Summary
EBER2 is an abundant nuclear noncoding RNA expressed by Epstein-Barr virus (EBV). Probing its possible chromatin localization by CHART revealed EBER2’s presence at the terminal repeats (TRs) of the latent EBV genome, overlapping previously identified binding sites for the B-cell transcription factor PAX5. EBER2 interacts with and is required for PAX5 localization to the TRs. EBER2 knockdown phenocopies PAX5 depletion in upregulating the expression of LMP2A/B and LMP1, genes nearest the TRs. Knockdown of EBER2 also decreases EBV lytic replication, underscoring the essential role of the TRs in viral replication. Recruitment of the EBER2-PAX5 complex is mediated by base-pairing between EBER2 and nascent transcripts from the TR locus. The interaction is evolutionarily conserved in the related primate herpesvirus CeHV15 despite great sequence divergence. Using base-pairing with nascent RNA to guide an interacting transcription factor to its DNA target site is a previously undescribed function for a trans-acting noncoding RNA.
Introduction
Epstein–Barr virus (EBV) is a human lymphotropic gamma-1 herpesvirus (or lymphocryptovirus, LCV) that expresses two noncoding RNAs called EBER1 (EBV-encoded RNA 1) and EBER2, which are 167 and 173 nts long, respectively (Lerner et al., 1981). They are expressed during all forms of EBV latency and also during lytic growth (Greifenegger et al., 1998; Rowe et al., 2009). EBER1 accumulates to ~106 and EBER2 to ~2.5×105 copies per infected cell (Moss and Steitz, 2013). The high copy number and the evolutionary conservation of EBERs in related primate LCVs point to a fundamental role of EBERs in the EBV life cycle (Howe and Shu, 1988; Rivailler et al., 2002b). To elucidate their function, recombinant EBV strains lacking EBERs have been engineered and introduced into host B lymphocytes. However, conflicting observations regarding possible effects on B-cell growth and transformation have been reported (Gregorovic et al., 2011; Swaminathan et al., 1991; Yajima et al., 2005). Thus, the physiological roles of EBERs have remained unclear. Likewise, only limited mechanistic insights have been gained from investigating the interacting partners of these noncoding RNAs. Both EBER1 and EBER2 bind the host RNA chaperone protein La (Lerner et al., 1981), whereas ribosomal protein L22 and AUF1 (AU-rich element binding factor 1)/hnRNP D (heterogeneous nuclear ribonucleoprotein D) additionally associate with EBER1 (Lee et al., 2012; Toczyski et al., 1994). A hallmark of EBV is its tumorigenic potential, and several EBV latent proteins have been shown to contribute to oncogenicity (Young and Rickinson, 2004). Intriguingly, EBERs by themselves, particularly EBER1, can cause tumors under certain conditions, but the exact molecular mechanism has not been elucidated even though EBER1’s interaction with L22 has been implicated in the process (Houmani et al., 2009; Komano et al., 1999; Repellin et al., 2010). The unidentified function(s) of EBERs must be confined to the nucleus, as they exhibit strictly nuclear localization and do not undergo nucleo-cytoplasmic shuttling (Fok et al., 2006).
The EBV genome in virions is linear, flanked on both ends by tandem terminal repeats (TRs). These direct repeat units are 538 and 544 bp long (in EBV type I and II strains, respectively) and contain high GC content (78%). Each viral genome contains a varying number of up to 20 TRs (Brown et al., 1988). Upon infection of a host cell, the viral genome circularizes at the TRs, possibly through a recombination event, and amplifies as multicopy episomes during latency (Lindahl et al., 1976; Sugden et al., 1979). Following genome circularization, the promoter region and exons of LMP2 (Latent Membrane Protein 2) located at opposite ends of the linear genome become juxtaposed to allow the expression of both LMP2A and LMP2B isoforms, which differ by alternative promoter usage (Raab-Traub and Flynn, 1986). Both isoforms modulate B-cell receptor signal transduction to prevent premature lytic reactivation (Miller et al., 1994; Rovedo and Longnecker, 2007). During lytic replication, the circular EBV genome is amplified giving rise to long concatemers that are subsequently processed into unit length genomes. Again, processing occurs in the TR region by a proposed recombination event in conjunction with enzymatic cleavage by a so-called terminase complex (Chiu et al., 2014; Zimmermann and Hammerschmidt, 1995). The TRs further provide an essential sorting signal for the linear genome to be packaged into capsids (Feederle et al., 2005). These observations underscore the important role(s) of the TR regions in EBV genome organization during both latency and the lytic cycle. The latency-lytic switch is subject to tight regulation, as an expanding body of evidence indicates that lytic replication contributes to oncogenesis (Katsumura et al., 2011; Ma et al., 2011).
B lymphocytes are the major cell type infected by EBV. The transcription factor PAX5 (Paired box protein 5) has been described as a master regulator of B lymphocyte development through promoting the expression of B-cell specific genes and repressing B-lineage inappropriate genes (Medvedovic et al., 2011). Specific DNA binding of PAX5 is achieved through the conserved paired box DNA-binding motif. Intriguingly, a recent study has shown that PAX5 binds to the TRs of EBV perhaps to coordinate viral genome organization (Arvey et al., 2012). PAX5 has further been shown to regulate EBV latent gene expression, as depletion of PAX5 results, for example, in upregulation of the expression of LMP1, the main transforming protein of EBV that acts as a classical oncogene, and of both LMP2 isoforms (Arvey et al., 2012).
Here, we performed Capture Hybridization Analysis of RNA Targets (CHART) for EBER2, a method comparable to chromatin IP (ChIP) but assaying the chromatin localization of an RNA of interest (Simon et al., 2011). In lieu of an antibody in ChIP experiments, CHART employs antisense oligonucleotides (ASOs) that hybridize to accessible regions of an RNA for selection (Figure S1). We report that EBER2 localizes to the TRs of the latent EBV genome and provide in vivo evidence that its recruitment involves an RNA-RNA interaction with nascent RNA transcripts. This process in turn is required for efficient association of PAX5 with its target sites within the TRs. Perturbation of EBER2-PAX5 localization affects expression of genes nearest its binding site as well as lytic viral DNA replication, with possible downstream effects on oncogenic processes.
Results
EBER2 co-localizes with PAX5 to the TRs of the EBV genome
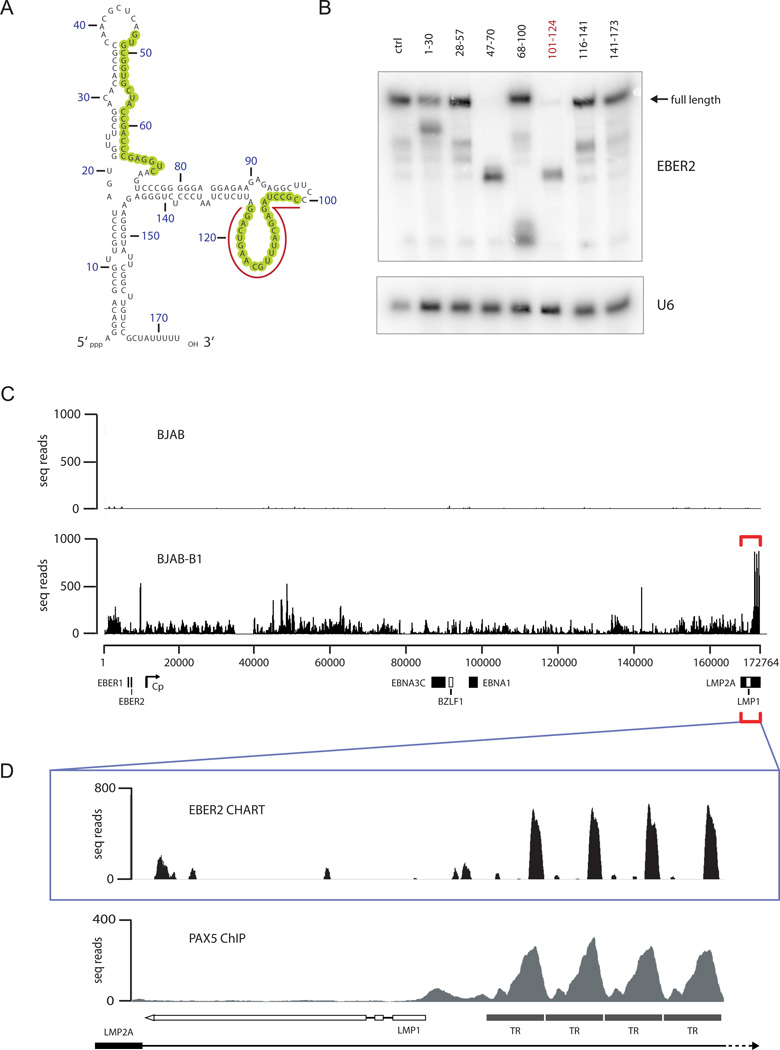
To identify an accessible region in EBER2 that could be targeted by an ASO for selection in CHART (Simon et al., 2011), we added ~30 nucleotide-long DNA oligonucleotides complementary to EBER2 to lysate from EBV-positive BJAB-B1 cells (which contain type II EBV). Formation of DNA-RNA hybrids at accessible regions in EBER2 induces cleavage by endogenous RNase H. Two such regions in EBER2 (nts 47–70 and 101–124) were detected by Northern blot analysis (Figure 1A, B). To select EBER2, we therefore coupled to agarose beads an RNA ASO targeting nts 101–124, as the secondary structure of this region is predicted to form an extensive loop (Figure 1A).
FIGURE 1.
EBER2 localizes to the TRs of the EBV genome. (A) Secondary structure model of EBER2. RNase H-sensitive regions (shown in B) are circled in green. The region hybridizing to the ASO used in CHART is underlined. (B) Northern blot of EBER2 after RNase H digestion using DNA oligonucleotides complementary to EBER2. The numbers on top correspond to the nucleotides targeted in EBER2. Arrow indicates the mobility of full-length EBER2 RNA. U6 RNA serves as a loading control. (C) EBER2-CHART results from BJAB and BJAB-B1 cells. Deep sequencing reads were mapped to the entire EBV genome (x-axis); the number of seq reads is plotted on the y-axis. Several EBV genes and the C promoter region are indicated. White boxes for BZLF1 and LMP1 indicate reverse gene orientation. (D) EBER2-CHART peaks in the TR region (bracket in C) and the PAX5 ChIP profile (from Arvey et al. (2012)) are shown. See also Figure S1.
We then used CHART to identify EBER2 binding sites on chromatin in the EBV-positive BJAB-B1 cell line; the isogenic EBV-deficient BJAB cell line served as a negative control (Figure S1). Deep sequencing libraries from both cell lines were prepared after CHART and subjected to Illumina massive parallel sequencing. When the sequencing reads were mapped to the host cell genome, no obvious EBER2 peaks were present in infected BJAB-B1 compared to BJAB cells (data not shown). However, prominent EBER2 binding sites mapped to the 3′ end of the annotated EBV genome (Figure 1C, bottom, bracketed region). Since very few sequence reads from control BJAB cells map to the EBV genome (Figure 1C, top), these peaks are unlikely to represent host sequences that misalign with viral DNA. A zoomed-in view shows that EBER2 localizes to the TR regions of the EBV genome (Figure 1D, top), its profile strikingly overlapping published ChIP data for the transcription factor PAX5 (Figure 1D). Because TRs represent tandem repeat sequences, as for PAX5 (Arvey et al., 2012), we cannot distinguish whether EBER2 binds to only one specific TR or is equally distributed across all TRs, as depicted here.
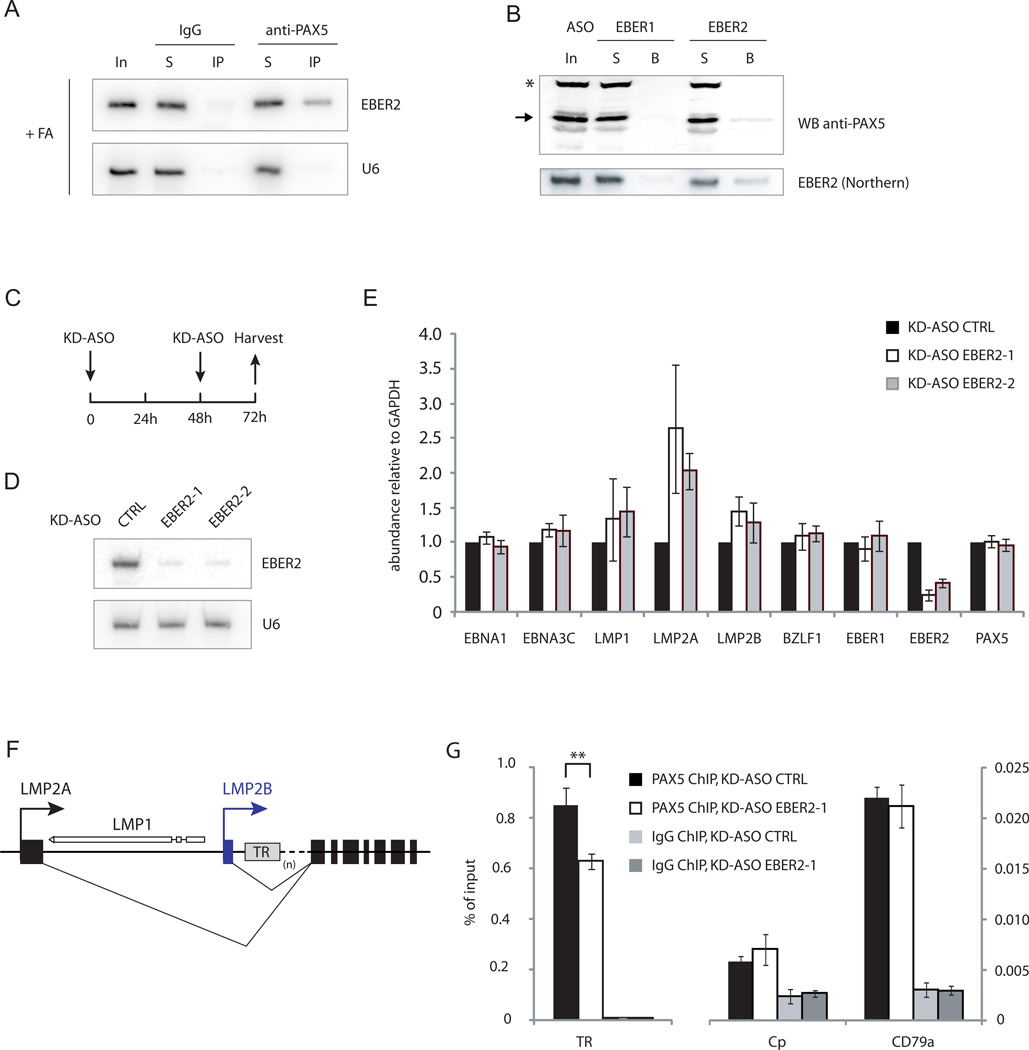
Given their co-localization on EBV chromatin, we asked whether EBER2 and PAX5 interact with each other. Co-immunoprecipitation after in-vivo formaldehyde crosslinking using anti-PAX5 antibody showed that EBER2 interacts with PAX5, while EBER2 was not co-precipitated using an IgG control antibody (Figure 2A, Figure S2A). A reciprocal experiment was performed using an EBER2 ASO (complementary to nts 101–124) that should select EBER2-associated proteins. As shown by Western blot analysis, PAX5 was enriched by the EBER2 ASO, while a control ASO against EBER1 failed to capture PAX5 (Figure 2B, Figure S2B). We asked whether EBER2 interacts directly with PAX5 by performing an RNA-IP assay under denaturing conditions after UV-crosslinking (Lee et al., 2012). EBER2 did not precipitate with anti-PAX5 antibody (Figure S2C), consistent with the fact that EBER2 does not exhibit a bandshift in the presence of recombinant Pax5 in electrophoretic mobility shift assays (EMSAs) (Figure S2D-G). Together, these results suggest that EBER2 and PAX5 interact, but the association may be indirect.
FIGURE 2.
EBER2 interacts with PAX5 and is required for efficient PAX5 binding to TRs. (A) Northern blot of EBER2 after IP with IgG (control) or anti-PAX5 antibody after formaldehyde crosslinking (+FA). In = 5% input, S = 5% supernatant, IP = 100%. (B) EBER1 and EBER2 ASOs were used to pull down associated proteins, followed by Western blot using anti-PAX5 antibody (top). Arrow indicates PAX5; asterisk indicates a non-specific band. In = 10% input, S = 10% supernatant, B = 100% beads. The same samples were subjected to Northern blot analysis to detect EBER2 (bottom). Quantification of panels A and B are shown in Figure S2A and B. (C) Experimental outline for EBER2 knockdown. (D) Northern blot for EBER2 was carried out after EBER2 knockdown with two different KD-ASOs (complementary to nts 101–124 and 39–62) that target the nucleotides circled in green in Figure 1A. The same blot was probed for U6 as a loading control. (E) RNA levels of several EBV genes were assessed by qRT-PCR after EBER2 knockdown. (F) The LMP locus of the episomal EBV genome. LMP1 is transcribed in the opposite direction to LMP2. The variable number of TRs is indicated by (n). (G) PAX5 localization at the TRs after EBER2 knockdown was measured by ChIP-qPCR. The cellular CD79a promoter region, a known PAX5 target site, served as a positive ChIP control. The C promoter region of the EBV genome (Cp), an active promoter region not bound by PAX5, was the negative control. All data represent the mean of three independent experiments +/− SD; ** p = 0.008 (Student’s t-test; n = 3). See also Figure S2 and Table S1.
Based on its interaction with PAX5, we reasoned that EBER2 might act in concert with PAX5 to regulate EBV latent genes. Therefore, we knocked down EBER2 using chimeric ASOs that induce endogenous RNase H-mediated degradation (Table S1) (Ideue et al., 2009) and assessed the mRNA levels of several EBV latent genes by RT-qPCR. Two knockdown ASOs (KD-ASOs) that target the available regions in EBER2 (nts 101–124 and 39–62; Figure 1A, B) efficiently depleted EBER2 to less than 20% of its original level upon nucleofection (Figure 2D). As latent gene expression at 48 h post nucleofection did not change (data not shown), we introduced a second KD-ASO nucleofection step at 48 h and harvested the cells after three days of depletion (Figure 2C). This procedure was necessary to maintain EBER2 at less than 20% its original level because EBER2 levels increased from ~18% at 24 h to ~46% at 48 h after a single knockdown.
Upon EBER2 depletion, we observed that expression of LMP2A, and to a lesser extent LMP1 and LMP2B, was upregulated (Figure 2E), phenocopying the results of PAX5 knockdown (Arvey et al., 2012). We observed no significant change for other EBV genes (e.g. EBNA1 and BZLF1), as reported for PAX5 depletion (Arvey et al., 2012), possibly because we examined the RNA levels at an earlier time point (after 3 days of EBER2 depletion as compared to 5 days of PAX5 depletion) in an effort to reduce potential secondary effects of knockdown. Since the TRs are located in the first intron of LMP2A/B positioned close to the transcription start site of LMP1 (Figure 2F), it is not unexpected that these genes would be most affected by lack of EBER2 localization to the TRs.
Finally, we asked whether EBER2 knockdown would affect PAX5 recruitment to the TRs. As shown by PAX5 ChIP, EBER2 depletion specifically reduced PAX5 localization at the TRs, whereas its binding was unaltered at a cellular PAX5 target, the CD79a promoter region (Figure 2G) (Revilla et al., 2012). In summary, our results show that EBER2 is required for PAX5 recruitment to the TRs and synergizes with PAX5 to regulate certain EBV latent genes.
Base pairing of EBER2 to nascent LMP2 transcripts recruits PAX5 to the TRs
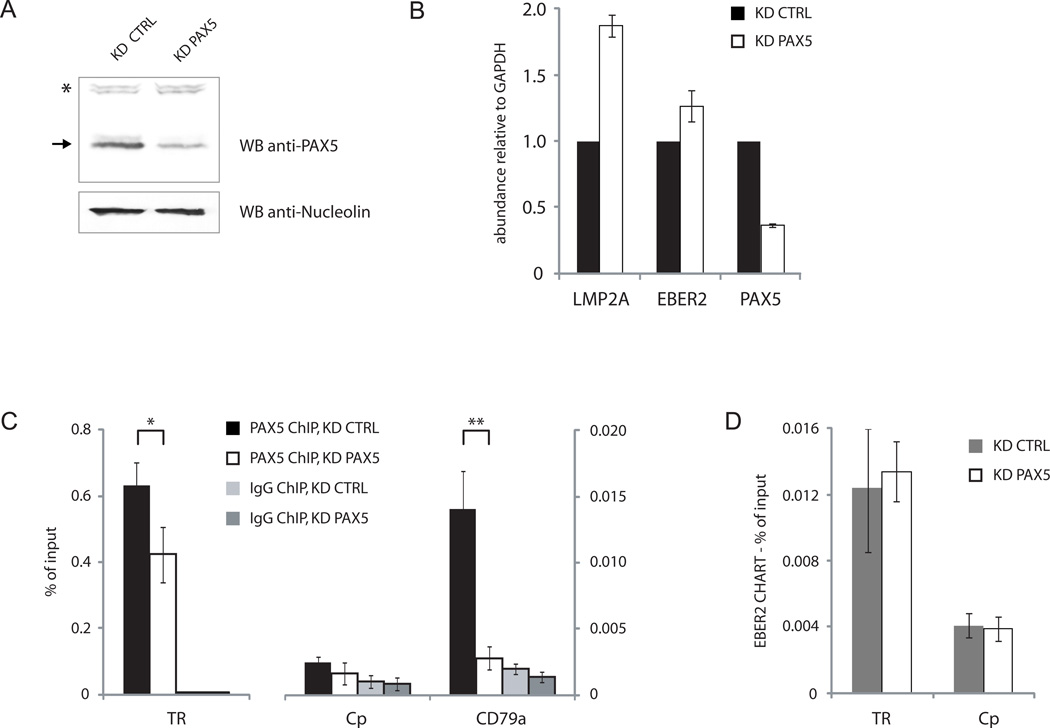
The TR sequence in EBV DNA contains two strong PAX5 consensus sequences (Arvey et al. 2012), suggesting that recruitment of the EBER2-PAX5 complex could be achieved through the DNA binding domain of PAX5. To test whether EBER2 is localized to the TRs by virtue of its interaction with PAX5, we knocked down PAX5 using lentivirally expressed short hairpin RNAs as described (Arvey et al., 2012). Efficient depletion of PAX5 in BJAB-B1 cells was confirmed by Western blot (Figure 3A); the knockdown efficiency (to ~40% of the original level) was sufficient to elicit upregulation of LMP2A as measured by RT-qPCR (Figure 3B), arguing that the transcriptional control imposed by EBER2-PAX5 was compromised under these conditions. As expected upon depletion, PAX5 localization assessed by ChIP was significantly reduced at both the TRs and the CD79a promoter region (Figure 3C). However, surprisingly, EBER2 localization at the TRs remained unaffected as measured by CHART coupled to qPCR analysis (Figure 3D). We conclude that EBER2 is required for PAX5 localization (Figure 2G), but PAX5 is dispensable for EBER2 recruitment to the TRs.
FIGURE 3.
PAX5 is dispensable for EBER2 recruitment to the TRs. (A) Knockdown efficiency of PAX5 by lentivirally expressed shRNA was determined by Western blot using anti-PAX5 antibody. Anti-Nucleolin antibody provided a loading control. (B) RT-qPCR analysis after PAX5 knockdown. (C) PAX5 ChIP-qPCR analysis after PAX5 knockdown. * p = 0.03, ** p = 0.002 (Student’s t-test; n = 3). (D) EBER2-CHART followed by qPCR analysis after PAX5 knockdown. All data represent the mean of three independent experiments +/− SD.
Since EBER2 appears to be the key recruiting entity of the EBER2-PAX5 RNP, we considered the possibility of EBER2 recruitment via an RNA-RNA interaction. The fact that EBER2 binding to the TRs as assessed by CHART was not affected when RNase H digestion preceded ASO selection argues against an RNA-DNA interaction (Figure S3A-B). On the other hand, a region within EBER2 that base pairs with an RNA transcribed from the TR region, such as the nascent transcripts of the two LMP2 isoforms, which contain the TRs in their first intron, might exist (Figure S4B). If this RNA-RNA mediated recruitment model were correct, we should be able to 1) identify the complementarity, 2) show that transcription through the TRs is required for EBER2 recruitment, and 3) obtain evidence for a physical interaction between EBER2 and the nascent TR sequence-containing transcript in vivo.
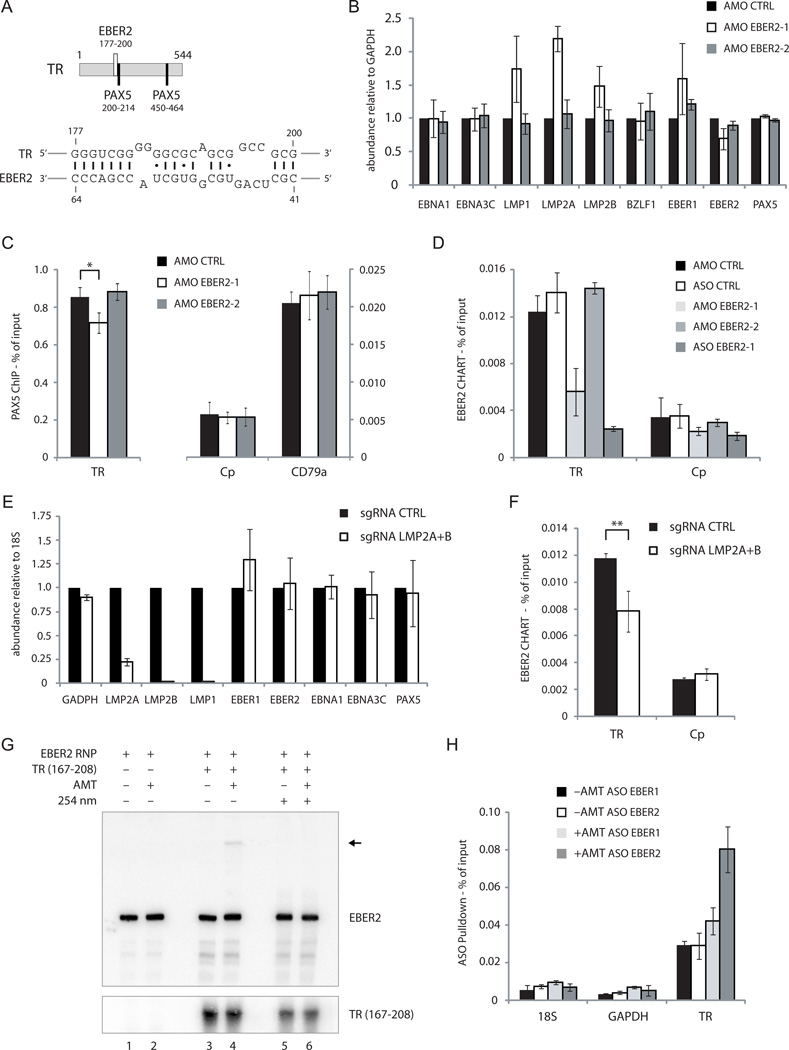
Potential base pairing between EBER2 and the EBV type II TR RNA sequence was examined using the RNAup program to search for short, stable RNA-RNA interactions (Muckstein et al., 2006). A putative 18-bp hybrid was identified with a predicted free energy of ΔG= −28.10 kcal/mol. This hybrid comprises EBV TR nts 177–200 and EBER2 nts 41–64 (Figure 4A). Intriguingly, this sequence in EBER2 coincides with one of two oligonucleotide-accessible sites identified by RNase H digestion (Figure 1B). Notably, the sequence within the TR transcript (nts 177–200) predicted to base pair with EBER2 lies adjacent to one of the PAX5 consensus sites (nts 200–214; Figure 4A). A second putative 17-bp RNA-RNA hybrid of comparable predicted stability was identified that also overlaps with the same oligonucleotide-accessible region within EBER2 (site A versus site B of EBV type II; Figure S5B-D).
FIGURE 4.
EBER2 is recruited to the TRs through base pairing with nascent RNA from the TR locus. (A) Predicted RNA-RNA interaction between EBER2 and a region within the TR (bottom). TR coordinates are also shown for the two PAX5 consensus sites (top). (B) RNA levels of several EBV genes were measured by RT-qPCR after three days of treatment with EBER2 AMO complementary to nts 35–59 (AMO EBER2-1) or nts 146–170 (AMO EBER2-2). (C) Quantification of the PAX5 ChIP at the TR, Cp, and CD79a loci after treatment with AMO EBER2-1 or EBER2-2. The control IgG ChIP data (not shown) were comparable to those in Figure 2G. * p = 0.03 (Student’s t-test, n = 3). (D) EBER2-CHART followed by qPCR analysis after AMO EBER2-1 or KD-ASO treatment. (E) RT-qPCR analysis after expressing sgRNAs targeting LMP2A and LMP2B (Figure S4B) in dCAS9-KRAB expressing BJAB-B1 cells. (F) EBER2 CHART was conducted after CRISPR/dCAS9-mediated transcriptional interference of LMP2 genes. ** p = 0.01 (Student’s t-test, n = 3). (G) EBER2 RNP containing cell lysate was incubated with an in vitro transcribed 42-nt RNA fragment from the TR region (nts 167–208) predicted to base pair with EBER2 as shown in (A). AMT was added where indicated and the reaction was exposed to long-wave UV-light (365nm). Short-wave UV-light irradiation (254 nm) was included as indicated to reverse crosslinks. RNA was isolated and Northern blotting was carried out on a denaturing urea-polyacrylamide gel, probing for EBER2 and the in vitro transcribed TR RNA. Arrow indicates EBER2 crosslinked to the TR RNA fragment. (H) EBV-positive cells were treated with long-wave UV-light in the presence or absence of AMT. EBER1 and EBER2 were selected using specific ASOs, and together with co-precipitated RNAs were reverse-transcribed for RT-qPCR analysis. The abundance of TR-sequence-containing RNA was measured in EBER1 and EBER2 selected samples. Primers detecting 18S rRNA and GAPDH mRNA were used as negative controls. All data represent the mean of three independent experiments +/− SD. See also Figure S3–5 and Table S1.
To show that the accessible region in EBER2 (nts 41–64) is indeed necessary for its recruitment to TRs, we used an antisense morpholino oligonucleotide (AMO) that anneals to this region (AMO EBER2-1; Table S1; Figure 5A, left panel, nucleotides in blue) to block the putative EBER2-TR base-pairing interaction. We analyzed mRNA levels for several EBV latent genes upon nucleofection of the 25-nt AMO EBER2-1 and observed that LMP2A, and to a lesser extent LMP1 and LMP2B, transcripts increased in level compared to a scrambled control AMO (AMO CTRL) and an AMO targeting EBER2 nts 146–170 (AMO EBER2-2; Table S1) that is not predicted to form RNA-RNA interactions (Figure 4B, Figure S3C). Moreover, AMO EBER2-1 treatment reduced PAX5 localization specifically at the TRs as measured by ChIP (Figure 4C). We were unable to use an AMO against the TR nts 177–200 to block base pairing of the nascent transcript with EBER2, as the nucleotide sequence of this AMO exhibits strong self-complementarity (data not shown). In summary, blocking the putative base-pairing region of EBER2 with an AMO results in the same phenotype as EBER2 depletion (Figure 2E and G). Importantly, CHART confirmed decreased EBER2 binding to the TRs in the presence of the AMO EBER2-1 that targets the predicted RNA-RNA interaction site (Figure 4D). These results argue that base pairing of EBER2 to the nascent LMP2A/B transcript could be instrumental in recruiting EBER2 and PAX5 to the TRs.
FIGURE 5.
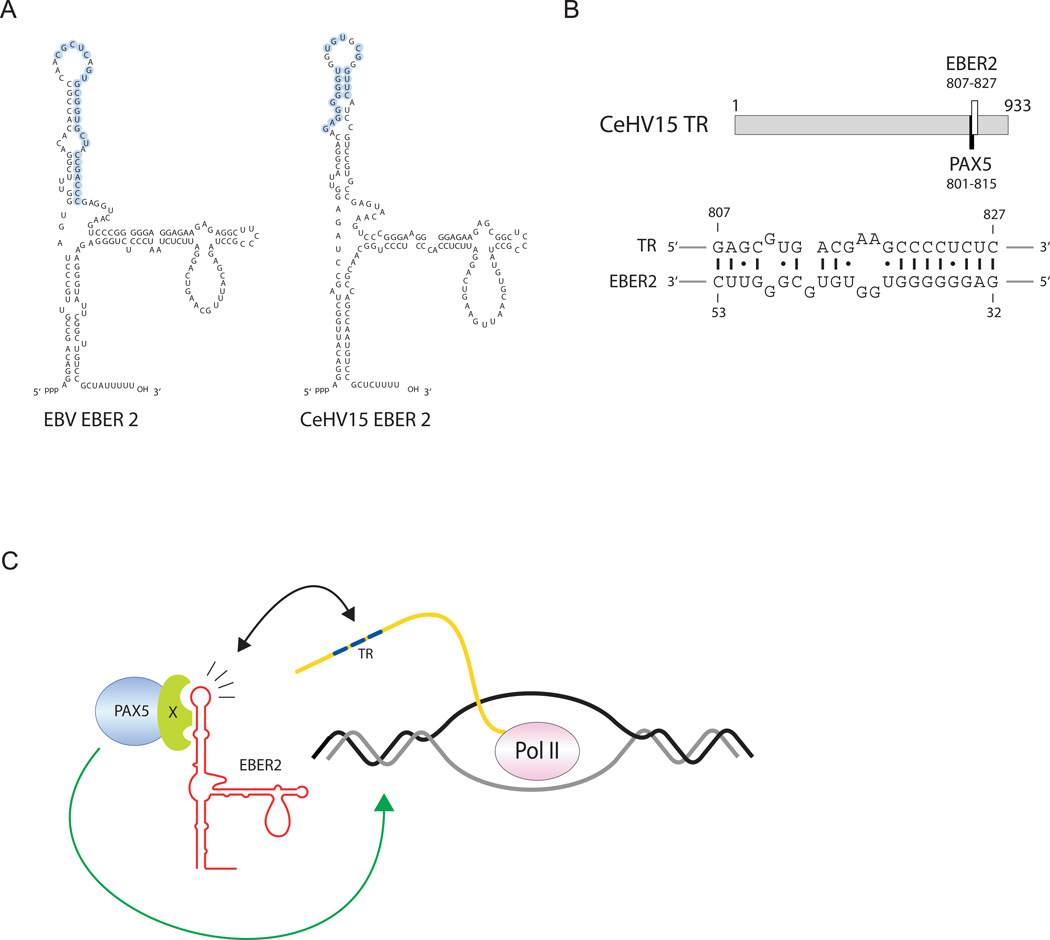
EBER2-guided recruitment of PAX5 to the TRs appears to be evolutionarily conserved in a related gamma-herpesvirus. (A) Secondary structure models of EBV EBER2 and the EBER2 homolog of rhesus LCV (CeHV15). Nucleotides predicted to base pair with TR RNA are circled in blue. (B) Relative position and coordinates within the TR, as well as the PAX5 consensus site, are shown (top). The predicted RNA-RNA interaction between CeHV15 EBER2 and the TR RNA is shown at the bottom. (C) Model for complementary base-pairing mediated recruitment of EBER2-PAX5 RNP to the TR region. See also Figure S3.
To demonstrate that transcription through the TRs is necessary for EBER2 recruitment, we interfered with LMP2 gene expression, which generates nascent transcripts containing TR sequences within the first intron (Figure 5C). We used CRISPR-mediated transcriptional interference by the catalytically inactive dCas9 protein to specifically silence both LMP2 isoforms (Gilbert et al., 2013). We generated stable dCas9-KRAB expressing BJAB-B1 cells and identified potent single guide RNAs (sgRNAs) against LMP2A and LMP2B (Figure 4E, Figure S4A-C). LMP1 expression was collaterally silenced by this approach, probably because its promoter is located close to the LMP2B transcription start site. Inhibiting the expression of nascent transcripts containing TR sequences by silencing both LMP2 isoforms resulted in decreased EBER2 binding to the TRs as determined by CHART (Figure 4F). Simultaneous usage of sgRNAs against both LMP2 isoforms was necessary to observe decreased EBER2 binding, as inhibiting one isoform alone did not affect EBER2 localization to the TRs (Figure S4D and data not shown).
Finally, to confirm in vivo the predicted base-pairing interaction between EBER2 and TR sequence-containing RNA, we used the psoralen derivative aminomethyltrioxsalen (AMT). AMT preferentially crosslinks pyrimidine bases on opposite strands of double-stranded RNA after irradiation with 365 nm UV-light; 254nm UV-light irradiation reverses the crosslinks (Cimino et al., 1985). First, we ascertained that AMT is able to crosslink the predicted interaction sites between EBER2 and nascent RNA containing the TR sequence. We in vitro transcribed a 42-nt RNA from the TR region (Table S1) that contains the sequence predicted to base pair with EBER2 and added it to an EBER2 RNP containing cell lysate. Only in the presence of AMT and the in vitro transcribed TR RNA did we observe a higher molecular weight band in an EBER2 Northern blot (Figure 4G, lane 4, arrow). This band disappeared when crosslinks were reversed by irradiating with 254 nm UV-light (Figure 4G, lane 6), indicating that the predicted base pairs form in vitro. In vitro crosslinking was also observed with the second EBER2-TR interaction site (Figure S5E), suggesting that both RNA-RNA interactions could contribute to association in vivo. To show that base-pairing interaction does occur in vivo, we enriched EBER2, and EBER1 as a control, using ASO beads under denaturing conditions from a lysate of cells after in vivo psoralen crosslinking. EBERs together with crosslinked RNAs were eluted from the ASO beads with tetraethylammonium chloride-containing buffer to minimize background (Figure S5A). TR RNA was enriched by EBER2 compared to EBER1 ASO beads only after psoralen crosslinking as measured by RT-qPCR (Figure 4H). No enrichment was observed for 18S rRNA or GAPDH mRNA. Together, these results argue that EBER2 base pairs with TR sequence-containing nascent transcripts of the LMP2A and 2B genes in vivo.
EBER2’s interaction with nascent RNA is evolutionarily conserved
Of the many EBV-related primate LCVs (Lacoste et al., 2010), complete genome sequences are available only for EBV (type I and type II), for the rhesus (Cercopithecine herpesvirus 15, CeHV15) and for the marmoset LCV (Callitrichine herpesvirus 3) (Rivailler et al., 2002a; Rivailler et al., 2002b). The genome of the last has apparently lost its EBER2 gene, while CeHV15 retains an EBER2 homolog. Even though only moderate sequence conservation (65%) is exhibited, CeHV15 EBER2 can be modeled to fold into a structure that is almost identical to that of EBV EBER2 (Figure 5A). The CeHV15 TR, on the other hand, has no obvious sequence similarity to the EBV TR sequence except for high overall GC content and a tandem repeat organization (Rivailler et al., 2002b); the repeat unit is considerably longer (933 bp) and contains only a single PAX5 consensus sequence (Figure 5B, top).
Using the RNAup program, we searched for an RNA-RNA interaction between CeHV15 EBER2 and its TR sequence. Only one stable hybrid was predicted with a free energy of ΔG = −25.60 kcal/mol (Figure 5B, bottom). Strikingly, the sequence within the CeHV15 EBER2 homolog predicted to base pair with the TR is in the same relative location as that in EBV EBER2 (Figure 5A, nucleotides in blue). Furthermore, despite great sequence divergence, the region within CeHV15 TR predicted to base pair with its EBER2 homolog overlaps the PAX5 consensus site, similar to its position in the EBV TR (compare Figure 5B and Figure 4A). Thus, the EBER2 guide function of PAX5 to the TRs appears to be evolutionarily conserved in the rhesus virus and possibly other yet unsequenced LCVs.
EBER2 knockdown affects viral lytic replication
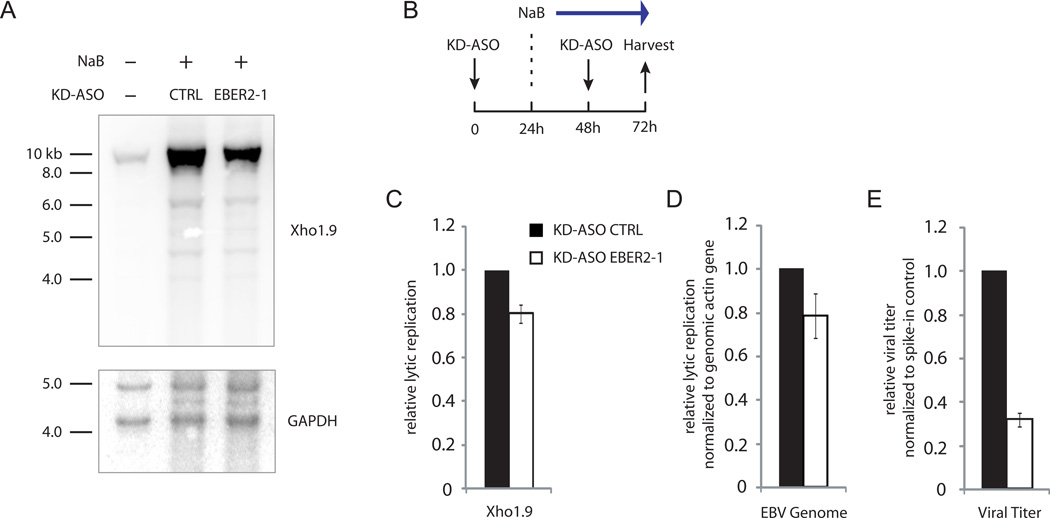
Given the localization of EBER2 at the TRs, which during the lytic cycle are the sites of viral genome linearization upon packaging into capsids (Zimmermann and Hammerschmidt, 1995), we asked whether viral lytic replication might be affected by EBER2 depletion. We treated the replication-permissive EBV-positive cell line HH514-16 with sodium butyrate (NaB) to induce lytic replication in combination with EBER2 KD-ASO nucleofection (Figure 6B, Figure S6A) (Ragoczy et al., 1998). Genomic DNA was isolated and subjected to Southern blot analysis using the Xho1.9 probe, which covers a unique sequence adjacent to the TR region, to quantify lytic replication (Figure S6B) (Raab-Traub and Flynn, 1986). After EBER2 depletion, lytic replication was decreased as analyzed by Southern blot and qPCR (Figure 6A, C and D). The viral titer in the culture medium was reduced to an even greater extent (Figure 6E). Depletion of PAX5 consistently resulted in comparable decreased lytic replication (Figure S6C-E), supporting the notion that EBER2 and PAX5 act in concert. NaB promotes the expression of Zebra, an EBV transcription activator encoded by the BZLF1 gene that acts to induce lytic replication (Miller et al., 2007). EBER2 knockdown did not affect Zebra expression (Figure S6F), ruling out the possibility that the decreased lytic replication was due to a lower level of Zebra. Interestingly, the decrease in viral replication was restricted to lytic replication, since latent replication, measured in the absence of NaB induction (see Figure 2C), was unaffected upon EBER2 knockdown (Figure S6G).
FIGURE 6.
EBER2 depletion results in decreased viral lytic replication. (A) Viral lytic replication after KD-ASO mediated EBER2 depletion was measured by Southern blot analysis using the Xho1.9 probe. The same blot was probed for the cellular GAPDH locus as a loading control. (B) Experimental outline for viral lytic induction by sodium butyrate (NaB) in combination with EBER2 knockdown by KD-ASO. (C) Quantification of three independent experiments as shown in (A). (D) Viral lytic replication was measured by qPCR analysis amplifying the EBV dyad symmetry DNA region normalized to the cellular actin gene. (E) Relative viral titer in supernatant was measured by qPCR normalized to spike-in control. All data represent the mean of three independent experiments +/− SD. See also Figure S6 and Table S1.
Discussion
Using CHART we identified EBER2 as localized to the TRs of the latent EBV genome. We were unable to examine EBER1 localization by CHART because no region in EBER1 is accessible for hybridization with an ASO (Lee et al., 2012). A potential function of EBER2 at the TRs was suggested by its chromatin co-localization and interaction with the B-cell master regulator PAX5. Even though two strong PAX5 consensus sequences are present in each TR unit, recruitment of the EBER2-PAX5 RNP does not appear to be achieved by direct PAX5 binding to DNA, but is greatly facilitated by nucleotide sequence information contributed by EBER2 (Figure 5C). Perhaps because of the degenerate nature of the PAX5 consensus sequence (Figure S2D), the EBER2 RNP is required for efficient recruitment to the DNA target site (Figure 2). Alternatively, the EBER2 RNP may be required to clear adjacent chromatin of interfering factor(s) or to stabilize PAX5-DNA binding. We identified a base-pairing interaction between EBER2 and nascent transcripts from the TR regions that could provide such enhanced targeting specificity (Figure 4A). Indeed, the process of transcription through the TR regions per se might be integral to the recruitment mechanism by opening the chromatin conformation and thus facilitating accessibility of the PAX5-EBER2 RNP to its target site.
We identified PAX5 as a novel EBER2 interacting protein, prompted by the observation that this transcription factor and the viral noncoding RNA co-localize at the TRs (Figure 1). The interaction appears to be indirect, based on the negative results of EMSAs and UV-crosslinking experiments (Figure S2C-G). We are currently attempting to further analyze the EBER2-PAX5 RNP, particularly focusing on the factor that bridges EBER2 and PAX5. Depletion of either EBER2 or PAX5 exhibits overlapping phenotypes, such as the upregulation of LMP genes (Figure 2E), suggesting a functional as well as physical interaction of the two factors. PAX5 depletion was reported to have a broader effect on EBV latent gene expression (Arvey et al., 2012) than we observe here, which might be explained by the different time points and methods used. While Arvey et al. achieved PAX5 knockdown by lentiviral expression of shRNAs and gene expression changes were examined after 5 days, we chose an earlier time point (3 days) for analyzing gene expression upon EBER2 depletion by KD-ASOs in an effort to exclude secondary and pleiotropic effects of long-term PAX5 knockdown.
Our attempts to recapitulate EBER2-PAX5 guided transcriptional silencing at EBV TRs in heterologous reporter systems were unsuccessful. When the entire LMP2A locus including the TRs in its first intron was cloned into a vector and expressed together with EBER2 and PAX5 in B lymphocytes, no effect on LMP2A expression was observed (Figure S3D). Similarly, incorporating TRs into the 3′ end of a luciferase reporter gene was also unresponsive to the presence of EBER2 and PAX5 (Figure S3E). Apparently, a nascent transcript from the TRs alone is not sufficient for PAX5 recruitment through EBER2, and other factors, possibly DNA elements, are necessary to establish a silenced chromatin architecture.
PAX5 at the TRs appears to exert a function other than acting as a classical transcription activator or a repressor, as normally found at promoters. The repeat organization of the TRs might be a crucial aspect of the PAX5 localization mechanism and perhaps also of function. Indeed, the related transcription factors, Pax3 and Pax9, have been reported to restrict RNA output from mouse satellite repeat sequences by binding and recruiting histone methyl transferases to silence repetitive DNA (Bulut-Karslioglu et al., 2012). In another uninfected cellular context, Pax5 action has been reported to regulate the immunoglobulin heavy chain (Igh) locus during VDJ recombination in pro-B cells (Ebert et al., 2011). Parallels include: 1) repeat regions bound by Pax5, the so-called Pax5-activated intergenic repeat elements (PAIRs) of which 14 are interspersed in the Igh locus, and 2) strikingly, the existence of a noncoding RNA expressed from the Igh locus. Expression of the RNA coincides with Pax5 binding to the PAIRs, whereas at later times in B-cell development when the RNA ceases to be made, Pax5 localization is no longer detected. It is tempting to speculate that the Igh noncoding transcript might contribute to Pax5 recruitment similarly to the nascent transcript emanating from the TR regions of EBV. Furthermore, Pax5 induces chromatin condensation of the Igh locus (Fuxa et al., 2004). If an analogous chromatin contraction occurs at the TRs of the EBV genome, transcriptional upregulation of LMP genes following perturbation of the EBER2-PAX5 mediated control mechanism might be explained by a looser chromatin conformation that facilitates transcription through the region.
Consistent with the possibility that genome organization is regulated by the EBER2-PAX5 interaction, EBER2 depletion does not result in immediate transcriptional upregulation of EBV genes nearest to its binding site; changes become apparent only after 3 days of knockdown. This observation suggests that the genome organization at the TRs, once established, remains stable unless the correct organization cannot be resumed following genome replication and/or dilution of regulating factors by knockdown. Importantly, we demonstrate that EBER2 depletion affects viral lytic replication and propose that decreased lytic replication might be a consequence of improper genome organization that hinders efficient replication. Perhaps latent replication is not affected because the TRs contribute differently to the replication of EBV episomes compared to the production of linear packaged virion DNA. It is of course possible that there are additional consequences of EBER2 knockdown that we have not assessed in this study. One such possibility, given the fact that both EBER2 and lytic replication have been implicated in promoting oncogenicity (Katsumura et al., 2011; Ma et al., 2011), is an interplay between the function of EBER2 at the TRs and the consequences of lytic replication on tumor formation. An indication that EBER2 could have function(s) in addition to recruiting PAX5 to the TRs stems from the fact that more EBER2 molecules are present than complementary TR binding sites; in an infected cell, there are 2.5×105 EBER2 molecules and up to 50 EBV episomes, each containing up to 20 TRs, although each TR probably harbors multiple nascent transcripts. On the other hand, the overabundance of EBER2 molecules compared to the number of TR binding sites during latency could be necessary to accommodate the massive increase in EBV genome copy number, and hence TR binding sites, occurring during viral lytic replication.
In recent years, more and more long noncoding RNAs (lncRNAs), arbitrarily defined as >200 nt in size, have been shown to fulfill a diversity of cellular functions (Cech and Steitz, 2014). In addition to post-transcriptional regulation, a common theme is the interaction of lncRNAs with chromatin-modulating factors to control gene expression (Huarte et al., 2010; Nagano et al., 2008; Tsai et al., 2010; Zhao et al., 2008). Thus, lncRNAs have been proposed in theory to act as targeting guides for effector proteins by base pairing with specific chromatin sites via RNA-RNA interactions, RNA-DNA interactions (Gilbert et al., 2013), or triplex formation (Schmitz et al., 2010). RNA-RNA interactions are the best-supported interaction mode experimentally for noncoding RNAs smaller than 200 nt, as exemplified by small nucleolar RNAs acting in RNA modification, small nuclear RNAs in pre-mRNA splicing, or microRNAs in targeting mRNAs (Kim et al., 2009; Watkins and Bohnsack, 2012; Will and Luhrmann, 2011). A guide function for targeting specific sites on chromatin thus far has been ascribed to tiny RNAs only, such as piRNA-mediated recruitment of PIWI in Drosophila, siRNA-mediated centromeric silencing in yeast, and siRNA-directed DNA methylation in plants (Lejeune et al., 2010; Malone and Hannon, 2009; Matzke et al., 2007). All previously reported lncRNAs appear to fulfill an architectural scaffolding function, often with chromatin regulating proteins. Here, for the first time, we provide evidence for a base-pairing interaction of a trans-acting moderately-sized noncoding RNA, EBER2, which facilitates the recruitment of an associated transcription factor to chromatin target sites.
The ability of EBER2 to help recruit PAX5 to the TRs appears to be evolutionarily conserved not only in both type I and type II EBV (Figure S5B-E), but also in a related rhesus LCV. Marmoset LCV, a virus that infects a new world primate, has lost an EBER2 homolog, but nonetheless retains a strong PAX5 consensus site within its TR (Rivailler et al., 2002a), suggesting that PAX5 binding occurs. This raises the question of whether PAX5 is recruited by an analogous mechanism involving a yet unidentified noncoding RNA, which has replaced EBER2, or whether a compensatory mechanism not involving an RNA-RNA interaction mediates recruitment of PAX5 in marmoset LCV. A better understanding of the precise EBER2-PAX5 RNP composition will be essential to distinguish between the two possibilities. Another open question is whether EBER2 is recruited to TRs in EBV-infected cells that do not express the B cell specific factor PAX5, such as nasopharyngeal carcinoma (NPC) cells, which are epithelial. As NPCs exhibit latency II (Rowe et al., 2009), characterized by robust expression of LMP1 and LMP2 genes, as well as EBER2, EBER2-PAX5 mediated transcription inhibition is unlikely to occur in these cells. Similarly, the questions of whether and how PAX5 is recruited to the TRs in EBV strains carrying a deletion of the EBER2 gene also remain to be answered.
Experimental Procedures
CHART assay
Endogenous RNase H cleavage assays were performed as described in Lee et al. (2012). CHART was carried out as described (Simon, 2013) with minor modifications. CHART-seq data was deposited in the Sequence Read Archive under accession no. SRR1640963. For detailed protocol, see Supplementary Information.
EBER2 and PAX5 knockdown
2.5×106 BJAB-B1 cells were nucleofected with 10 µl of 100 mM KD-ASO/AMO stock solution in SF solution with program EN-150 using the Lonza 4D-Nucleofector System. HH514-16 cells were nucleofected using the Lonza 2b Device with solution V and program A-023. On the day following nucleofection, cells were separated from debris using Lymphocyte Separation Medium (Corning Cellgro) according to the manufacturer’s instructions. For KD-ASO and AMO sequence information, see Table S1. KD-ASOs were designed (Ideue et al., 2009) with DNA bases flanked by 2′-O-methyl bases and consisting of a phosphorothioate backbone (for increased stability) to induce cleavage by endogenous RNase H. RNAi against PAX5 was performed by lentiviral shRNA expression using MISSION shRNA clones TRC0000016061 and TRC0000016062 (SIGMA) as described (Arvey et al., 2012; Cozma et al., 2007). Lentiviruses were produced as described (Lee et al., 2012). Cells were cultured under puromycin selection 1 day after infection and harvested 4 days post infection, as a decrease in proliferation rate became apparent at this time point.
Psoralen crosslinking of RNAs
A 42-nt or 36-nt RNA within the TR region (nts 167–208 and 74–109 of EBV type II, respectively) predicted to base pair with EBER2 was in vitro transcribed with T7 polymerase from an oligonucleotide template (see Supplementary Table 1 for sequence). For psoralen crosslinking of EBER2 RNP to the in vitro transcribed TR fragment, nuclei were isolated by lysing 107 BJAB-B1 cells in 10 mM Tris pH 8.0, 0.32 M sucrose, 3 mM CaCl, 0.1 mM EDTA, 0.1% NP-40 and resuspended in 100 µl of 10 mM HEPES pH 7.4, 150 mM KCl, 5 mM MgCl2, 0.2 mM DTT, 10 % glycerol, 0.5 % NP-40 to generate nuclear extract. The lysate was cleared by a 3-min centrifugation step at full speed in a table-top centrifuge. 200 ng of in vitro transcribed TR fragment was added to 10 µl of nuclear extract in presence of 40 µg/ml aminomethyltrioxsalen (AMT), 0.2 µg tRNA, 40 U RNase inhibitor, and incubated 30 min at RT before the reaction was irradiated for 30 min on ice covered with a 2 mm-thick glass plate from a distance of 2.5 cm with a handheld 365 nm-UV lamp. Extracts were treated with 254 nm UV-irradiation for 10 min on ice where applicable. After crosslinking, RNA was isolated with TRIZOL and subjected to Northern blot analysis.
For in vivo crosslinking of intact cells, 2×107 cells were resuspended in 1 ml of growth medium containing 50 µg/ml AMT, incubated for 5 min at 37°C before chilling the cells on ice, and irradiated with UV-light as described above. Cells were washed with PBS, and RNA was isolated with TRIZOL and DNase-treated. 20 µg total RNA was heated in 100 µl TE buffer at 95°C for 3 min and chilled on ice before 50 µl Denaturant buffer and 150 µl 2x Hybridization buffer were added (see CHART protocol above). 25 µl of biotinylated (EBER1 or EBER2) ASO-streptavidin Dynabeads were added and incubated overnight at RT. Beads were washed three times with CHART wash buffer, once with 2.4 M tetraethylammonium chloride (TEACl) at 25°C, and bound RNAs were eluted with 2.4 M TEACl for 5 min at 40°C followed by phenol-chloroform extraction prior to Northern blot or RT-qPCR analyses.
Supplementary Material
Acknowledgments
We thank Drs. George Miller (Yale), Ayman El-Guindy (Yale), and Nancy Raab-Traub (University of North Carolina) for EBV reagents and discussions; Matthew Simon (Yale), Paul Lieberman (Wistar), Tom Cech (UC Boulder), Thomas Jenuwein (MPI Freiburg), Sandra Weller (UConn Health), and Joel Rozowsky (Yale) for advice; and Jessica Brown, Kazimierz Tycowski, and Mingyi Xie for helpful comments. This work was supported by grant CA16038 from the NCI. JAS is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Information
Supplementary Information includes Extended Experimental Procedures, seven figures, and one table and can be found with this article online.
References
- Arvey A, Tempera I, Tsai K, Chen HS, Tikhmyanova N, Klichinsky M, Leslie C, Lieberman PM. An atlas of the Epstein-Barr virus transcriptome and epigenome reveals host-virus regulatory interactions. Cell Host Microbe. 2012;12:233–245. doi: 10.1016/j.chom.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NA, Liu CR, Wang YF, Garcia CR. B-cell lymphoproliferation and lymphomagenesis are associated with clonotypic intracellular terminal regions of the Epstein-Barr virus. J Virol. 1988;62:962–969. doi: 10.1128/jvi.62.3.962-969.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulut-Karslioglu A, Perrera V, Scaranaro M, de la Rosa-Velazquez IA, van de Nobelen S, Shukeir N, Popow J, Gerle B, Opravil S, Pagani M, et al. A transcription factor-based mechanism for mouse heterochromatin formation. Nat Struct Mol Biol. 2012;19:1023–1030. doi: 10.1038/nsmb.2382. [DOI] [PubMed] [Google Scholar]
- Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157:77–94. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Chiu SH, Wu MC, Wu CC, Chen YC, Lin SF, Hsu JT, Yang CS, Tsai CH, Takada K, Chen MR, Chen JY. Epstein-Barr virus BALF3 has nuclease activity and mediates mature virion production during the lytic cycle. J Virol. 2014;88:4962–4975. doi: 10.1128/JVI.00063-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino GD, Gamper HB, Isaacs ST, Hearst JE. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- Cozma D, Yu D, Hodawadekar S, Azvolinsky A, Grande S, Tobias JW, Metzgar MH, Paterson J, Erikson J, Marafioti T, et al. B cell activator PAX5 promotes lymphomagenesis through stimulation of B cell receptor signaling. J Clin Invest. 2007;117:2602–2610. doi: 10.1172/JCI30842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert A, McManus S, Tagoh H, Medvedovic J, Salvagiotto G, Novatchkova M, Tamir I, Sommer A, Jaritz M, Busslinger M. The distal V(H) gene cluster of the Igh locus contains distinct regulatory elements with Pax5 transcription factor-dependent activity in pro-B cells. Immunity. 2011;34:175–187. doi: 10.1016/j.immuni.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Feederle R, Shannon-Lowe C, Baldwin G, Delecluse HJ. Defective infectious particles and rare packaged genomes produced by cells carrying terminal-repeat-negative Epstein-Barr virus. J Virol. 2005;79:7641–7647. doi: 10.1128/JVI.79.12.7641-7647.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fok V, Friend K, Steitz JA. Epstein-Barr virus noncoding RNAs are confined to the nucleus, whereas their partner, the human La protein, undergoes nucleocytoplasmic shuttling. J Cell Biol. 2006;173:319–325. doi: 10.1083/jcb.200601026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxa M, Skok J, Souabni A, Salvagiotto G, Roldan E, Busslinger M. Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev. 2004;18:411–422. doi: 10.1101/gad.291504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorovic G, Bosshard R, Karstegl CE, White RE, Pattle S, Chiang AK, Dittrich-Breiholz O, Kracht M, Russ R, Farrell PJ. Cellular gene expression that correlates with EBER expression in Epstein-Barr Virus-infected lymphoblastoid cell lines. J Virol. 2011;85:3535–3545. doi: 10.1128/JVI.02086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greifenegger N, Jager M, Kunz-Schughart LA, Wolf H, Schwarzmann F. Epstein-Barr virus small RNA (EBER) genes: differential regulation during lytic viral replication. J Virol. 1998;72:9323–9328. doi: 10.1128/jvi.72.11.9323-9328.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmani JL, Davis CI, Ruf IK. Growth-promoting properties of Epstein-Barr virus EBER-1 RNA correlate with ribosomal protein L22 binding. J Virol. 2009;83:9844–9853. doi: 10.1128/JVI.01014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe JG, Shu MD. Isolation and characterization of the genes for two small RNAs of herpesvirus papio and their comparison with Epstein-Barr virus-encoded EBER RNAs. J Virol. 1988;62:2790–2798. doi: 10.1128/jvi.62.8.2790-2798.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideue T, Hino K, Kitao S, Yokoi T, Hirose T. Efficient oligonucleotide-mediated degradation of nuclear noncoding RNAs in mammalian cultured cells. RNA. 2009;15:1578–1587. doi: 10.1261/rna.1657609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumura KR, Maruo S, Takada K. EBV lytic infection enhances transformation of B-lymphocytes infected with EBV in the presence of T-lymphocytes. J Med Virol. 2011;84:504–510. doi: 10.1002/jmv.23208. [DOI] [PubMed] [Google Scholar]
- Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- Komano J, Maruo S, Kurozumi K, Oda T, Takada K. Oncogenic role of Epstein-Barr virus-encoded RNAs in Burkitt's lymphoma cell line Akata. J Virol. 1999;73:9827–9831. doi: 10.1128/jvi.73.12.9827-9831.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste V, Lavergne A, de Thoisy B, Pouliquen JF, Gessain A. Genetic diversity and molecular evolution of human and non-human primate Gammaherpesvirinae. Infect Genet Evol. 2010;10:1–13. doi: 10.1016/j.meegid.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Lee N, Pimienta G, Steitz JA. AUF1/hnRNP D is a novel protein partner of the EBER1 noncoding RNA of Epstein-Barr virus. RNA. 2012;18:2073–2082. doi: 10.1261/rna.034900.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune E, Bayne EH, Allshire RC. On the connection between RNAi and heterochromatin at centromeres. Cold Spring Harb Symp Quant Biol. 2010;75:275–283. doi: 10.1101/sqb.2010.75.024. [DOI] [PubMed] [Google Scholar]
- Lerner MR, Andrews NC, Miller G, Steitz JA. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1981;78:805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T, Adams A, Bjursell G, Bornkamm GW, Kaschka-Dierich C, Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976;102:511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- Ma SD, Hegde S, Young KH, Sullivan R, Rajesh D, Zhou Y, Jankowska-Gan E, Burlingham WJ, Sun X, Gulley ML, et al. A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. J Virol. 2011;85:165–177. doi: 10.1128/JVI.01512-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone CD, Hannon GJ. Molecular evolution of piRNA and transposon control pathways in Drosophila. Cold Spring Harb Symp Quant Biol. 2009;74:225–234. doi: 10.1101/sqb.2009.74.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke M, Kanno T, Huettel B, Daxinger L, Matzke AJ. Targets of RNA-directed DNA methylation. Curr Opin Plant Biol. 2007;10:512–519. doi: 10.1016/j.pbi.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Medvedovic J, Ebert A, Tagoh H, Busslinger M. Pax5: a master regulator of B cell development and leukemogenesis. Adv Immunol. 2011;111:179–206. doi: 10.1016/B978-0-12-385991-4.00005-2. [DOI] [PubMed] [Google Scholar]
- Miller CL, Lee JH, Kieff E, Longnecker R. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc Natl Acad Sci U S A. 1994;91:772–776. doi: 10.1073/pnas.91.2.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, El-Guindy A, Countryman J, Ye J, Gradoville L. Lytic cycle switches of oncogenic human gammaherpesviruses. Adv Cancer Res. 2007;97:81–109. doi: 10.1016/S0065-230X(06)97004-3. [DOI] [PubMed] [Google Scholar]
- Moss WN, Steitz JA. Genome-wide analyses of Epstein-Barr virus reveal conserved RNA structures and a novel stable intronic sequence RNA. BMC Genomics. 2013;14:543. doi: 10.1186/1471-2164-14-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muckstein U, Tafer H, Hackermuller J, Bernhart SH, Stadler PF, Hofacker IL. Thermodynamics of RNA-RNA binding. Bioinformatics. 2006;22:1177–1182. doi: 10.1093/bioinformatics/btl024. [DOI] [PubMed] [Google Scholar]
- Nagano T, Mitchell JA, Sanz LA, Pauler FM, Ferguson-Smith AC, Feil R, Fraser P. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science. 2008;322:1717–1720. doi: 10.1126/science.1163802. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N, Flynn K. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell. 1986;47:883–889. doi: 10.1016/0092-8674(86)90803-2. [DOI] [PubMed] [Google Scholar]
- Ragoczy T, Heston L, Miller G. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J Virol. 1998;72:7978–7984. doi: 10.1128/jvi.72.10.7978-7984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repellin CE, Tsimbouri PM, Philbey AW, Wilson JB. Lymphoid hyperplasia and lymphoma in transgenic mice expressing the small non-coding RNA, EBER1 of Epstein-Barr virus. PLoS One. 2010;5:e9092. doi: 10.1371/journal.pone.0009092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revilla IDR, Bilic I, Vilagos B, Tagoh H, Ebert A, Tamir IM, Smeenk L, Trupke J, Sommer A, Jaritz M, Busslinger M. The B-cell identity factor Pax5 regulates distinct transcriptional programmes in early and late B lymphopoiesis. EMBO J. 2012;31:3130–3146. doi: 10.1038/emboj.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivailler P, Cho YG, Wang F. Complete genomic sequence of an Epstein-Barr virus-related herpesvirus naturally infecting a new world primate: a defining point in the evolution of oncogenic lymphocryptoviruses. J Virol. 2002a;76:12055–12068. doi: 10.1128/JVI.76.23.12055-12068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivailler P, Jiang H, Cho YG, Quink C, Wang F. Complete nucleotide sequence of the rhesus lymphocryptovirus: genetic validation for an Epstein-Barr virus animal model. J Virol. 2002b;76:421–426. doi: 10.1128/JVI.76.1.421-426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovedo M, Longnecker R. Epstein-Barr virus latent membrane protein 2B (LMP2B) modulates LMP2A activity. J Virol. 2007;81:84–94. doi: 10.1128/JVI.01302-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M, Kelly GL, Bell AI, Rickinson AB. Burkitt's lymphoma: the Rosetta Stone deciphering Epstein-Barr virus biology. Semin Cancer Biol. 2009;19:377–388. doi: 10.1016/j.semcancer.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz KM, Mayer C, Postepska A, Grummt I. Interaction of noncoding RNA with the rDNA promoter mediates recruitment of DNMT3b and silencing of rRNA genes. Genes Dev. 2010;24:2264–2269. doi: 10.1101/gad.590910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MD. Capture hybridization analysis of RNA targets (CHART) Curr Protoc Mol Biol. 2013:25. doi: 10.1002/0471142727.mb2125s101. Chapter 21, Unit 21. [DOI] [PubMed] [Google Scholar]
- Simon MD, Wang CI, Kharchenko PV, West JA, Chapman BA, Alekseyenko AA, Borowsky ML, Kuroda MI, Kingston RE. The genomic binding sites of a noncoding RNA. Proc Natl Acad Sci U S A. 2011;108:20497–20502. doi: 10.1073/pnas.1113536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B, Phelps M, Domoradzki J. Epstein-Barr virus DNA is amplified in transformed lymphocytes. J Virol. 1979;31:590–595. doi: 10.1128/jvi.31.3.590-595.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S, Tomkinson B, Kieff E. Recombinant Epstein-Barr virus with small RNA (EBER) genes deleted transforms lymphocytes and replicates in vitro. Proc Natl Acad Sci U S A. 1991;88:1546–1550. doi: 10.1073/pnas.88.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toczyski DP, Matera AG, Ward DC, Steitz JA. The Epstein-Barr virus (EBV) small RNA EBER1 binds and relocalizes ribosomal protein L22 in EBV-infected human B lymphocytes. Proc Natl Acad Sci U S A. 1994;91:3463–3467. doi: 10.1073/pnas.91.8.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins NJ, Bohnsack MT. The box C/D and H/ACA snoRNPs: key players in the modification, processing and the dynamic folding of ribosomal RNA. Wiley Interdiscip Rev RNA. 2012;3:397–414. doi: 10.1002/wrna.117. [DOI] [PubMed] [Google Scholar]
- Will CL, Luhrmann R. Spliceosome structure and function. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima M, Kanda T, Takada K. Critical role of Epstein-Barr Virus (EBV)-encoded RNA in efficient EBV-induced B-lymphocyte growth transformation. J Virol. 2005;79:4298–4307. doi: 10.1128/JVI.79.7.4298-4307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322:750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann J, Hammerschmidt W. Structure and role of the terminal repeats of Epstein-Barr virus in processing and packaging of virion DNA. J Virol. 1995;69:3147–3155. doi: 10.1128/jvi.69.5.3147-3155.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.