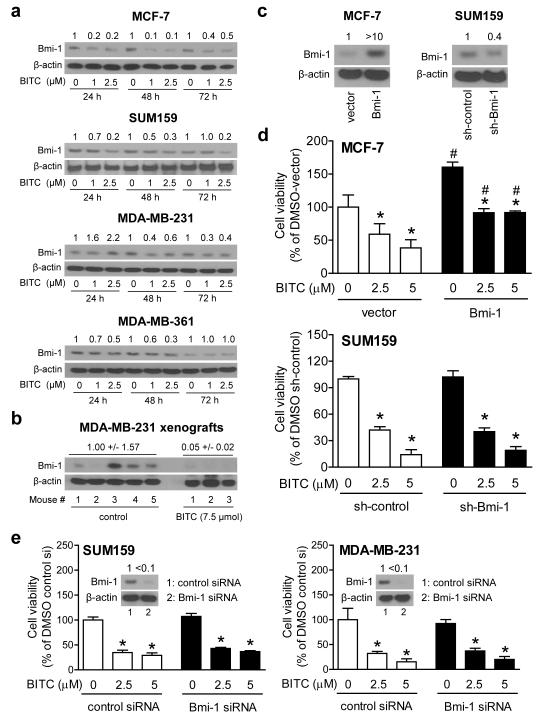
We used a panel of human breast cancer cells (MCF-7, MDA-MB-231, MDA-MB-361,and SUM159) representing major subtypes and pharmacologically relevant BITC concentrations [25-26] to study its effect treatment on Bmi-1 protein level. The western blots shown in Figure 1a revealed downregulation of Bmi-1 protein after treatment with BITC in each cell line, although cell line-specific differences were also discernible. For example, BITC-mediated suppression of Bmi-1 protein level was clearly evident at all three time points and at both drug concentrations in MCF-7 cells (Fig. 1a). In contrast, downregulation of Bmi-1 protein expression post-BITC treatment was observed only at 48 h (40-60% decrease) and 72 h time points (60-70 % decrease) in MDA-MB-231 cells (Fig 1a).
Fig. 1.
BITC decreases Bmi-1 protein level. a Western blotting for Bmi-1 protein using lysates from the indicated cells after 24, 48, and 72 h treatment with DMSO (control) or BITC. Numbers above bands represent changes in Bmi-1 protein level relative to corresponding DMSO-treated control. b Western blotting Bmi-1 using MDA-MB-231 xenograft supernatants from control and BITC-treated athymic mice (n = 5 for control and n = 3 for BITC-treated). c Western blotting for Bmi-1 protein in MCF-7 cells stably transfected with empty pcDNA3.1 vector or the same vector encoding for Bmi-1, and SUM159 cells stably transfected with control shRNA or shRNA targeting Bmi-1. d The bar graphs show cell viability in MCF-7 and SUM159 cells with altered Bmi-1 expression after 72 h treatment with DMSO or BITC. e. Viability of SUM159 and MDAMB-231 cells transiently transfected with control siRNA or Bmi-1 targeted siRNA and treated for 72 h with DMSO or BITC, Inset shows western blotting for Bmi-1 protein. Results in panels d and e are expressed as mean ± SD (n = 3). Significantly different (P<0.05) compared with *respective DMSO-treated control, and # between empty vector transfected and Bmi-1 overexpressing MCF-7 cells by one-way ANOVA followed by Bonferroni’s multiple comparison test. Each experiment was repeated twice, and representative data from one such experiment are shown.
Consistent with cellular results (Fig. 1a), inhibition of MDA-MB-231 xenograft growth in vivo after BITC administration [13] was accompanied by a decrease in Bmi-1 protein level (Fig. 1b). Bmi-1 expression was visually obvious in 4 of 5 MDA-MB-231 xenografts from the control group (Fig. 1b). The reason for variability in Bmi-1 protein level in control xenografts is unclear, but its expression was barely detectable in each MDA-MB-231 xenograft from the BITC treatment group. Collectively, these results indicated that the BITC-mediated downregulation of Bmi-1 protein was neither cell line-specific nor restricted to cultured breast cancer cells.
The role of Bmi-1 in BITC-mediated inhibition of breast cancer cell viability
In agreement with published data [27], overexpression of Bmi-1 protein (Fig. 1c) led to a significant (1.6-fold) increase in MCF-7 cell viability (Fig. 1d). In addition, Bmi-1 overexpressing MCF-7 cells exhibited a modest but statistically significant resistance to cell viability inhibition by BITC (Fig. 1d). In sharp contrast to MCF-7 cells [27], viability of SUM159 cells was not affected by stable knockdown of Bmi-1 protein (Fig. 1c,d). Furthermore, SUM159 cells stably transfected with the control shRNA and the Bmi-1-targeted shRNA were equally sensitive to BITC-mediated inhibition of cell viability. Consistent with these results, cell viability inhibition by BITC was not affected by knockdown of Bmi-1 protein (>90% knockdown) by transient transfection with siRNA in either SUM159 or MDA-MB-231 cells (Fig 1e). Similarly, cell viability inhibition by BITC, determined by trypan blue dye exclusion assay, was not affected by RNA interference of Bmi-1 at least in SUM159 cells (results not shown). These results indicated cell line-specific role for Bmi-1 in regulation of cell viability.
Bmi-1 was dispensable for proapoptotic effect of BITC in MCF-7 and SUM159 cells
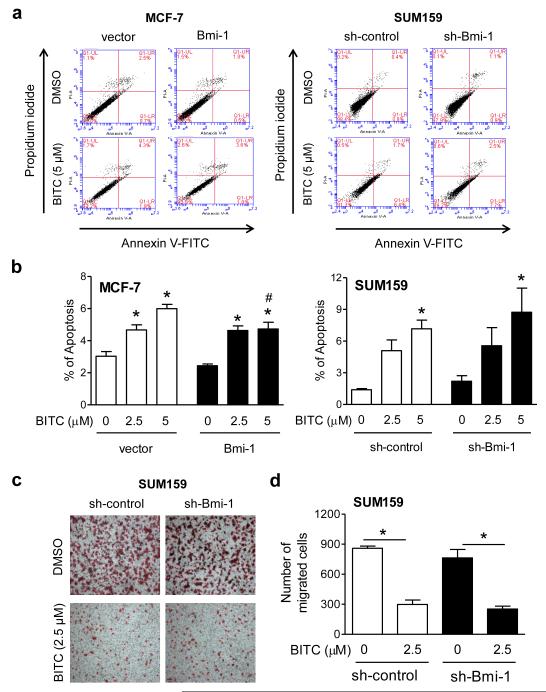
Breast cancer growth retardation upon treatment with BITC is accompanied by apoptosis induction in vitro and in vivo [15, 28]. Even though the published literature on the role of Bmi-1 in regulation of apoptosis is ambiguous [27, 29], we were tempted to test if the proapoptotic response to BITC was affected by overexpression or knockdown of Bmi-1 protein. Figure 2a shows early (Annexin Vhigh and propidium iodidelow) and late apoptotic fraction (Annexin Vhigh and propidium iodidehigh) in MCF-7 and SUM159 cells after 24 h treatment with DMSO or BITC. The basal apoptosis was not affected by stable overexpression (MCF-7) or stable knockdown (SUM159) of the Bmi-1 protein (Fig. 2a). With the exception of a modest attenuation of cell death induction at the higher BITC dose in MCF-7 cells, the proapoptotic effect of this agent was generally not affected by alteration of Bmi-1 expression (Fig. 2b). Our interpretation of these results is that Bmi-1 is largely dispensable for apoptosis induction by BITC.
Fig. 2.
Bmi-1 is largely dispensable for BITC-induced apoptosis or cell migration inhibition. a Representative flow histograms depicting apoptotic fraction in the indicated cells after 24 h treatment with DMSO or BITC. b The bar graphs show quantitation of apoptotic fraction. Results are expressed as mean ± SD (n = 3). Significantly different (P<0.05) compared with *respective DMSO-treated control, and # between empty vector transfected control and Bmi-1 overexpressing MCF-7 cells by one-way ANOVA followed by Bonferroni’s multiple comparison test. c Representative migration images (Boyden chamber assay) after 24 h treatment with DMSO or BITC (100× magnification). d Quantitation of cell migration. Results shown are mean ± SD (n = 3). *Significantly different (P<0.05) between the indicated groups by one-way ANOVA followed by Bonferroni’s multiple comparison test.
BITC-mediated inhibition of SUM159 cell migration was not affected by Bmi-1 knockdown
Overexpression of Bmi-1 protein in immortalized human mammary epithelial cells was previously shown to increase cell migration, whereas a decrease in propensity for migration of MDA-MB-435S cancerous breast cells was discernible after its knockdown [30]. Even though stable knockdown of Bmi-1 protein resulted in a modest decrease in SUM159 cell migration, the difference was insignificant (Fig. 2c,d). Nevertheless, the extent of cell migration inhibition by BITC was comparable in sh-control and sh-Bmi-1 cells (Fig. 2d).
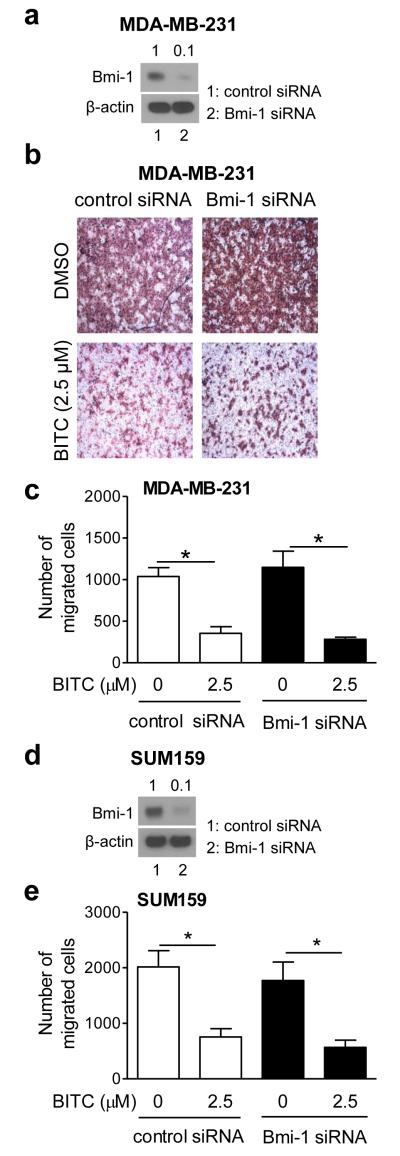
Because of incomplete knockdown of the protein using Bmi-1-targeted shRNA (Fig. 1c), we carried out experiments using a Bmi-1-targeted siRNA (Fig. 3). BITC-mediated inhibition of MDA-MB-231 (Fig. 3a-c) and SUM159 (Fig. 3d,e) cell migration was not affected even after 90% knockdown of Bmi-1 protein with transient RNA interference (Fig. 3a,d). Similar results were observed in cells transfected with control and Bmi-1 siRNA even at lower (1 μM) BITC concentration (results not shown). These results indicated that Bmi-1 knockdown did not affect cell migration inhibition by BITC.
Fig. 3.
Effect of Bmi-1 RNA interference on cell migration inhibition by BITC. a Western blotting for Bmi-1 in MDA-MB-231 cells transiently transfected with control siRNA or Bmi-1-targeted siRNA. b Representative migration images (Boyden chamber assay) for MDA-MB-231 cells transfected with control siRNA or Bmi-1-targeted siRNA after 24 h treatment with DMSO or 2.5 μM BITC (100× magnification). c Quantitation of cell migration in MDA-MB-231 cells. d Western blotting for Bmi-1 in SUM159 cells transiently transfected with control siRNA or Bmi-1 targeted siRNA. e Quantitation of cell migration for SUM159 cells. Results in panel c and e are mean ± SD; n=3. *Significantly different (P<0.05) between the indicated groups by one-way ANOVA followed by Bonferroni’s multiple comparison test.
The role of Bmi-1 in bCSC inhibition by BITC
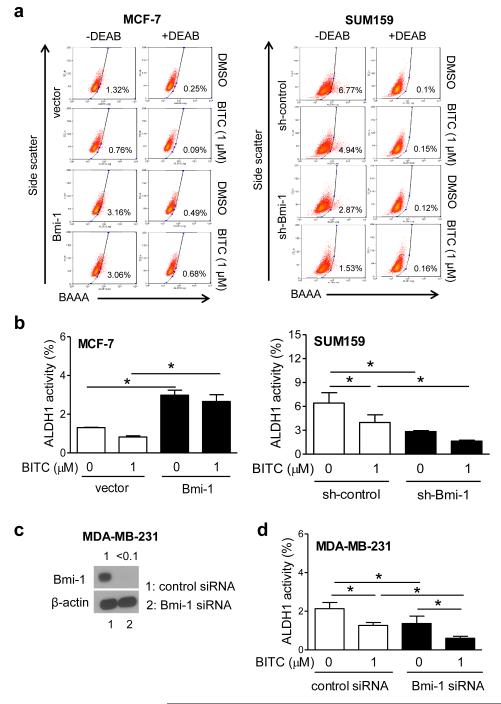
Two independent assays, including ALDH1 activity determination and mammosphere formation, were performed to explore potential involvement of Bmi-1 in BITC-mediated suppression of bCSC activity. Figure 4a shows flow histograms for ALDH1 activity with or without treatment with BITC or ALDH1 inhibitor diethylaminobenzaldehyde (DEAB). The ALDH1 activity was increased by about 2.3-fold upon overexpression of Bmi-1 itself in MCF-7 cells (Fig. 4b). Treatment of empty vector transfected MCF-7 cells with 1 μM BITC for 72 h led to a 37% decrease in ALDH1 activity compared with DMSO control, but the difference was not significant (Fig. 4b). A decrease of only about 10% (non-significant) in ALDH1 activity was observed by a similar treatment in MCF-7 cell stably overexpressing Bmi-1 in comparison with DMSO control (Fig. 4b). The difference in ALDH1 activity between BITC-treated empty vector and BITC-treated Bmi-1 overexpressing cells was significant (Fig. 4b). The ALDH1 activity was decreased by about 56% after knockdown of Bmi-1 protein (P<0.05) in SUM159 cells (Fig. 4b). BITC treatment resulted in a 38% decrease in ALDH1 activity in SUM159 cells stably transfected with control shRNA (P<0.05). In addition, the BITC-mediated inhibition of ALDH1 activity was augmented by Bmi-1 knockdown in SUM159 cells (P<0.05) (Fig. 4b). Similar results were seen in MDA-MB-231 cells transiently transfected with Bmi-1-targeted siRNA (Fig. 4c,d).
Fig. 4.
The role of Bmi-1 in bCSC inhibition by BITC. a Representative flow histograms showing ALDH1 activity after 72 h treatment with DMSO or BITC. DEAB, diethylaminobenzaldehyde; BAAA- BODIPY™ aminoacetaldehyde. The ALDH1 inhibitor DEAB was used as a control. b The bar graphs show quantitation of ALDH1 activity (mean ± SD; n = 3). *Significantly different (P<0.05) between the indicated groups by one-way ANOVA followed by Bonferroni’s multiple comparison test. c Western blotting for Bmi-1 in MDA-MB-231 cells transiently transfected with control siRNA or Bmi-1 targeted siRNA. d The bar graph shows quantiation of ALDH1 activity after 72 h treatment with DMSO or BITC (mean ± SD; n = 3). *Significantly different (P<0.05) between the indicated groups by one-way ANOVA followed by Newman-Keuls test. Representative data from replicative experiments are shown.
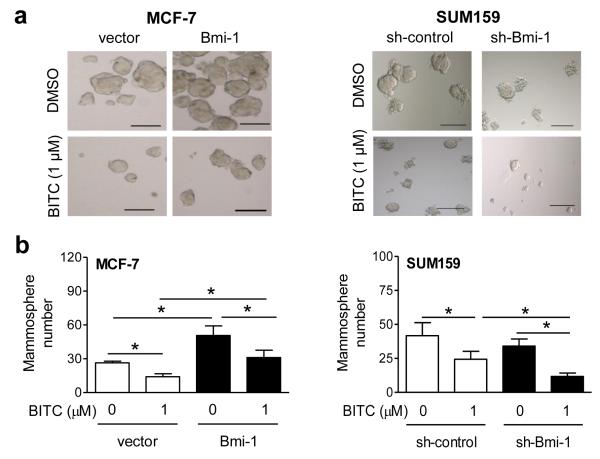
Figure 5a depicts primary mammospheres resulting after 5 days (MCF-7) or 7 days (SUM159) of treatment with BITC or DMSO (control). Quantitation of mammospheres revealed 1.9-fold enrichment in MCF-7 cells after Bmi-1 overexpression (P<0.05) (Fig. 5b). Mammosphere number was decreased by 47% (P<0.05) after BITC treatment in empty vector transfected MCF-7 cells (Fig. 5b). Overexpression of Bmi-1 in MCF-7 cells conferred significant protection against mammosphere inhibition by BITC (P<0.05). Mammosphere frequency was reduced by 42% (non-significant) in Bmi-1 knockdown SUM159 cells (Fig. 5b). BITC-mediated inhibition of mammosphere number was augmented after stable knockdown of Bmi-1 in SUM159 cells (P<0.05). The general conclusions from the mammosphere formation assays were consistent with those of ALDH1 activity data.
Fig. 5.
The role of Bmi-1 in mammosphere inhibition by BITC. a Representative mammosphere images after 5 (MCF-7) or 7 (SUM159) days of treatment with DMSO or BITC (100× magnification; scale bar- 100 μm). b The bar graphs show quantitation of mammosphere number (mean ± SD; n=3). *Significantly different (P<0.05) between the indicated groups by one-way ANOVA followed by Newman-Keuls test. Representative data from replicative experiments are shown.
Notch4 activation by BITC impeded its effect on bCSC fraction
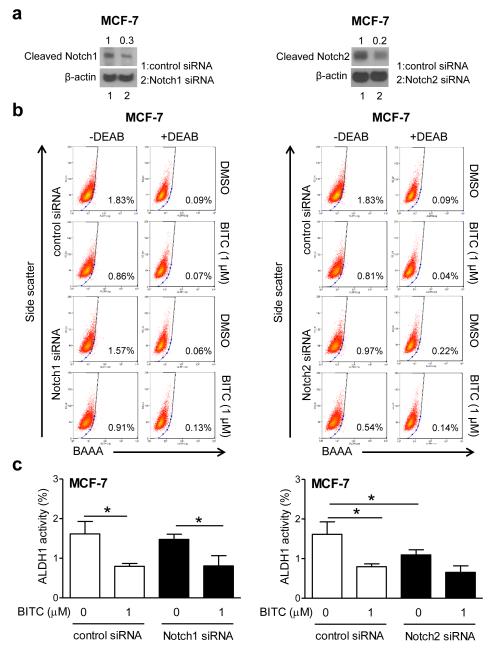
The transcription factors belonging to the Notch family, especially Notch1 and Notch4 have emerged as critical mediators of stemness in breast cancer [18, 20]. At the same time, a tumor suppressor role for Notch2 has also been suggested in breast cancer cells [31]. Our own previous work has revealed that BITC treatment increases levels of cleaved Notch1, Notch2 and Notch4 leading to transcriptional activation of Notch in a panel of human breast cancer cells, including MCF-7 and SUM159 cell lines [21]. Furthermore, the BITC-mediated activation of Notch was accompanied by induction of γ-secretase complex component Nicastrin [21]. Therefore, it was of interest to test whether anti-bCSC effect of BITC was compromised due to Notch activation. A series of RNA interference studies were conducted using MCF-7 cells to explore this possibility. RNA interference of Notch2 (Fig. 6a) decreased ALDH1 activity (Fig. 6b), but BITC elicited more or less similar response in cells transfected with control siRNA and Notch1 or Notch2 siRNA (Fig. 6c). RNA interference of Notch4 resulted in 60-90% decrease in its protein level in MCF-7 and SUM159 cells (Fig. 7a). In MCF-7 cells, the ALDH1 activity was statistically significantly inhibited by BITC treatment (Fig. 7b) as well as by Notch4 knockdown alone (Fig. 7b,c, left panel). The overall pattern was the same in SUM159 cells, but the difference did not reach significance for Notch4 knockdown alone compared with cells transfected with control siRNA (Fig. 7c, right panel). In both cells, however, BITC-mediated inhibition of ALDH1 activity was statistically significantly augmented by Notch4 knockdown (Fig. 7c: compare 2nd and 4th columns). We next explored whether activation of Notch4 was affected by Bmi-1 status. As can be seen in Fig. 7d, Notch4 activation was marginally affected by overexpression (MCF-7) and knockdown of Bmi-1 (SUM159).
Fig. 6.
Notch1 and Notch2 are dispensable for BITC-mediated inhibition of bCSC. a Western blotting for cleaved Notch1 and cleaved Notch2 in MCF-7 cells transiently transfected with control siRNA and Notch1 or Notch2-targeted siRNA. b Representative flow histograms for ALDH1 activity in MCF-7 cells after 48 h treatment with DMSO or BITC. c Quantitation of ALDH1 activity (mean ± SD; n=3). *Significantly different (P<0.05) between the indicated groups by one-way ANOVA followed by Newman-Keuls test.
Fig. 7.
Notch4 knockdown augments BITC-mediated inhibition of bCSC. a Western blots showing knockdown of cleaved Notch4 after its RNA interference in MCF-7 and SUM159 cells. b Representative flow histograms showing ALDH1 activity after 48 h treatment with DMSO or BITC. c Quantitation of ALDH1 activity (mean ± SD; n=3). *Significantly different (P<0.05) between the indicated groups by one-way ANOVA followed by Newman-Keuls test. d Western blotting for Bmi-1 and cleaved Notch4 protein in MCF-7 cells stably transfected with empty vector or the same vector encoding for Bmi-1, and in SUM159 cells stably transfected with control shRNA or shRNA targeting Bmi-1. Representative data from replicative experiments are shown.
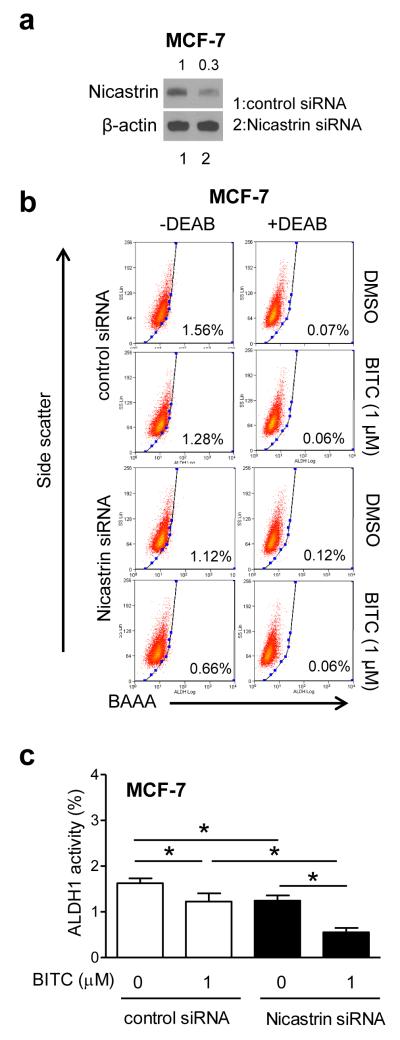
Transient knockdown of Nicastrin protein (Fig. 8a) resulted in potentiation of ALDH1 activity inhibition by BITC in MCF-7 cells (Fig. 8b,c). Consistent with published literature [32], Nicastrin knockdown alone was inhibitory against MCF-7 stemness (Fig. 8c). These results indicated that: (a) Notch1 and Notch2 are largely dispensable for bCSC inhibition by BITC, and (b) Notch4 activation by BITC, likely due to induction of Nicastrin, impeded its inhibitory effect on ALDH1 activity.
Fig. 8.
Effect of Nicastrin RNA interference on BITC-mediated inhibition of bCSC. a Expression of Nicastrin in MCF-7 cells transiently transfected with control siRNA or Nicastrin-targeted siRNA. b Representative flow histograms showing ALDH1 activity in MCF-7 cells transiently transfected with control siRNA or Nicastrin-targeted siRNA and treated for 48 h with DMSO or BITC. c The bar graph shows quantitation of ALDH1 activity. Results shown are mean ± SD (n=3). *Significantly different (P<0.05) between the indicated groups by one-way ANOVA followed by Bonferroni’s multiple comparison test.
Discussion
The role of the polycomb group protein Bmi-1 in regulation of cell viability or apoptosis is not fully understood. One study showed a decrease in cell viability in MCF-7 cells after knockdown of the Bmi-1 protein [27]. The present study reveals that while Bmi-1 expression affects MCF-7 cell viability (Fig. 1d), this relationship is not obvious in SUM159 or MDA-MB-231 cells (Fig. 1e). Thus, the association of Bmi-1 expression level with cell viability seems unique to MCF-7 cells. This scenario is partially supported by literature data showing no effect of Bmi-1 knockdown on viability of MDA-MB-435S cells [30]. Nevertheless, the present study reveals that the Bmi-1 expression has marginal impact on BITC’s ability to inhibit viability or induce apoptotic cell death in cells other than MCF-7. We also found no meaningful impact of Bmi-1 knockdown on SUM159 cell migration.
The present study shows that overexpression of Bmi-1 confers protection against bCSC inhibition by BITC in MCF-7 cells. In agreement with these results, bCSC inhibition by BITC is augmented by Bmi-1 knockdown in SUM159 and MDA-MB-231 cells. Bmi-1 can cause mammary epithelial cells to evade senescence leading to immortalization [33]. High Bmi-1 expression in human breast cancer specimens correlates with clinical and pathologic classification. Immunohistochemical analysis of Bmi-1 in breast tumors suggests that its expression is an independent prognostic factor associated with basal-like phenotype and poor survival [34]. Even though examples also exist arguing against the association of high Bmi-1 expression with unfavorable prognosis in breast cancer [35], the results shown herein indicate, for the first time, that Bmi-1 is a novel target of bCSC inhibition by BITC. We also propose that Bmi-1 suppression may represent a viable pharmacodynamic biomarker of BITC exposure because the expression of Bmi-1 is decreased after BITC treatment both in vitro and in vivo.
The present study building upon our previous findings [21] highlights that the activation of Notch4 by BITC attenuates its inhibitory effect on bCSC activity. Knockdown of γ-secretase complex component Nicastrin also augments bCSC inhibition by BITC. However, a similar relationship is not observed for Notch1 and Notch2 despite their activation by BITC [21]. The mechanism by which Notch4 regulates bCSC activity is not fully understood, but interaction with peptidyl-prolyl isomerase Pin1 leading to escape from proteasomal degradation has been observed in human breast cancer cells [36]. We are tempted to speculate that mammary cancer prevention by BITC is likely augmented by a combination regimen involving Notch4 inhibitor, but validation of this hypothesis requires investigation.
In summary, data shown herein indicate that: (a) BITC decreases expression of Bmi-1 protein in vitro; (b) expression of Bmi-1 protein is decreased in vivo after BITC administration to MDA-MB-231 xenograft bearing athymic mice; (c) Bmi-1 is largely dispensable for BITC-mediated inhibition of cell viability and migration; (d) BITC’s ability to induce apoptosis in human breast cancer cells is not altered by Bmi-1 expression level; (e) Bmi-1 plays an important role in bCSC inhibition by BITC; and (f) Notch4 activation by BITC hinders its inhibitory effect on bCSC.
Acknowledgements
This study was supported by the National Cancer Institute, National Institutes of Health grant RO1 CA129347-07. This study used the Flow Cytometry Facility supported in part by Cancer Center Support Grant P30 CA047904).
Abbreviations
- ALDH1
Aldehyde dehydrogenase 1
- BAAA
BODIPY™ aminoacetaldehyde
- BITC
benzyl isothiocyanate
- bCSC
breast cancer stem cells
- Bmi-1
B-lymphoma Moloney murine leukemia virus insertion region-1
- DEAB
diethylaminobenzaldehyde
- DMSO
dimethyl sulfoxide
- ER
estrogen receptor
- shRNA
small hairpin RNA
- siRNA
small interfering RNA
Footnotes
Conflict of interest: None of the authors has any conflict of interest.
References
- 1.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel project P-1 study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 2.Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, McTierman A, Robbins J, Johnson KC, Martin LW, Winquist E, Sarto GE, Garber JE, Fabian CJ, Pujol P, Maunsell E, Farmer P, Gelmon KA, Tu D, Richardson H, NCIC CTG MAP.3 Study Investigators Exemestane for breast-cancer prevention in postmenopausal women. N Eng J Med. 2011;364(25):2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 3.Higgins MJ, Baselga J. Breast cancer in 2010: novel targets and therapies for a personalized approach. Nature Rev Clin Oncol. 2011;8(2):65–66. doi: 10.1038/nrclinonc.2010.217. [DOI] [PubMed] [Google Scholar]
- 4.van Zitteren M, van der Net JB, Kundu S, Freedman AN, van Duijn CM, Janssens AC. Genome-based prediction of breast cancer risk in the general population: a modeling study based on meta-analyses of genetic associations. Cancer Epidemiol Biomarkers Prev. 2011;20(1):9–22. doi: 10.1158/1055-9965.EPI-10-0329. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 6.Velasco-Velázquez MA, Homsi N, De La Fuente M, Pestell RG. Breast cancer stem cells. Int J Biochem Cell Biol. 2012;44(4):573–577. doi: 10.1016/j.biocel.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien CS, Farnie G, Howell SJ, Clarke RB. Breast cancer stem cells and their role in resistance to endocrine therapy. Horm Cancer. 2011;2(2):91–103. doi: 10.1007/s12672-011-0066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surh YJ. Cancer Chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3(10):768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 9.Vyas AR, Singh SV. Molecular targets and mechanisms of cancer prevention and treatment by withaferin A, a naturally occurring steroidal lactone. AAPS J. 2014;16(1):1–10. doi: 10.1208/s12248-013-9531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sehrawat A, Singh SV. Molecular mechanisms of cancer chemoprevention with benzyl isothiocyanate. In: Kong AN, editor. Inflammation, Oxidative Stress, and Cancer. Taylor & Francis; New York, USA: 2013. pp. 447–462. 2013 (DOI 10.1201/b15323-32) [Google Scholar]
- 11.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134(5):1134–1138. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- 12.Wattenberg LW. Inhibition of carcinogenic effects of polycyclic hydrocarbons by benzyl isothiocyanate and related compounds. J Natl Cancer Inst. 1977;58(2):395–398. doi: 10.1093/jnci/58.2.395. [DOI] [PubMed] [Google Scholar]
- 13.Warin R, Xiao D, Arlotti JA, Bommareddy A, Singh SV. Inhibition of human breast cancer xenograft growth by cruciferous vegetable constituent benzyl isothiocyanate. Mol Carcinog. 2010;49(5):500–507. doi: 10.1002/mc.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim EJ, Hong JE, Eom SJ, Lee JY, Park JH. Oral administration of benzylisothiocyanate inhibits solid tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells in BALB/c mice. Breast Cancer Res Treat. 2011;130(1):61–71. doi: 10.1007/s10549-010-1299-8. [DOI] [PubMed] [Google Scholar]
- 15.Warin R, Chambers WH, Potter DM, Singh SV. Prevention of mammary carcinogenesis in MMTV-neu mice by cruciferous vegetable constituent benzyl isothiocyanate. Cancer Res. 2009;69(24):9473–9480. doi: 10.1158/0008-5472.CAN-09-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SH, Sehrawat A, Singh SV. Dietary chemopreventative benzyl isothiocyanate inhibits breast cancer stem cells in vitro and in vivo. Cancer Prev Res. 2013;6(8):782–790. doi: 10.1158/1940-6207.CAPR-13-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66(12):6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, Bundred NJ, Clarke RB. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70(2):709–718. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paranjape AN, Balaji SA, Mandal T, Krushik EV, Nagaraj P, Mukherjee G, Rangarajan A. Bmi1 regulates self-renewal and epithelial to mesenchymal transition in breast cancer cells through Nanog. BMC Cancer. 2014;14:785. doi: 10.1186/1471-2407-14-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez ME, Moore HM, Li X, Toy KA, Huang W, Sabel MS, Kidwell KM, Kleer CG. EZH2 expands breast stem cells through activation of NOTCH1 signaling. Proc Natl Acad Sci USA. 2014;111(8):3098–3103. doi: 10.1073/pnas.1308953111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SH, Sehrawat A, Singh SV. Notch2 activation by benzyl isothiocyanate impedes its inhibitory effect on breast cancer cell migration. Breast Cancer Res Treat. 2012;134(3):1067–1079. doi: 10.1007/s10549-012-2043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24(5):891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 23.Xiao D, Lew KL, Kim YA, Zeng Y, Hahm ER, Dhir R, Singh SV. Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin Cancer Res. 2006;12(22):6836–6843. doi: 10.1158/1078-0432.CCR-06-1273. [DOI] [PubMed] [Google Scholar]
- 24.Sakao K, Singh SV. D,L-Sulforaphane-induced apoptosis in human breast cancer cells is regulated by the adapter protein p66Shc. J Cell Biochem. 2012;113(2):599–610. doi: 10.1002/jcb.23386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, Los G, Slamon DJ. PD0332991, a selective cyclin D1 kinase 4/6 inhibitor, preferentially inhibits proliferation and luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boreddy SR, Pramanik KC, Srivastava SK. Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of P13K/AKT/FOXO pathway. Clin Cancer Res. 2011;17(7):1784–1795. doi: 10.1158/1078-0432.CCR-10-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Z, Liu H, Lv X, Liu Y, Li S, Li H. Knockdown of the Bmi-1 oncogene inhibits cell proliferation and induces cell apoptosis and is involved in the decrease of Akt phosphorylation in the human breast carcinoma cell line MCF-7. Oncol Rep. 2011;25(2):409–418. doi: 10.3892/or.2010.1078. [DOI] [PubMed] [Google Scholar]
- 28.Xiao D, Vogel V, Singh SV. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Mol Cancer Ther. 2006;5(11):2931–2945. doi: 10.1158/1535-7163.MCT-06-0396. [DOI] [PubMed] [Google Scholar]
- 29.Wu XM, Liu X, Bu YQ, Sengupta J, Cui HJ, Yi FP, Liu T, Yuan CF, Shi YY, Song FZ. RNAi-mediated silencing of the Bmi-1 gene causes growth inhibition and enhances doxorubicin-induced apoptosis in MCF-7 cells. Genet Mol Biol. 2009;32(4):697–703. doi: 10.1590/S1415-47572009005000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo BH, Feng Y, Zhang R, Xu LH, Li MZ, Kung HF, Song LB, Zeng MS. Bmi-1 promotes invasion and metastasis, and its elevated expression is correlated with an advanced stage of breast cancer. Mol Cancer. 2011;10(1):10. doi: 10.1186/1476-4598-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Neill CF, Urs S, Cinelli C, Lincoln A, Nadeau RJ, León R, Toher J, Mouta-Bellum C, Friesel RE, Liaw L. Notch2 signaling induces apoptosis and inhibits human MDA-MB-231 xenograft growth. Am J Pathol. 2007;171(3):1023–1036. doi: 10.2353/ajpath.2007.061029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lombardo Y, Filipović A, Molyneux G, Periyasamy M, Giamas G, Hu Y, Trivedi PS, Wang J, Yagüe E, Michel L, Coombes RC. Nicastrin regulates breast cancer stem cell properties and tumor growth in vitro and in vivo. Proc Natl Acad Sci USA. 2012;109(41):16558–16563. doi: 10.1073/pnas.1206268109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimri GP, Martinez JL, Jacobs JJ, Keblusek P, Itahana K, Van Lohuizen M, Campisi J, Wazer DE, Band V. The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res. 2002;62(16):4736–4745. [PubMed] [Google Scholar]
- 34.Wang Y, Zhe H, Ding Z, Gao P, Zhang N, Li G. Cancer stem cell marker Bmi-1 expression is associated with basal-like phenotype and poor survival in breast cancer. World J Surg. 2012;36(5):1189–1194. doi: 10.1007/s00268-012-1514-3. [DOI] [PubMed] [Google Scholar]
- 35.Choi YJ, Choi YL, Cho EY, Shin YK, Sung KW, Hwang YK, Lee SJ, Kong G, Lee JE, Kim JS, Kim JH, Yang JH, Nam SJ. Expression of Bmi-1 protein in tumor tissues is associated with favorable prognosis in breast cancer patients. Breast Cancer Res Treat. 2009;113(1):83–93. doi: 10.1007/s10549-008-9909-4. [DOI] [PubMed] [Google Scholar]
- 36.Rustighi A, Zannini A, Tiberi L, Sommaggio R, Piazza S, Sorrentino G, Nuzzo S, Tuscano A, Eterno V, Benvenuti F, Santarpia L, Aifantis I, Rosato A, Bicciato S, Zambelli A, Del Sal G. Prolyl-isomerase Pin1 controls normal and cancer stem cells of the breast. EMBO Mol Med. 2014;6(1):99–119. doi: 10.1002/emmm.201302909. [DOI] [PMC free article] [PubMed] [Google Scholar]