Abstract
Introduction
[11C]PBR28 is a high-affinity ligand for the Translocator Protein 18kDa (TSPO), which is considered to be a marker for microglial activation. Volume of distribution (VT) estimated with an arterial plasma input function is the gold standard for quantitation of [11C]PBR28 binding. However, arterial sampling is impractical at many PET sites for multiple reasons. Reference region modeling approaches are not ideal for TSPO tracers, as the existence of a true reference region cannot be assumed. Given that it would be desirable to have a non-invasive index of [11C]PBR28 binding, we elected to study the utility of the semi-quantitative metric, standardized uptake value (SUV) for use in brain [11C]PBR PET studies. The primary goal of this study was to determine the relationship between SUV and VT.
Methods
We performed a retrospective analysis of data from sixteen [11C]PBR28 PET scans acquired in baboons at baseline and at multiple time points after IV injection of lipopolysaccharide, an endotoxin that transiently induces neuroinflammation. For each scan, data from 14 brain regions of interest were studied. VT was estimated with the Logan plot, using metabolite-corrected input functions. SUV was calculated with data from 30–60 minutes after [11C]PBR28 injection.
Results
Within individual PET studies, SUV tended to correlate well with VT. Across studies, the relationship between SUV and VT was variable.
Conclusions
From study to study, there was variability in the degree of correlation between [11C]PBR28 VT and SUV. There are multiple physiological factors that may contribute to this variance.
Advances in Knowledge
As currently applied, the non-invasive measurement of SUV does not appear to be a reliable outcome variable for [11C]PBR28. Additional work is needed to discover the source of the discrepancy in SUV between [11C]PBR28 scans.
Implications for Patient Care
There is a need to develop alternatives to arterial plasma input functions for TSPO ligands in order to facilitate multi-center trials.
Keywords: PET, PBR28, TSPO, SUV, validity, inflammation
INTRODUCTION
[11C]PBR28 is a high-affinity ligand for the Translocator Protein 18kDa (TSPO), which is expressed on the outer mitochondrial membrane [1, 2]. The first in vivo imaging studies with [11C]PBR28 were published in 2007, and included brain imaging in rats [3] and the initial characterization of dosimetry and biodistribution of [11C]PBR28 in non-human primates and humans [4]. Increased expression of TSPO has been documented in activated macrophages and microglia [5, 6], and thus is often considered as a marker for microglial activation. Volume of distribution (VT) estimated with an arterial plasma input function is the gold standard for quantitation of radioligands, including [11C]PBR28 binding [7, although see 8]. However, arterial sampling is impractical at many PET sites for multiple reasons: it is invasive, can be painful, and can be difficult in populations such as the elderly. In rodents, arterial sampling requires vascular cannulation of a femoral or carotid artery. While this may be an acceptable procedure for acute, single scan studies, it is problematic for longitudinal experimental designs because of the difficulty in maintaining patency of arterial catheters over the course of weeks to months. Because of this, and the volume of blood needed for accurate measurement of radiolabeled parent and metabolite species, arterial cannulation is prohibitive for longitudinal studies in rodents. “Reference region” approaches (which use information from a target-free region as a surrogate for a plasma input function) have been proposed for the prototypical TSPO ligand, [11C]PK11195 [9–12]. These methods rely on the assumption that a true reference region exists for the TSPO (which may be problematic, given that the TSPO is a cholesterol binding site common to all mitochondria). These reference methods also have not been validated for [11C]PBR28. Image-derived input functions are sometimes a viable alternative to arterial input functions, but this methodology is not recommended for [11C]PBR28 [7]. Availability of a non-invasive index of [11C]PBR28 binding would help facilitate the use of high-affinity TSPO ligands in both human and small animal PET studies. We elected to study the utility of the semi-quantitative index, standardized uptake value (SUV), for use in brain [11C]PBR28 PET studies by comparing the performance of SUV to the accepted quantitative metric, VT. To accomplish this objective, we performed a retrospective analysis of [11C]PBR28 brain image data in non-human primates that were recently published [6].
MATERIALS AND METHODS
General
This study utilized data from a recently published report that demonstrated the effects of peripheral endotoxin administration on neuroinflammation [6]. All procedures were carried out in accordance with the Animal Welfare Act, other federal regulations governing the care and responsible use of animals for research, and under the recommended principles set forth in the Guide for Care and Use of Laboratory Animals [13]. The protocol was approved by the Yale University Institutional Animal Care and Use Committee. Details regarding the prior study of six of the animals can be found in Hannestad et al. [6]. The present work also includes a baseline scan from a seventh baboon. Briefly, animals received a bolus injection of 172 ± 12.4 MBq of [11C]PBR28, and dynamic data were acquired for 120 minutes on an ECAT EXACT HR+ (Siemens, Knoxville TN). Animals were scanned at baseline (n = 7), and at 1 hr (n = 4), 4 hr (n = 3), and 22 hr (n = 2) after IV administration of 0.1 mg/kg lipopolysaccharide (LPS). Arterial sampling was conducted during PET scanning for analysis of parent [11C]PBR28 and radiolabeled metabolites. Image processing was as described previously [6]. A total of sixteen [11C]PBR28 PET studies and results from the corresponding metabolite assays were available for this analysis.
Estimation of VT and Calculation of SUV
For each baboon, time-activity curves (TACs) of [11C] radioactivity in the brain were extracted from 14 standard brain regions of interest (ROIs; brainstem, caudate, centrum semiovale, cerebellum, cerebellum without vermis, cingulate cortex, frontal cortex, pallidum, insula, accumbens, occipital cortex, pons, putamen, and thalamus). For each ROI, total volume of distribution (VT) was estimated with Logan graphical analysis with the metabolite-corrected plasma input function [14], using 0–60 min of data, and t* = 10.
Calculation of SUV
Standardized uptake value (SUV) is defined as:
where QROI is the radionuclide quantity in an ROI, MROI is the mass of the ROI, ID is the injected dose of the radiopharmaceutical, and MSubject is the mass of the subject. A single SUV was calculated from each brain time-activity curve (TAC) using 30–60 min of data. To generate a single representative number for the whole brain for each study, the SUV values from the 14 ROIs were averaged.
LPS Effects on SUV
Paired t-tests were used to test for effects of LPS between baseline and 1-hour post- LPS (n = 4) and between baseline and 4-hours post-LPS (n = 3).
Comparison of SUV to VT
Linear regression analysis was used to determine the correspondence of SUV to VT within each [11C]PBR28 PET study.
RESULTS
Power of SUV to detect effects of LPS on [11C]PBR28 uptake
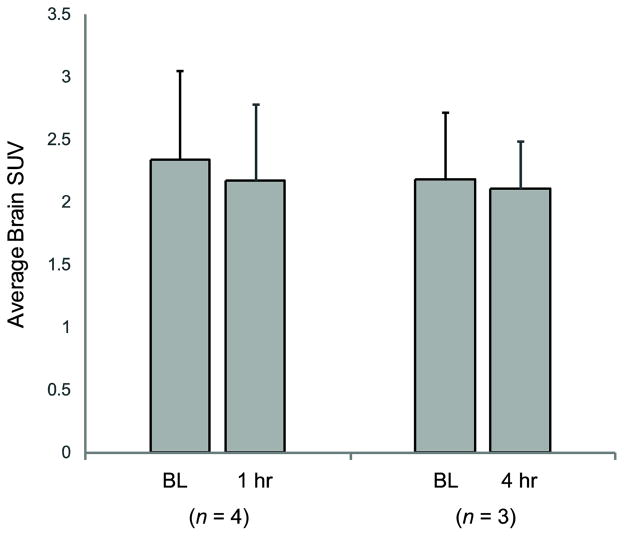
Figure 1 shows the average whole brain [11C]PBR28 SUV for the two groups of baboons scanned at baseline and at 1hr and 4hr post-LPS. There were no statistical differences between the baseline and LPS conditions. The average baseline SUV was 2.2 ± 0.72 (n = 7).
Figure 1.
Mean ± s.d. [11C]PBR28 standardized uptake values (SUV) for the whole brain at baseline (BL) and one and four hours after LPS injection (1 hr, 4 hr).
SUV vs VT
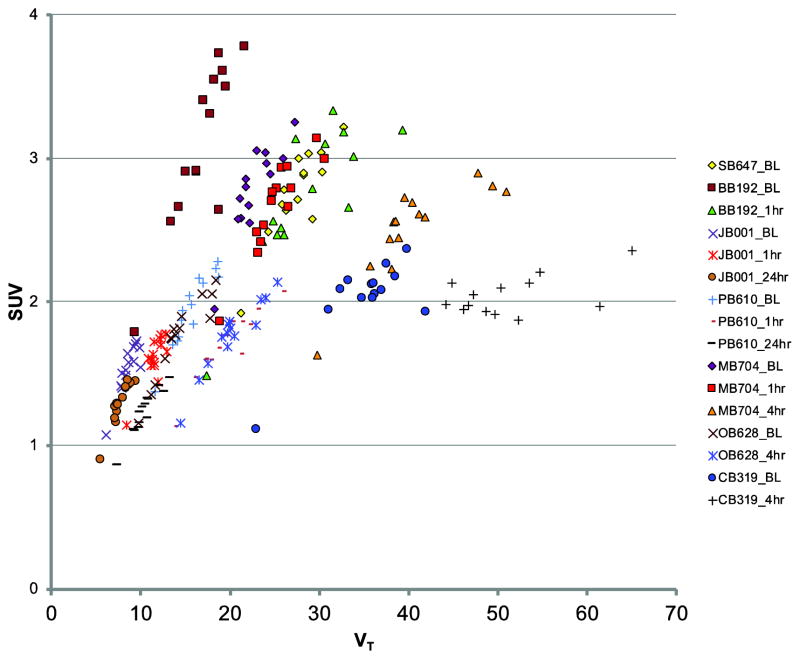
In fifteen of the sixteen [11C]PBR28 PET studies, regional SUV was highly correlated with regional VT (Figure 2). The r-value range was 0.78 to 0.96, and the p-value range was 0.001 – 1×10-06. However, the slope of the relationship between SUV and VT varied across studies, even within subjects. In general, the robustness of agreement between SUV and VT appeared to decrease substantially with higher values of VT.
Figure 2.
Relationships between [11C]PBR28 standardized uptake value (SUV) and volume of distribution (VT). Each set of symbols corresponds to a single [11C]PBR28 PET study; individual points within each set are from 14 different brain regions of interest (see text for details). Individual baboon IDs are two letter, three number designations, and are followed by the type of study, identified by: BL, baseline; 1hr, one hour post-lipopolysaccharide (LPS); 4hr, four hours post-LPS; 24hr, twenty-four hours post-LPS. In general, SUV correlates with VT within a given study (with the exception of CB319_4hr). However, the wide range of slopes across studies (even within animals) indicates that this relationship is not equivalent for each study.
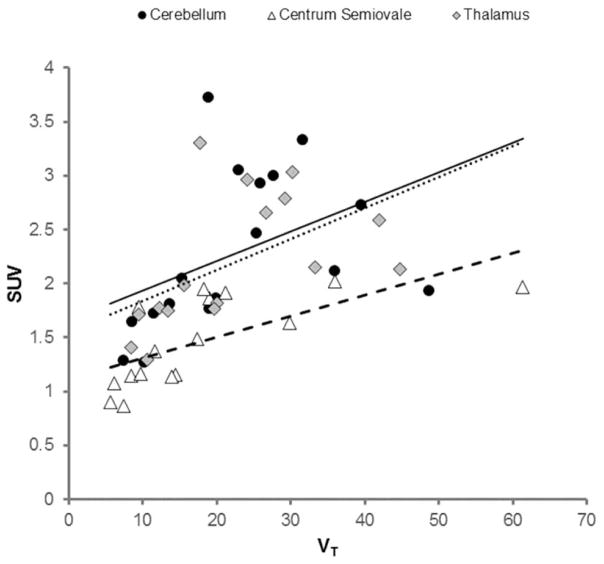
Figure 3 illustrates the relationship of SUV to VT for three ROIs with high, medium, and low [11C]PBR28 uptake.
Figure 3.
Relationships between [11C]PBR28 SUV and VT for regions of high (thalamus; gray diamonds, dotted line), medium (cerebellum; black circles, solid line) and low (centrum semiovale; white triangles, dashed line) [11C]PBR28 uptake. Data points are from the sixteen [11C]PBR28 PET studies described in the text.
DISCUSSION
This was retrospective analysis of sixteen [11C]PBR28 brain imaging datasets acquired in baboons at baseline, and at several time points after lipopolysaccharide (LPS) administration to induce an inflammatory state. We examined the relationship between the quantitative volume of distribution, VT, and the semi-quantitative standardized uptake values (SUV) for characterization of brain uptake of [11C]PBR28. The primary observation was that, within a single PET study, there was a consistent proportional relationship between SUV and VT sampled at different locations. Unfortunately, the proportionality between SUV and VT was not consistent from scan to scan. Additionally, with VT as an endpoint, Hannestad et al. [6] found a significant effect of 1hr and 4hr LPS; however, using SUV, no LPS effect was detected at either timepoint. This is likely due, in part, to the sample size (n = 4, n = 3). Small group numbers are unlikely to provide enough power for detecting effects with SUV, especially under conditions of high variance across studies. The degree of variance of the SUV values observed here (~30%) is similar to variability observed with estimations of brain VT for [11C]PBR28 in both humans [15–18] and the data from the baboons in the present study [6]. Given the SUV data above, a basic power analysis for a paired t-test (with α = 0.05 and power = 0.80) indicates that n = 24 would be needed to detect a 20% difference in SUV. Sample sizes would decrease to 12 and 6 for 30% and 50% effects, respectively. In the case of a between-groups design, a power analysis for an independent t-test results in n = 44 to detect a 20% effect, and 20 and 8, respectively, for 30% and 50% differences between groups [19]. Although [11C]PBR28 SUV has proven sensitive to the human TSPO binding affinity phenotype [20], the TSPO genotype effect is relatively large relative to what may be expected in disease states.
In order to discern likely sources of variability in SUV, it is helpful to consider the definition of SUV presented above. Conceptually, SUV represents the ratio of the radionuclide quantity observed in a tissue region to the quantity that would be observed in this region if the radionuclide was uniformly distributed throughout the body of the subject. In order for the SUV to serve as a reliable surrogate marker of TSPO expression within a tissue of interest, two criteria should be met: (1) the temporal characteristics of the concentration of the radiopharmaceutical in the arterial blood after intravenous injection follow a consistent pattern from study to study, and (2) the area under the arterial blood curve within the tissue of interest must be proportional to the ratio of the injected radiopharmaceutical dose to the mass of the subject. Violation of either/both of these two assumptions will lead to increased variability and/or bias in SUV. Breakdown of these assumptions may occur in several ways. For example, if TSPO expression varies from study to study, the shape of the arterial blood curve of the parent radiotracer will differ across studies [21], which would violate the first assumption (or possibly both), and could result in increased variability in the SUV measurement. Indeed, in this cohort, lower plasma parent fraction was observed after LPS administration, presumably as a function of increased TSPO expression via microglial activation [6; although see below]. It also possible that variable levels of isoflurane anesthesia across scans may contribute to differential TSPO binding via alterations of microglial activation [22, 23]. Another potential situation is that organs which constitutively express high levels of TSPO (e.g., lung, heart, kidney, endothelial tissue) have undergone changes in e.g., post-translational modification or mitochondrial morphology that result in apparent altered levels of TSPO. Yet another possible source of variance is differential metabolism of parent [11C]PBR28, which may significantly alter how much [11C]PBR28 is present in the arterial blood. In fact, the lower parent fraction observed post-LPS in the present cohort was attributed to faster metabolic rate [6]. However, in the present dataset, accounting for the rate of metabolism of parent within the SUV calculation did not improve the relationship of SUV to VT (data not shown). Regardless of the cause, changes in the temporal behavior of the parent radiopharmaceutical concentration in the blood between studies would lead to violation of one, or both, of the criteria listed above. Finally, another consideration is that, for radiopharmaceuticals like [11C]PBR28, which have a high first-pass extraction fraction and tissue retention, the delivery of tracer to a tissue of interest is dependent on blood flow to the tissue. This dependence on blood flow means that tracer delivery will have a very weak relationship, if any, to body mass, which would violate the second assumption above. In this circumstance, body mass becomes a source of variability to SUV.
CONCLUSION
In baboons, at baseline and under conditions of LPS-induced TSPO expression, SUV is not consistently representative of specific binding of [11C]PBR28 in the brain as defined by VT. It is unclear how these results would translate into human disease conditions that involve more subtle levels of neuroinflammation. Additional work is needed to determine the source of discrepancy in SUV across [11C]PBR28 scans, and to develop alternatives to arterial plasma input functions (e.g., image-derived methods), for quantitation of [11C]PBR28.
Acknowledgments
The authors would like to thank John West for computational support. The Department of the Army (W81XWH-08-2-0701, JH), the Society for Nuclear Medicine (Molecular Imaging Research Grant for Junior Medical Faculty, JH), and NIH (K12DA00167, JH; K01DA020651, KPC) provided funding and/or salary support. The U.S. Army Medical Research Acquisition Activity, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. The information does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, et al. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–9. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Papadopoulos V, Lecanu L. Translocator protein (18 kDa) TSPO: an emerging therapeutic target in neurotrauma. Exp Neurol. 2009;219:53–7. doi: 10.1016/j.expneurol.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imaizumi M, Kim HJ, Zoghbi SS, Briard E, Hong J, Musachio JL, et al. PET imaging with [11C]PBR28 can localize and quantify upregulated peripheral benzodiazepine receptors associated with cerebral ischemia in rat. Neurosci Lett. 2007;411:200–5. doi: 10.1016/j.neulet.2006.09.093. [DOI] [PubMed] [Google Scholar]
- 4.Brown AK, Fujita M, Fujimura Y, Liow JS, Stabin M, Ryu YH, et al. Radiation dosimetry and biodistribution in monkey and man of 11C-PBR28: a PET radioligand to image inflammation. J Nucl Med. 2007;48:2072–9. doi: 10.2967/jnumed.107.044842. [DOI] [PubMed] [Google Scholar]
- 5.Cosenza-Nashat M, Zhao ML, Suh HS, Morgan J, Natividad R, Morgello S, et al. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol. 2009;35:306–28. doi: 10.1111/j.1365-2990.2008.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hannestad J, Gallezot JD, Schafbauer T, Lim K, Kloczynski T, Morris ED, et al. Endotoxin-induced systemic inflammation activates microglia: [(1)(1)C]PBR28 positron emission tomography in nonhuman primates. Neuroimage. 2012;63:232–9. doi: 10.1016/j.neuroimage.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanotti-Fregonara P, Liow JS, Fujita M, Dusch E, Zoghbi SS, Luong E, et al. Image-derived input function for human brain using high resolution PET imaging with [C](R)-rolipram and [C]PBR28. PLoS One. 2011;6:e17056. doi: 10.1371/journal.pone.0017056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzo G, Veronese M, Tonietto M, Zanotti-Fregonara P, Turkheimer FE, Bertoldo A. Kinetic modeling without accounting for the vascular component impairs the quantification of [(11)C]PBR28 brain PET data. J Cereb Blood Flow Metab. 2014;34:1060–9. doi: 10.1038/jcbfm.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kropholler MA, Boellaard R, Schuitemaker A, Folkersma H, van Berckel BN, Lammertsma AA. Evaluation of reference tissue models for the analysis of [11C](R)-PK11195 studies. J Cereb Blood Flow Metab. 2006;26:1431–41. doi: 10.1038/sj.jcbfm.9600289. [DOI] [PubMed] [Google Scholar]
- 10.Kropholler MA, Boellaard R, van Berckel BN, Schuitemaker A, Kloet RW, Lubberink MJ, et al. Evaluation of reference regions for (R)-[(11)C]PK11195 studies in Alzheimer’s disease and mild cognitive impairment. J Cereb Blood Flow Metab. 2007;27:1965–74. doi: 10.1038/sj.jcbfm.9600488. [DOI] [PubMed] [Google Scholar]
- 11.Tomasi G, Edison P, Bertoldo A, Roncaroli F, Singh P, Gerhard A, et al. Novel reference region model reveals increased microglial and reduced vascular binding of 11C-(R)-PK11195 in patients with Alzheimer’s disease. J Nucl Med. 2008;49:1249–56. doi: 10.2967/jnumed.108.050583. [DOI] [PubMed] [Google Scholar]
- 12.Turkheimer FE, Edison P, Pavese N, Roncaroli F, Anderson AN, Hammers A, et al. Reference and target region modeling of [11C]-(R)-PK11195 brain studies. J Nucl Med. 2007;48:158–67. [PubMed] [Google Scholar]
- 13.Council NR. Guide for the Care and Use of Laboratory Animals. Washington, D.C: The National Academies Press; 1996. [PubMed] [Google Scholar]
- 14.Logan J, Fowler J, Volkow N, Wolf A, Dewey S, Schlyer D, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–7. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 15.Fujita M, Imaizumi M, Zoghbi SS, Fujimura Y, Farris AG, Suhara T, et al. Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. Neuroimage. 2008;40:43–52. doi: 10.1016/j.neuroimage.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannestad J, DellaGioia N, Gallezot JD, Lim K, Nabulsi N, Esterlis I, et al. The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [(1)(1)C]PBR28 PET study. Brain, behavior, and immunity. 2013;33:131–8. doi: 10.1016/j.bbi.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hines CS, Fujita M, Zoghbi SS, Kim JS, Quezado Z, Herscovitch P, et al. Propofol decreases in vivo binding of 11C-PBR28 to translocator protein (18 kDa) in the human brain. J Nucl Med. 2013;54:64–9. doi: 10.2967/jnumed.112.106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N, et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain. 2013;136:2228–38. doi: 10.1093/brain/awt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen J. Statistical Power Analyses for the Behavioral Sciences. 2. Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- 20.Yoder KK, Nho K, Risacher SL, Kim S, Shen L, Saykin AJ. Influence of TSPO Genotype on 11C-PBR28 Standardized Uptake Values. J Nucl Med. 2013;54:1320–2. doi: 10.2967/jnumed.112.118885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briard E, Zoghbi SS, Imaizumi M, Gourley JP, Shetty HU, Hong J, et al. Synthesis and evaluation in monkey of two sensitive 11C-labeled aryloxyanilide ligands for imaging brain peripheral benzodiazepine receptors in vivo. J Med Chem. 2008;51:17–30. doi: 10.1021/jm0707370. [DOI] [PubMed] [Google Scholar]
- 22.Kim JA, Li L, Zuo Z. Delayed treatment with isoflurane attenuates lipopolysaccharide and interferon gamma-induced activation and injury of mouse microglial cells. Anesthesiology. 2009;111:566–73. doi: 10.1097/ALN.0b013e3181af5b3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X, Kim JA, Zuo Z. Isoflurane preconditioning reduces mouse microglial activation and injury induced by lipopolysaccharide and interferon-gamma. Neuroscience. 2008;154:1002–8. doi: 10.1016/j.neuroscience.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]