Abstract
The retina is a delicate tissue that detects light, converts photochemical energy into neural signals, and transmits the signals to the visual cortex of the brain. A detailed protein inventory of the proteome of the normal human eye may provide a foundation for new investigations into both the physiology of the retina and the pathophysiology of retinal diseases. To provide an inventory, proteins were extracted from five retinas of normal eyes and fractionated using SDS-PAGE. After in-gel digestion, peptides were analyzed in duplicate using LC-MS/MS on an Orbitrap Elite mass spectrometer. A total of 3,436 non-redundant proteins were identified in the human retina, including 20 unambiguous protein isoforms, of which 8 have not previously been demonstrated to exist at the protein level. The proteins identified in the retina included most of the enzymes involved in the visual cycle and retinoid metabolism. One hundred and fifty-eight proteins that have been associated with age-related macular degeneration were identified in the retina. The MS proteome database of the human retina may serve as a valuable resource for future investigations of retinal biology and disease. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD001242.
Keywords: Age-related macular degeneration, Eye, Human, Retina, Phototransduction, Proteome
The human retina is a delicate, light-sensitive tissue that lines the inner surface of the posterior segment of the eye. The retina lies between the vitreous humor and the retinal pigment epithelium. The main function of the retina is to detect light, convert photochemical energy into neural signals, and transmit the signals to the visual cortex of the brain. The retina consists of six layers: nerve fiber layer, ganglion cell layer, inner plexiform layer, inner nuclear layer, outer plexiform layer, and outer nuclear layer. The cells of the retina include endothelial cells and pericytes, astrocytes, Müller cells, ganglion, horizontal, amacrine, bipolar, and photoreceptors. The retina is supplied by blood carried in the retinal vasculature and the choriocapillaris.
Important causes of visual disability and blindness in older adults that involve the retina include age-related macular degeneration (AMD), diabetic retinopathy, primary open angle glaucoma, epiretinal membrane, macular hole, and retinitis pigmentosa [1–6]. Despite drug therapies, surgical interventions, and laser treatment, many people with retinal disease progress to blindness. The proteome of the human retina has not been well characterized and may provide new insights into biological pathways that underlie the pathology of retinal disease. As late as 2013, published studies using proteomic approaches and MS had only identified 672 non-redundant proteins in the human retina [7]. The Human Eye Proteome Project was founded in 2012 as part of the international Human Proteome Organization (HUPO) [7]. The main goal of the Human Eye Proteome Project is “to characterize the proteome of the human eye in health and disease in order to gain insight into the pathophysiology of eye diseases and to contribute to new preventive and therapeutic modalities for the prevention of visual disability and blindness” [7]. The specific aim of this hypothesis-free project is to characterize the proteome of the normal human retina. To address this aim, we analyzed the proteome of normal human retina in middle- to older-aged adults.
Five human donor eyes were obtained from the Lions Eye Institute, Tampa, FL. Right eyes were selected from five adults (4 males, 1 female), age 51–76 y, with no history of eye disease or previous eye surgery. The causes of death were myocardial infarction, end-stage renal disease (2), and cancer (2, not on chemotherapy). Time of death to enucleation was 3–7 h for all subjects. Globes were placed in eye bank vials and refrigerated until received at the Wilmer Eye Institute. Time from enucleation to tissue freezing was 24–36 h for all subjects. The Institutional Review Board of the Johns Hopkins School of Medicine approved this study.
A corneal surgeon (S.F.) dissected the globes following a standardized protocol for isolation of human eye tissues [8]. Retina was dissected free from retinal pigment epithelium/choroid and vitreous, taking great care in the dissection to obtain pure retina samples. Retina samples were immediately snap frozen and stored at −80°C until processing. Retinas were suspended in 1 mL of lysis buffer (10 mM HEPES, 42 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol [DTT], 1× phosphatase inhibitor [Pierce, PI78420], 1× protease inhibitor [Sigma, P8340]), and homogenized using a motorized handheld Eppendorf mortar/pestle until tissue was no longer clumped. Samples were then sonicated three times for ten seconds, storing on ice to prevent overheating between sonications. Sodium dodecyl sulfate was added for a final concentration of 2% (w/v). Samples were incubated at room temperature for 10 min to lyse the cells and extract the proteins. The samples were then spun for 45 min, 14,000 × g, at room temperature. Protein concentration of the supernatant was determined using the Pierce microBCA kit.
Proteins were separated using SDS-PAGE gels. Double distilled H2O was added to the samples to achieve 50 μg of protein in 45 μL volume in a low retention Eppendorf tube. Four mL of 4× lithium dodecyl sulfate + 100 mM DTT was added to each tube. Tubes were vortexed, spun briefly, and placed on a 106°C heat block for 10 min to denature the proteins. Tubes were vortexed and spun twice. Fifty μg of sample was run in each lane on a 1D SDS gel using NuPAGE 4–12% BT 1.5, 200V for 35 min. Gels were stained with Coomassie blue (250 mL methanol, 50 mL acetic acid, 200 mL dH2O, 0.5 g Coomassie brilliant blue) on a rotating table at room temperature for 30 min. Gels were destained (50 mL methanol, 75 mL acetic acid, 875 mL dH2O) overnight. Each gel was cut into 12 bands. Gel pieces were placed in separate vials and disrupted with forceps. Gels were destained, dehydrated with acetonitrile, and dried down with a Speedvac concentrator. Samples were reduced and alkylated with 10 mM DTT in 25 mM ammonium bicarbonate, washed with ultrapure water, dehydrated with 100% acetonitrile, and dried. Samples were digested with trypsin/LysC (Promega, V5073) at 37°C overnight with shaking. Supernatant with peptides was removed, incubated with 100% acetonitrile, and then dried. Peptides were resuspended in 0.1% formic acid in water, placed in a desalting column (Pierce Spin-Tip), spun, eluted with 0.1% formic acid in 80% acetonitrile, and then dried. Samples were resuspended in 0.1% formic acid in water and spiked with 5 fmol/μL calibration peptides (Pierce Peptide Retention Time Calibration Mixture).
Samples were run LC-MS/MS using a one-hour gradient of 2–30% acetonitrile (Fisher Scientific) with 0.1% formic acid (Sigma-Aldrich) using an EASY-Spray source (Thermo Scientific) coupled with an Orbitrap Elite (Thermo Scientific) mass spectrometer. EASY-Spray source was run at 35°C using a 25 cm × 50 μm integrated spray tip column. Peptides were trapped at 980 bar on a 2 cm × 75 μm trapping column. The trap was a 3 μm particle, and the column was 2 μm Acclaim PepMap C18. All individual samples were run with two technical replicates.
MS raw data were batch processed using i3D (Shimadzu and Integrated Analysis), X!Tandem and OMSSA search engines, and the UniProt Sequence database. UniProt database included all human sequences, and parameters allowed for 2 missed tryptic cleavages, a fragment ion mass error of 1 Da, and potential protein modifications for carbamidomethylation, oxidation, phosphorylation, deamidation, and carbamylation. Peptide identifications were accepted if they could be established at greater than 95.0% probability by the Peptide Prophet algorithm [9]. Protein identifications were accepted if they could be established at ≥90.0% probability and contained at least 1 identified peptide. Protein Prophet algorithm was used to assign protein probabilities [10] using a false discovery rate of 0.13% for peptides and 0.81% for proteins. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony. Proteins sharing significant peptide evidence were grouped into clusters.
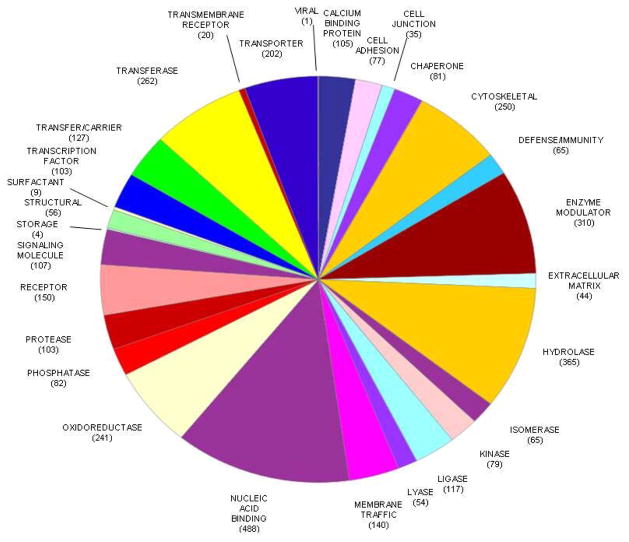
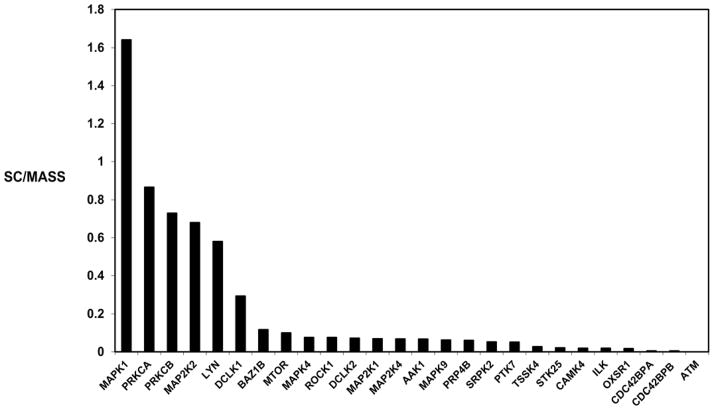
A total of 19,083 peptides were identified, resulting in 3,130 protein groups and 3,436 non-redundant proteins identified in the normal human retina (Supporting Information Table 1). The percentage of proteins that were identified on the basis of a match of ≥5, 4, 3, 2, peptides and 1 peptide was 43.7%, 8.9%, 10.8%, 15.0%, and 21.5%, respectively. One peptide matches were manually validated. MS/MS spectra are shown for peptides used to identify proteins on the basis of a single peptide [PXD001242]. Protein isoforms were identified by further verifying putative isoforms from i3D processing. Peptide sequences were manually compared with sequence alignment of the different isoforms in order to confirm unambiguous identification of isoforms. PANTHER analysis was used to classify protein function [11] (Figure 1). Four examples of MS/MS spectra of peptides used for identification of proteins in the human retina, interphotoreceptor matrix proteoglycan 1, alpha-crystallin B chain, interphotoreceptor matrix proteoglycan 2, and complement factor H are shown (Supporting Information Figure 1). A heat map of the total unique peptides in human retina is presented (Supporting Information Figure 2), showing the normalized unique peptide distribution in retina sample fractions. The overlap between the 3,436 proteins identified in the present study with 672 previously published proteins identified in the retina using proteomic and MS approaches [7], and 1,929 plasma proteins in the Plasma Peptide Atlas [12] are shown (Supporting Information Figure 3). Using ProteinCenter (Thermo Fisher), we also compared the 3436 proteins with the 15058 proteins deposited in Human Peptide Atlas (updated 07-31-2014), and with 267 transcripts detected in retina previously in Human Protein Atlas by using immunohistochemistry approaches. There were 781 proteins in the present study that were not in the current Human Peptide Atlas (Venn diagram in Supporting Information Figure 4), 2,640 proteins that overlapped with those described in the current Atlas, and 87 proteins with transcripts previously detected in retina. Twenty-six protein kinases were identified (Figure 2). We compared the proteins identified in the retina with a recent curated list of genes associated with AMD [13]. There were 158 proteins in the retina that have been associated with AMD (Supporting Information Table 2).
Figure 1.
Pie diagram of the distribution of protein functions using PANTHER.
Figure 2. Kinome of the human retina, shown by spectral count/mass (kDa).
Mitogen-activated protein kinase 1 (MAPK1); protein kinase C alpha type (PRKCA) and beta type (PRKCB); dual specificity mitogen-activated protein kinase 1 (MAPK1), 2 (MAPK2), and 4 (MAPK4); tyrosine protein kinase LYN (LYN) and BAZ1B (BAZ1B); serine/threonine protein kinase DCLK1 (DCLK1), DCLK2 (DCLK2), mTOR (MTOR), P4P4 homolog (PRP4B), 25 (STK25), OSR1 (OXSR1), MRCK alpha (CDC42BPA), and MRCK beta (CDC42BPB); mitogen-activated protein kinase 4 (MAPK4) and 9 (MAPK9); Rho-associated protein kinase (ROCK1); AP2-associated protein kinase (AAK1); SRSF protein kinase (SRPK2); inactive tyrosine protein kinase 8 (PTK7); testis-specific serine/threonine protein kinase 4 (TSSK4); calcium/calmodulin-dependent protein kinase type IV (CAMK4); integrin-linked protein kinase (ILK); serine protein kinase ATM (ATM).
Many enzymes involved in the visual cycle and retinoid metabolism were identified, including rhodopsin, rhodopsin kinase, retinol-binding proteins 1, 3, and 4, retinol dehydrogenases 10, 11, 12, and 14, 11-cis retinol dehydrogenase, retinaldehyde-binding protein 1, retinal dehydrogenases 1, retinal guanylyl cyclase 1, retinal-specific ATP-binding cassette transporter, RPE-retinal G-protein-coupled receptor, and retinal rod rhodopsin-sensitive cGMP 3′,5′-cyclic phosphodiesterase subunit delta.
Several apolipoproteins were identified in the retina, including apolipoprotein (apo) A-I and apoB, which have been implicated in diabetic retinopathy [1], and apoE, which is implicated in AMD [2]. ApoA-1, apoA-IV, and apoE have previously been described in human retina using proteomic and mass spectrometry-based approaches, whereas apoA-II, apoB, apoCIII, and apoD are newly described using these methods.
There were 20 unambiguous protein isoforms identified in the present study (Table 1). Eight of these protein isoforms had no previous experimental confirmation available in UniProt annotation, including isoforms of Bcl-2-like protein 2 (implicated in promoting cell survival), microtubule-associated protein 2 (involved in stabilizing microtubules), coronin-7 (involved in membrane trafficking), and programmed cell death 6-interacting protein (thought to be involved in apoptosis).
Table 1.
Twenty protein isoforms identified in human retina
| UniProt ID | Protein Name | Distinguishing Peptide(s) |
|---|---|---|
| P49419-2 | Alpha-aminoadipic semialdehyde dehydrogenase, isoform 2 | MSTLLINQPQYAWLK |
| Q92843-2* | Bcl-2-like protein 2, isoform 3 | TSLALDESLFR |
| Q9ULU8-4 | Calcium-dependent secretion activator 1, isoform 4 | LCSMEMGQEFAK |
| P60953-1 | Cell division control protein 42 homolog, isoform 1 | YVECSALTQR |
| P57737-3* | Coronin-7, isoform 3 | SAAASNLSGLSLQEAQQILNVSK |
| P56545-2 | C-terminal-binding protein 2, isoform 2 | EAVYNSVAAR EYYSDPSGAAR MAYETYEADLSTFQQPGGK |
| Q14195-2 | Dihydropyrimidinase-related protein 3, isoform LCRMP-4 | EESREPAPASPAPAGVEIR |
| Q9NRG7-2 | Epimerase family protein SDR39U1, isoform 2 | TLATGYQYSFPELGAALK |
| Q5QNW6-2* | Histone H2B type 2-F, isoform 2 | LIGPILWK |
| Q9P260-2* | LisH domain and HEAT repeat-containing protein KIAA1468, isoform 2 | QVNETLVAQR |
| P84157-3* | Matrix-remodeling-associated protein 7, isoform 3 | IELTSDLTSL |
| P51608-2 | Methyl-CpG-binding protein 2, isoform B | AAAAAAAPSGGGGGGEEERLEEK |
| P11137-4* | Microtubule-associated protein 2, isoform 3 | FAALEQPEVER |
| Q14697-2 | Neutral alpha-glucosidase AB, isoform 2 | VNLTLGSIWDK |
| Q8N490-2 | Probable hydrolase PNKD, isoform 2 | ASSQSAPSPDVGSGVQT |
| Q8WUM4-2* | Programmed cell death 6-interacting protein, isoform 2 | YFYFQEVFPVLAAK |
| P14618-2 | Pyruvate kinase isozymes M1/M2, isoform M1 | CLAAALIVLTESGR |
| Q8TBG9-2* | Synaptoporin, isoform 2 | MDPVSQLASAGTFR |
| P61764-2 | Syntaxin-binding protein 1, isoform 2 | VSFEDQAPTME |
| O15294-3 | UDP-N-acetylglucosamine-peptide N-acetylglucosaminyltransferase 110 kDa subunit, isoform 1 | ASSVGNVADSTGLAELAHR |
UniProt website (https://www.uniprot.com) noted for the specific isoform that “No experimental confirmation available” (07/15/14)
This MS proteome database of the human retina may serve as a valuable resource for future investigations of retinal biology and disease. Future work is needed to identify protein PTMs and isoforms that are specific to certain eye tissues and differentially expressed during development and aging and in health and disease.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grants R01 EY024596, R01 AG027012, and Research to Prevent Blindness.
Abbreviations
- AMD
age-related macular degeneration
Footnotes
Craig Dufresne is an employee of Thermo Fisher Scientific. The remaining authors have declared no conflict of interest.
References
- 1.Jager RD, Meiler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 2.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 3.Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360:1113–1124. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joshi M, Agrawal S, Christoforidis JB. Inflammatory mechanisms of idiopathic epiretinal membrane formation. Mediators Inflamm. 2013;2013:192582. doi: 10.1155/2013/192582. Epub 2013 Nov 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumagai K, Ogino N, Hangai M, Larson E. Percentage of fellow eyes that develop full-thickness macular hole in patients with unilateral macular hole. Arch Ophthalmol. 2012;130:393–394. doi: 10.1001/archopthalmol.2011.1427. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari S, Di Iorio E, Barbaro V, Ponzin D, Sorrentino FS, Parmeggiani F. Retinitis pigmentosa: genes and disease mechanisms. Curr Genomics. 2011;12:238–249. doi: 10.2174/138920211795860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semba RD, Enghild JJ, Venkatraman V, Dyrlund TF, Van Eyk JE. The Human Eye Proteome Project: perspectives on an emerging proteome. Proteomics. 2013;13:2500–2511. doi: 10.1002/pmic.201300075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skeie JM, Mahajan VB. Dissection of human vitreous body elements for proteomic analysis. J Vis Exp. 2011;47:e2455. doi: 10.3791/2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 10.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 11.Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8:1551–1566. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrah T, Deutsch EW, Omenn GS, Campbell DS, Sun Z, Bletz JA, Mallick P, Katz JE, Malmström J, Ossola R, Watts JD, Lin B, Zhang H, Moritz RL, Aebersold R. A high-confidence human plasma proteome reference set with estimated concentrations in Peptide Atlas. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman AM, Gallo NB, Hancox LS, Miller NJ, Radeke CM, Maloney MA, Cooper JB, Hageman GS, Anderson DH, Johnson LV, Radeke MJ. Systems-level analysis of age-related macular degeneration reveals global biomarkers and phenotype-specific functional networks. Genome Med. 2012;4:16. doi: 10.1186/gm315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.