Abstract
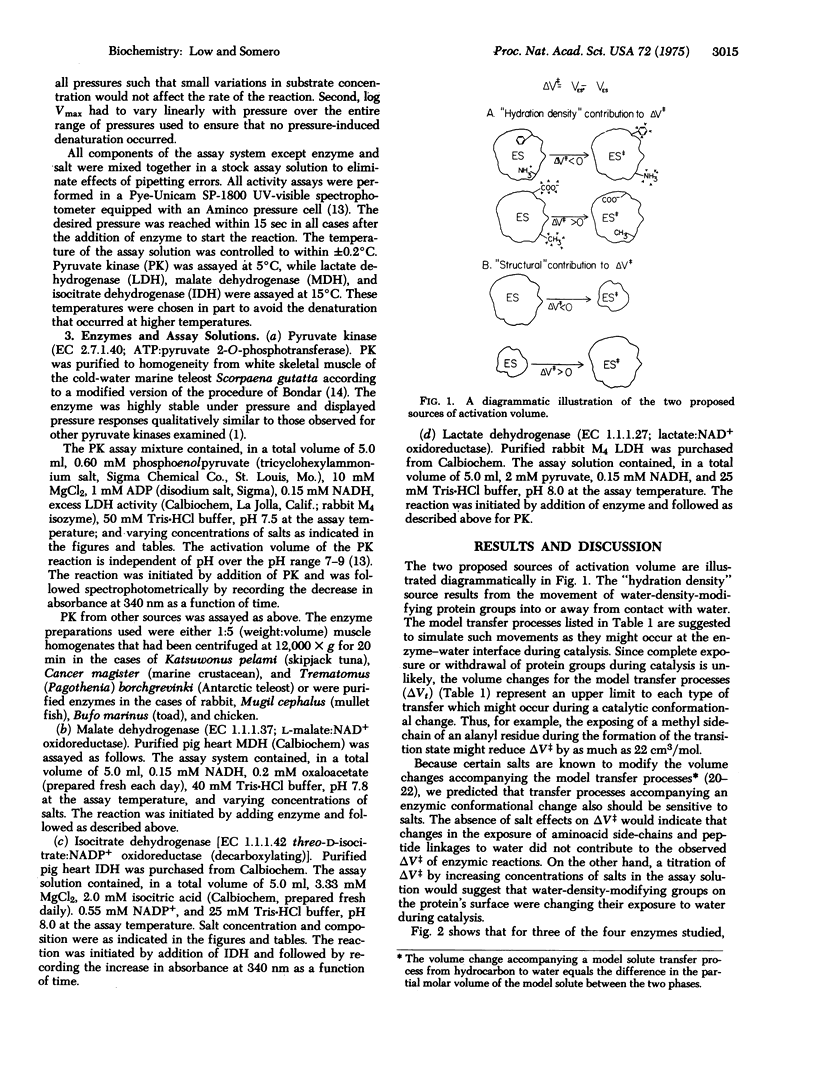
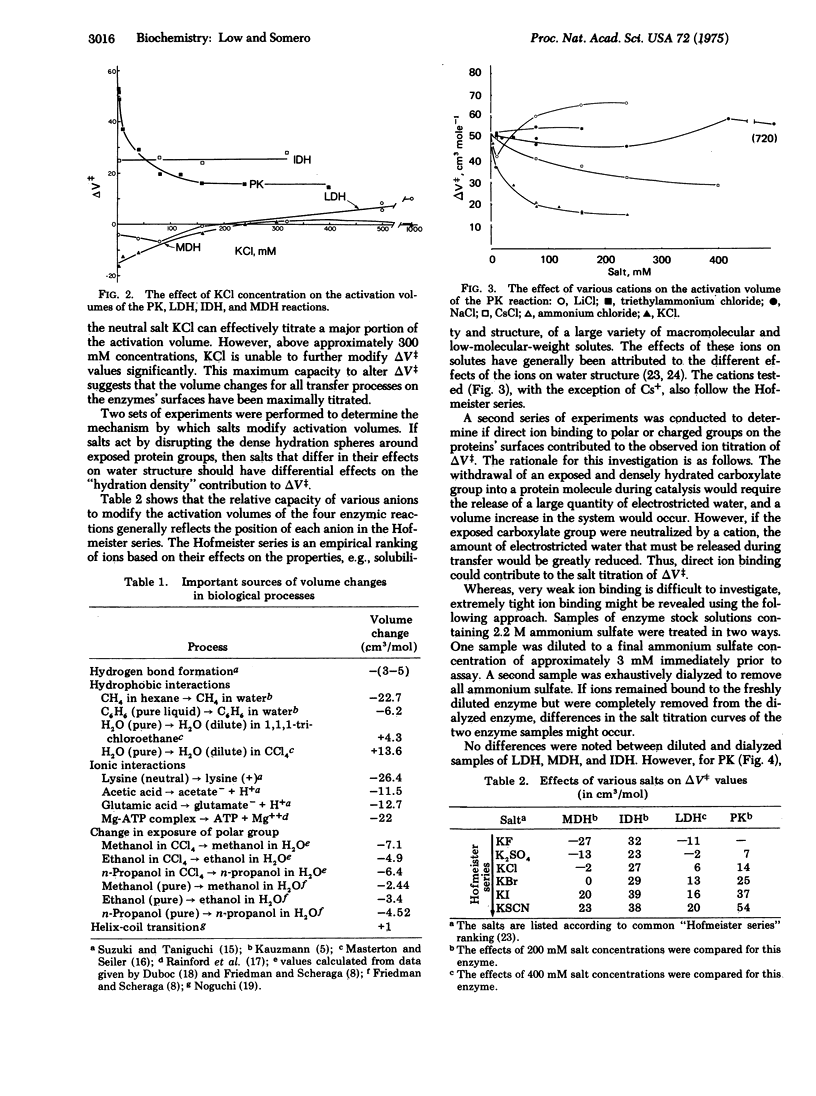
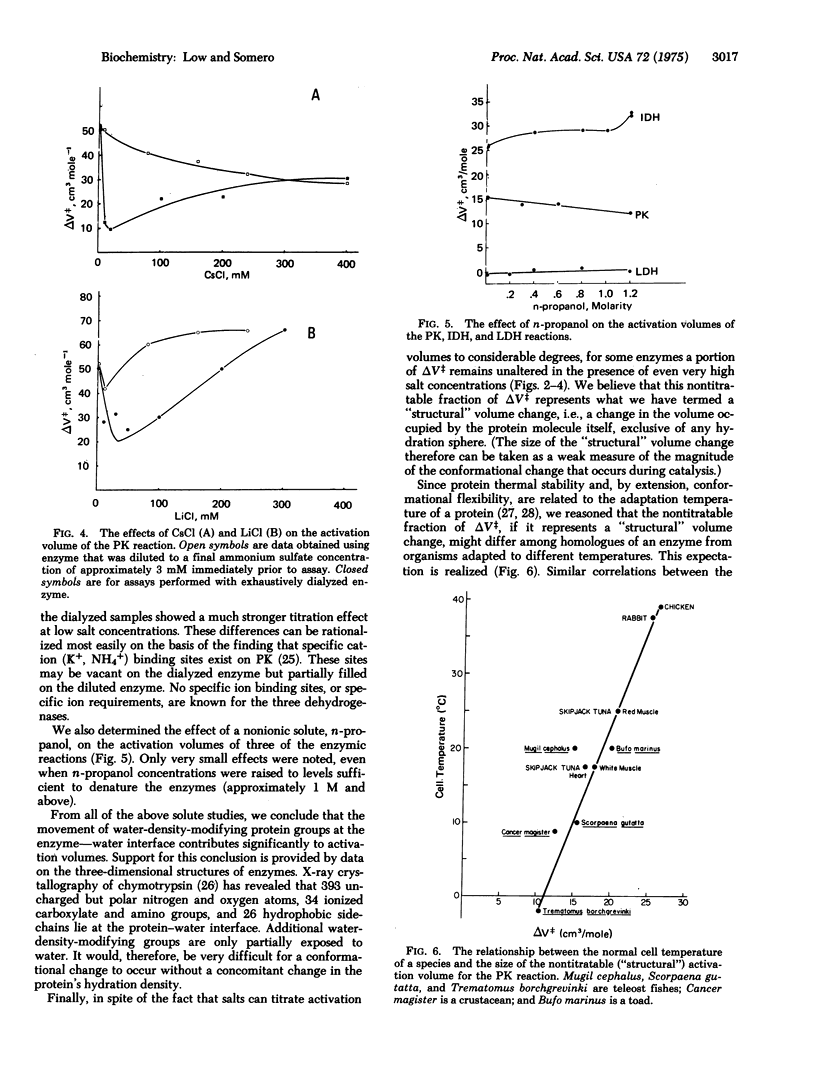
Changes in enzyme conformation are often accompanied by large changes in volume. Model compound studies suggest that these volume changes may derive from two sources: (i) "hydration density" effects due to changes in the exposure to solvent of protein groups which modify water density, and (ii) "structural" contributions arising from changes in the volume of the protein itself. An experimental approach was developed to test the validity of the predictions based on model compound studies for catalytic conformational changes. By examining the effects of different solutes on the activation volumes of different enzymic reactions, we show that both sources of volume change provide significant contributions to the activation volume.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birktoft J. J., Blow D. M. Structure of crystalline -chymotrypsin. V. The atomic structure of tosyl- -chymotrypsin at 2 A resolution. J Mol Biol. 1972 Jul 21;68(2):187–240. doi: 10.1016/0022-2836(72)90210-0. [DOI] [PubMed] [Google Scholar]
- Bondar R. J., Pon N. G. Purification of rabbit muscle pyruvate kinase by CM-sephadex and evidence for an endogenous inhibitor. Biochim Biophys Acta. 1969;191(3):743–747. doi: 10.1016/0005-2744(69)90376-3. [DOI] [PubMed] [Google Scholar]
- Brandts J. F., Oliveira R. J., Westort C. Thermodynamics of protein denaturation. Effect of pressu on the denaturation of ribonuclease A. Biochemistry. 1970 Feb 17;9(4):1038–1047. doi: 10.1021/bi00806a045. [DOI] [PubMed] [Google Scholar]
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- LAIDLER K. H. The influence of pressure on the rates of biological reactions. Arch Biochem. 1951 Feb;30(2):226–236. [PubMed] [Google Scholar]
- Nowak T. Monomethylammonium ion as a magnetic resonance probe for monovalent cation activators. The monovalent cation in pyruvate kinase catalysis. J Biol Chem. 1973 Oct 25;248(20):7191–7196. [PubMed] [Google Scholar]
- STERLING T. D. TOWARD AN UNDERGRADUATE MATHEMATICS PROGRAM FOR FUTURE RESEARCHERS IN THE FIELDS OF BIOLOGY AND MEDICINE. Fed Proc. 1965 Jan-Feb;24:5–9. [PubMed] [Google Scholar]
- Singleton R., Jr, Amelunxen R. E. Proteins from thermophilic microorganisms. Bacteriol Rev. 1973 Sep;37(3):320–342. doi: 10.1128/br.37.3.320-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipp A., Kauzmann W. Pressure denaturation of metmyoglobin. Biochemistry. 1973 Oct 9;12(21):4217–4228. doi: 10.1021/bi00745a028. [DOI] [PubMed] [Google Scholar]



