Abstract
Rationale
Depletion of medial smooth muscle cell (SMC) is a major pathological characteristic of abdominal aortic aneurysm (AAA), although the mechanism by which these cells are eliminated remains incompletely understood. We reasoned that necroptosis, a recently described form of necrosis mediated by receptor-interacting protein kinase 3 (RIP3), may contribute to AAA pathology through the induction of SMC death and the significant production of inflammatory cytokines.
Objective
To test the hypothesis that RIP3-mediated necroptosis is actively involved in aneurysm pathogenesis.
Methods and Results
RIP3 and RIP1 levels were found to be elevated in human AAAs, most noticeably in SMCs. Elevations of RIP3 and SMC necrosis were also observed in the elastase-induced mouse model of AAAs. Deletion of one or both copies of Rip3 prevented AAA formation. By transplanting Rip3+/- aortae to Rip3+/+ mice, we demonstrated that reduced Rip3 expression in arterial wall was the primary cause of aneurysm resistance. In vitro, adenoviral overexpression of RIP3 was sufficient to trigger SMC necroptosis. PKCδ contributed to TNFα-induced SMC necroptosis by regulating Rip3 expression. Furthermore, Rip3 deficiency impaired TNFα-induced inflammatory gene expression in aortic SMCs, which was at least in part due to attenuation of p65 Ser536 phosphorylation. In vivo, the lack of RIP3 diminished activation of p65 in SMCs, implicating a necrosis independent function of RIP3 in aneurysms.
Conclusions
Enhanced RIP3-signaling in aneurysmal tissues contributes to AAA progression by causing SMC necroptosis as well as stimulating vascular inflammation, and therefore may serve as a novel therapeutic target for AAA treatment.
Keywords: Abdominal aortic aneurysm, RIP3, smooth muscle cells, Protein Kinase C [delta], necroptosis, nuclear factor-kappa B, necrosis, apoptosis
Introduction
Abdominal aortic aneurysm (AAA), which is the progressive weakening and dilation of the infrarenal segment of the aorta, is a major potentially lethal aortic disease with no available pharmacological treatment.1, 2 The pathophysiology of AAA remains incompletely understood.
Studies using human specimens and animal models have shown that the infiltrating inflammatory cells, such as macrophages and mast cells, are the major source of both pro-inflammatory cytokines and matrix-degrading enzymes accumulated in the aneurysmal aortae.3-6
Medial smooth muscle cells (SMCs) are generally believed to influence the development and progression of AAA, although the mechanisms remain obscure.7, 8 Medial SMC paucity has been noted in human pathological studies and consistently reproduced in mouse aneurysmal models9-11, though it is not entirely clear how SMC die and whether the cell death is an active pathological event or simply a consequence of tissue deterioration. We have previously shown that inhibition of SMC apoptosis with a pan caspase inhibitor Q-VD-OPh preserves extracellular matrix (ECM) integrity and prevents aneurysm formation in angiotensin II-infused apoE deficient mice.11 However, Q-VD-OPh provides little benefit in stopping aneurysm progression when administered one week after initiating the disease, which indicates the contribution of apoptosis is somewhat limited to the initial phase of aneurysm formation.
Historically, necrosis and apoptosis have been described as two opposite forms of cell death. Apoptosis is regulated by sequential activation of caspases and does not normally trigger immune responses due to efficient engulfment of intact cells (or membrane-bound apoptotic bodies) by neighboring healthy cells or by professional phagocytes such as macrophages.12 In contrast, necrosis was thought to be accidental, associated with rapid loss of cell membrane integrity and inflammation.12 However, experimental evidence accumulated over the past decade asserts that certain forms of necrosis are actually regulated by well-orchestrated signaling networks, and should be considered as programmed necrosis or necroptosis.13, 14 Receptor-interacting serine/threonine-protein kinase 3 (RIP3) and a closely related kinase called RIP1 have been identified as critical mediators in initiating the necroptosis process.13, 14 Expression of RIP3 is crucial in determination of necroptotic cell fate.15, 16 Cells deficient in RIP3 or treated with RIP1 kinase inhibitors are protected from necroptosis.15, 17-19
Mice lacking Rip3 develop normally and are fertile17, providing an invaluable tool for in vivo investigations of necroptosis. In contrast, Rip1-/- mice die shortly after birth.20 Necrostatin-1 and an optimized derivative of necrostatin-1 (7-Cl-O-Nec-1) are small molecules inhibitors of RIP1.18, 19, 21 The use of RIP3 deficient mice as well as necrostatin-1 and 7-Cl-O-Nec-1 has demonstrated the significance of necroptosis in several animal models of human diseases including ischemic brain injury18, photoreceptor loss22, myocardial infarction23, systemic inflammatory stress syndrome (SIRS)24, and atherosclerosis16.
Necroptosis as well as activation of RIP3 can be triggered by a variety of signals including cytokines, pathogen-associated molecular patterns, and oxidative stress, all of which are known to be elevated in AAA.13 In this study, we tested the hypothesis that RIP3 contributes to aneurysm pathophysiology and therefore serves as a significant therapeutic target. Using an AAA model induced by elastase, we demonstrated that Rip3 deficiency prevented aneurysm formation likely through mediating aortic cell death and inflammation. Our data further identified a necroptosis-independent function of RIP3 in NF-κB–mediated inflammatory response. Finally, we investigated the role of PKCδ in the regulation of RIP3 expression and SMC necroptosis. our results provide new insights into pathophysiology of AAA as well as other vascular diseases associated with cell death or NF-κB signaling dysregulation.
Methods
An expanded Materials and Methods section is available in Online Data Supplement.
Mice
Rip3+/- mice17 on a C57BL/6 background were generously provided by Dr. Vishva M. Dixit (Genentech, South San Francisco, CA). Prkcd+/- mice47, generously providing by Dr. Keiichi I. Nakayama of Kyushu University in Japan, were maintained on a mixed background of C57BL/6 and 129/Sv. Heterozygous mice were intercrossed to generate littermates of three genotypes for the study. C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). Male mice of 8∼12 weeks old were used for experiments. All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Madison (Protocol M02284).
Cell culture
Primary mouse aortic SMCs were isolated from the abdominal aorta as described previously.48, 49 Cells between three and seven passages were used. The mouse aortic SMC cell line MOVAS cells and the mouse fibroblast cell line L929 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA).
Elastase-induced murine abdominal aortic aneurysm
Male mice aged 8-12 weeks underwent an elastase-induced AAA model as described previously.4, 10, 27 All the elastase treated mice were perfused with elastase for 5 min. As a control, a separate group of mice were treated with equal concentration of heat-inactivated (100°C for 15 min) elastase for 5 min. The diameter of the largest portion of an abdominal aorta was measured and recorded prior to elastase perfusion and at sacrifice. A percentage increase in maximal external aortic diameter was calculated. As shown in Figure 2A and supplemental Figure I, aortic infusion with heat-inactivated elastase or elastase caused a 29.889%±4.644% (0.71mm±0.02mm) or 157.366%±9.973% (1.42mm±0.06mm) expansion in the wildtype mice, respectively. This is consistent with what reported in the literature.27
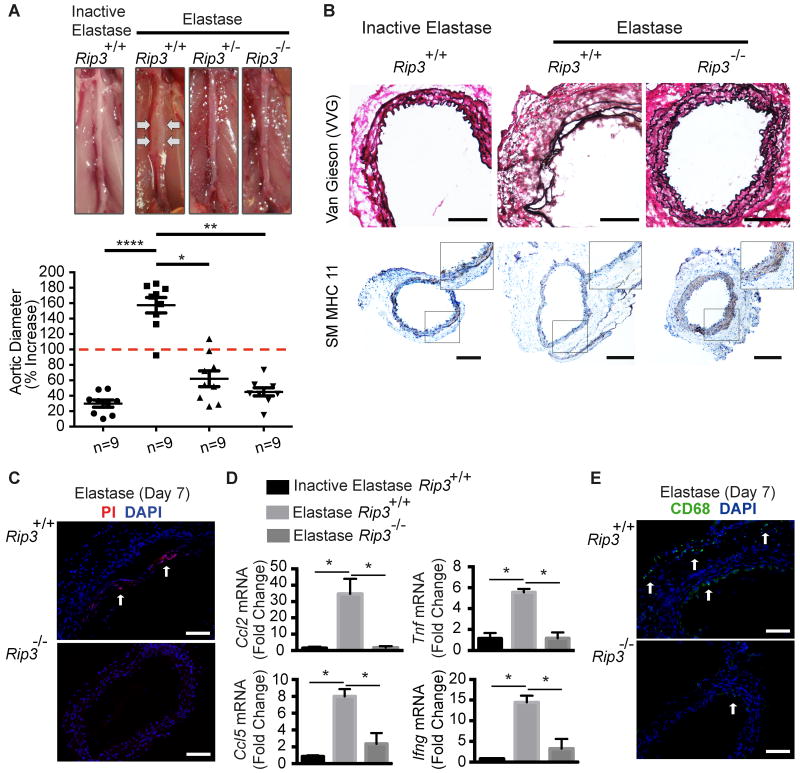
Figure 2. Rip3 deficiency protects mice from developing abdominal aortic aneurysm.
(A) Mice of three genotypes were subjected to aneurysm induction by elastase perfusion. Animals were sacrificed 14 days after. Upper: Representative photos of abdominal aortae taken during sacrifice. Bottom: Aortic dilatation measured as the percentage increase of maximal external aortic diameter between pre-perfusion and on day 14 after perfusion. An AAA is defined as a percentage increase in aortic diameter greater than 100% (red dashed line). n=9. (B) Cross sections of harvested aortae were stained for Verhoeff-Van Gieson (VVG) in which elastin fibers are stained in dark purple or for smooth muscle myosin heavy chain 11 (SM MHC11). Inset photos are higher magnified views of highlighted regions. n=3. Scale bars=200μm. (C) Representative photos of aortic sections from mice that were injected with propidium iodide (PI) 2 hours prior to sacrifice on Day 7 post elastase perfusion. The necrotic cells (PI-positive) were indicated by white arrows. n=3. Scale bars=100μm. (D) Rip3+/+ and -/- mice were subjected to elastase perfusion and sacrificed 14 days after. Aortic levels of pro-inflammatory cytokines were analyzed by real-time PCR. n=4. (E) Representative photographs of immunostaining for a macrophage marker CD68 (green, arrows) in the elastase-perfused aortae. n=3. Scale bars=100μm. *P<0.05; **P<0.005; ****P<0.0001. All values represent mean±SEM.
Orthotopic allograft transplantation
Orthotopic allograft transplantation of elastase-treated abdominal aorta was carried out as we reported recently.28
Adenoviral vectors and infection
Adenoviral vectors expressing PKCδ (AdPKCδ) and empty vector (AdNull) were constructed and purified by CsCl gradient centrifuging as previously described.48, 49 The adenoviral vector expressing RIP3 were constructed and purified by Welgen, Inc (Worcester, MA). In vitro adenovirus infection was carried out as described previously.31
Flow cytometric analysis
Cell death was evaluated by using an Annexin V-PE/7-AAD staining Kit (BD Biosciences, San Jose, CA) and a Becton Dickinson Biosciences FACSCalibur (BD Biosciences, San Jose, CA).
In vivo Propidium Iodide (PI) staining
In vivo cell necrosis was examined by intraperitoneal (IP) injection of propidium iodide (PI).22 Two hours after PI administration, mice were euthanized and perfusion fixed with 4% formaldehyde. 6-μm-thick cryosections were cut. Staining was immediately visualized with a Nikon Eclipse Ti inverted microscope system and digital images were acquired using a Nikon DS-Ri1 digital camera.
Statistical analysis
Data are presented as mean±SEM. Two-tailed Student's t-test for normally distributed data and Mann-Whitney nonparametric test for skewed data that deviate from normality were used to compare two conditions. One-way ANOVA with Bonferroni's post-hoc test for normally distributed data and Kruskal-Wallis nonparametric test for skewed data were used to compare three or more means. Differences with P <0.05 were considered statistically significant.
Results
Increased expression of RIP3 and elevated necrosis in abdominal aortic aneurysm (AAA)
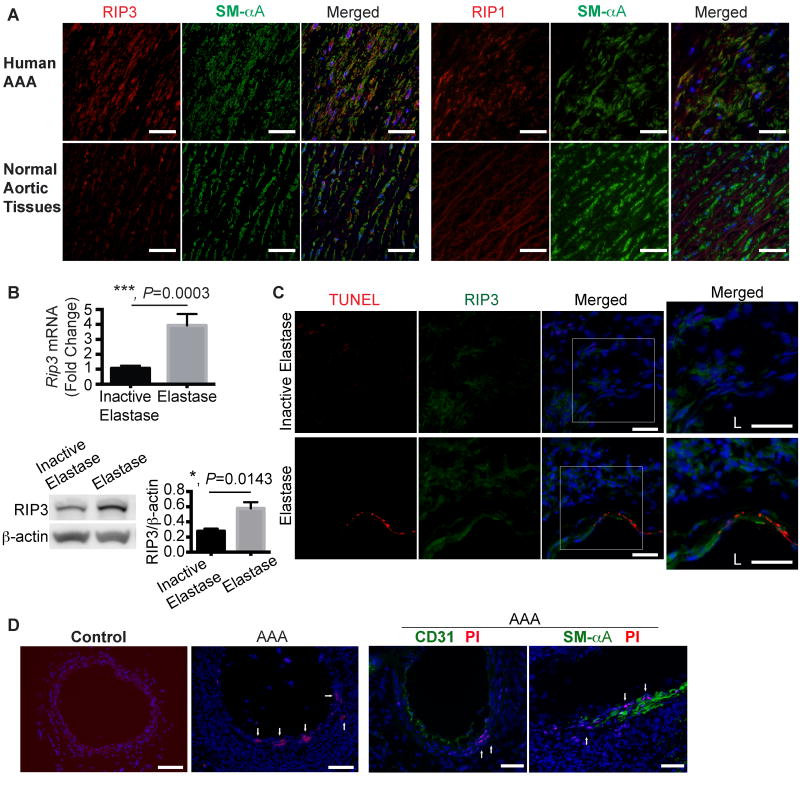
To examine RIP3 expression in aneurysm disease, we stained AAA tissues derived from aneurysm patients who were treated with elective open surgical repair. Abdominal aortic tissues from transplant donors served as controls. Immunostaining analysis demonstrated elevations of RIP3 as well as RIP1, another key protein in mediating necroptosis25, in the SMC layer of human AAA tissues (Figure 1A and Online Figure I). The diminished staining of smooth muscle-specific α-actin (SM-αA) in AAA tissues reflected the loss of muscle cells as well as a reduction in contractile proteins in those tissues. We next induced aneurysm in C57BL/6 mice by transient perfusion of infrarenal aortae with porcine pancreatic elastase, an established AAA model.26, 27 Mice that were perfused with heat-inactivated elastase were used as controls. The affected aortic tissues were harvested for RNA or protein extraction 14 days after aneurysm induction and analyzed by real-time PCR or Western blotting. As shown in Figure 1B, the aneurysm induction by elastase increased levels of Rip3 mRNA and protein in the injured aortic wall by 3.93±0.763 fold (p=0.0003, n=8) and 2.07 fold (p=0.0143, n=4) over the controls, respectively. In contrast, increases in Rip1 mRNA and protein were marginal and did not reach statistical significance (Online Figure II A and B). To further characterize the RIP1 and RIP3 positive cells, we co-stained aortic tissues with TUNEL. No colocalization was noted between RIP3 and TUNEL staining (Figure 1C), while some high RIP1-expressing cells were also positive for TUNEL (Online Figure II C). Due to the lack of specific markers for necroptotic cells, we used propidium iodide (PI, administered to mice via intraperitoneal (IP) injection 2 hours prior to sacrifice) to identify necrotic cells with disrupted cell membrane.22 As shown in Figure 1D and Online Figure III, compared to control aortae, necrosis increased in the SMC-rich medial layer of elastase-treated aortae. Importantly, those PI positive cells were positive for smooth muscle specific-α-actin (SM-αA) but not CD31, an endothelial cell marker, suggesting necrosis occurs primarily in SMCs in the elastase-induced AAA model.
Figure 1. Increased receptor interacting protein 3 (RIP3) expression and cell necrosis in abdominal aortic aneurysm tissues.
(A) Representative photographs of human aneurysmal and normal aortic cross sections that were double stained for RIP3 (or RIP1, red) and smooth muscle α-Actin (SM-αA, green). n=4. Scale bars=50μm. (B) Real-time PCR (n=8) and Western blotting (n=4) analyses of Rip3 expression in C57BL/6 mouse arteries perfused with elastase or heat-inactivated elastase on Day 14 post-perfusion. Data are mean±SEM. (C) Representative photographs of aortic cross-sections harvested from C57BL/6 mice on Day 3 post-surgery. Sections were co-stained for RIP3 and TUNEL. Higher magnified views of highlighted regions were shown on the right. L indicates lumen. n=3. Scale bars=50μm. (D) Representative photographs of aortic cross sections of normal or elastase-perfused aortae (Day 7 post-perfusion). Propidium iodide (PI) was administered to mice via intraperitoneal injection 2 hours prior to sacrifice. Following PI staining, aortic sections were also stained for CD31 or SM-αA. n=3. Scale bars=100μm.
Rip3 deficiency attenuates AAA formation
It has been demonstrated in several disease models including atherosclerosis and photoreceptor death that Rip3 deficiency inhibits necroptosis but not apoptosis.16, 22 Therefore, we used Rip3 mutants to probe the potential role of necroptosis in aneurysm pathophysiology. Male Rip3+/+, Rip3+/-, Rip3-/- mice, 8-12 week old, were subjected to aneurysm induction by elastase. Western blotting of aortic tissue extracts confirmed the absence of RIP3 protein in Rip3-/- mice and a markedly reduced RIP3 expression in Rip3+/- mice (Online Figure IV). Levels of RIP1 appeared to be comparable in all three genotypes. Fourteen days after elastase perfusion, mice were sacrificed for evaluation of aneurysm, defined in this model as 100% or more increase in the maximal external diameter compared to that measured before perfusion.27 Rip3 gene deletion profoundly inhibited aneurysm formation: 0 out of the 9 Rip3-/- mice and 2 out of the 9 Rip3+/- mice (22.22%) developed aneurysms as compared to 8 out of the 9 (88.89%) aneurysm incidence in the Rip3+/+ mice. The mean aortic expansion caused by elastase infusion was 45.088%±5.247% (0.77mm±0.03mm) and 62.140%±10.175% (0.85mm±0.04mm) in Rip3-/- and Rip3+/- mice, respectively, which was significantly smaller than the 157.366%±9.973% (1.42mm±0.06mm) expansion observed in elastase treated wildtype mice. The aortic expansions in Rip3+/- mice appeared to be slightly larger than that seen in Rip3-/- mice, however, the difference in aortic diameters between the two mutant genotypes did not reach statistical significance (P=0.16) (Figure 2A and Online Figure V). Histologically, the Rip3 gene deficiency protected aortic tissue integrity. Representative images in Figure 2B demonstrate elastin fragmentation and SMC paucity in Rip3+/+ aortae on Day 14 after elastase perfusion. These aneurysm associated characteristics were profoundly diminished in elastase-perfused Rip3-/- aortae. Consistent with the known function of RIP3 in necroptosis, in vivo PI staining showed a significant decrease in the number of necrotic cells detected in Rip3-/- mice on day 7 after elastase perfusion (Figure 2C). Since necrotic cells are highly immunogenic, we tested whether Rip3 gene deficiency attenuated vascular inflammation by assessing mRNA levels of cytokines in the elastase-perfused infrarenal region of aorta where aneurysmal expansion occurs. As shown in Figure 2D, the loss of Rip3 profoundly reduced mRNA levels of examined proinflammatory cytokines including CCL2 (MCP-1), TNFα, CCL5, and IFNγ. Immunofluorescent staining correspondingly revealed reduced macrophage infiltration in arteries harvested from Rip3-/- mice compared to that in Rip3+/+ mice (Figure 2E).
Rip3 deficiency in arterial wall is sufficient to inhibit aneurysm formation
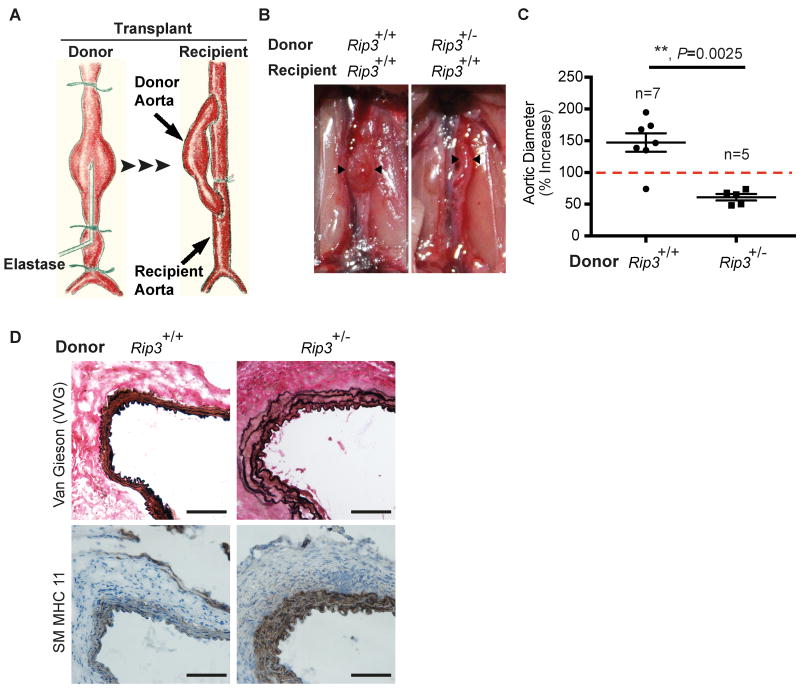
The formation and growth of aortic aneurysm involves a complex interplay between resident cells of the aortic wall and infiltrating inflammatory cells. To delineate the significance of RIP3 in resident aortic cells versus circulating inflammatory cells, we employed an aorta transplant model which we have recently developed (Figure 3A).28 We subjected a Rip3+/- mouse to aneurysm induction via elastase perfusion, as described for the conventional elastase model, for 5 minutes. The aortic segment was excised immediately following perfusion and transplanted to the abdominal aorta of a wildtype recipient mouse (Rip3+/- → Rip3+/+). A Rip3+/+ to Rip3+/+ transplantation was also carried out as a control. Consistent with what we have reported using C57BL/6 mice28, the elastase-perfused Rip3+/+ donor aortae in the Rip3+/+ recipients became dilated (147.2%±14.59%, 1.39mm±0.09mm) (Figure 3B and C; Online Figure VI) and displayed typical aneurysm characteristics including elastin fragmentation and SMC depletion (Figure 3D). In contrast, none of the 5 elastase-perfused Rip3+/- donor aortae developed aneurysmal dilatation in Rip3+/+ recipients (61.01%±5.05%, 0.87mm±0.02mm) (Figure 3B and C; Supplemental Figure II). Additionally, elastin fragmentation and SMC depletion in the Rip3+/- donor aortae were absent (Figure 3D). These results suggest that reducing the Rip3 gene by one copy in the aortic wall is sufficient to attenuate aneurysm development, which makes RIP3 an attractive therapeutic target.
Figure 3. Rip3 in the vascular wall is necessary for abdominal aortic aneurysm formation.
(A) Graphic illustration of a mouse aortic transplant model. Prior to tissue transplantation, donor abdominal aorta was subjected to aneurysm induction via elastase perfusion for 5 minutes. Injured aortic segment was excised immediately following perfusion and anastomosed to a recipient abdominal aorta in an end-to-side fashion. (B) Representative photos taken 14 days after transplantation. Donor aortae are indicated by arrowheads. (C) Maximal external diameters of donor aortae were measured at sacrifice and prior to elastase perfusion. Aortic dilatation calculated as the percentage increase of maximal external aortic diameter between pre-perfusion and on day 14 after perfusion. An AAA is defined as a percentage increase in aortic diameter greater than 100% (red dashed line). Data represent mean±SEM. n=5∼7. (D) Cross sections of harvested aortae were stained for Verhoeff-Van Gieson (VVG) in which elastin fibers are stained in dark or for smooth muscle myosin heavy chain 11 (SM MHC11). n=3. Scale bars=50μm.
RIP3-dependent SMC necroptosis
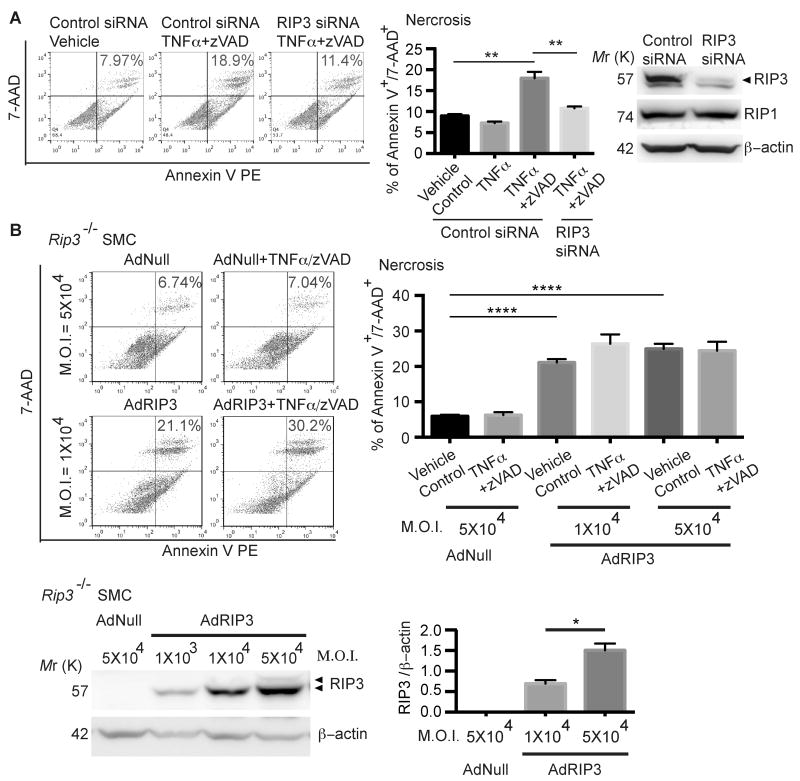
Results from the aorta transplant study indicate that RIP3 upregulation in resident cells of the aortic wall, rather than circulating inflammatory cells, is crucial for aneurysm formation and/or progression. Since SMCs are most abundant in the aortic wall, we further hypothesized that RIP3 is responsible for death and inflammation of vascular SMCs during aneurysm. To test this hypothesis, we challenged isolated aortic SMCs with a combination of TNFα (50 ng/ml) and pan caspase inhibitor zVAD (40μM), an established in vitro necroptosis induction protocol.15, 25, 29 As expected, aortic SMCs isolated from C57BL/6 mice (Figure 4A) responded to TNFα/zVAD with necrosis. Inhibition of RIP3 by either siRNA-mediated gene silencing (Rip3+/+ SMCs) or by gene deletion (Rip3-/- SMCs) (Figure 4A and B, Online Figure VII A) significantly diminished the ability of SMCs to undergo necrosis in response to TNFα/zVAD, which established that the form of necrosis detected here was RIP3-dependent necroptosis. By infecting Rip3-/- aortic SMCs with increasing concentrations of a RIP3 expressing-adenoviral vector (AdRIP3), we dose-dependently restored RIP3 protein expression (Figure 4B). Interestingly, Rip3-/- SMCs that were infected with 1×104 M.O.I. of AdRIP3 displayed spontaneous necroptosis (21.080±0.922%), which was further enhanced by the TNFα/zVAD treatment (Figure 4B). AdRIP3 at 5×104 M.O.I. caused even higher level of spontaneous necroptosis (24.920±1.414%), and rendered cells insensitive to the TNFα/zVAD treatment (Figure 4B).
Figure 4. Aortic smooth muscle cells (SMCs) undergo RIP3-dependent necroptosis in vitro.
(A) Aortic SMCs isolated from C57BL/6 mice were transfected with control siRNA or RIP3 siRNA. Cells were treated with DMSO (vehicle control), TNFα or TNFα plus zVAD for 24 hours. Cells were stained with PE Annexin V and 7-AAD and analyzed by flow cytometry. Necrotic cells were identified as PE Annexin V+/7-AAD+. The Western blot shown on the rightmost confirms the gene silence efficacy and specificity of RIP3 siRNA. Cell lysates were collected 36 hours post-transfection. **P<0.005. Data represent mean±SEM. n=3. (B) Rip3-/- aortic SMCs were infected with the empty adenoviral vector (AdNull) or the recombinant adenoviral vector expressing RIP3 (AdRIP3) at indicated multiplicity of infection (M.O.I.) units. After 24 hours, cells were treated with DMSO (vehicle control) or TNFα plus zVAD for 24 hours. Cell necrosis was determined by flow cytometry following staining with PE Annexin V and 7-AAD. Necrotic cells were identified as PE Annexin V+/7-AAD+. AdRIP3-mediated RIP3 expression in Rip3-/- SMCs was confirmed by Western blot. *P<0.05, ****P<0.0001. Data represent mean±SEM. n=3∼5.
Together, these data demonstrate that the level of RIP3 is a primary determinant of SMC necroptosis.
RIP3 mediates the inflammatory response of SMCs
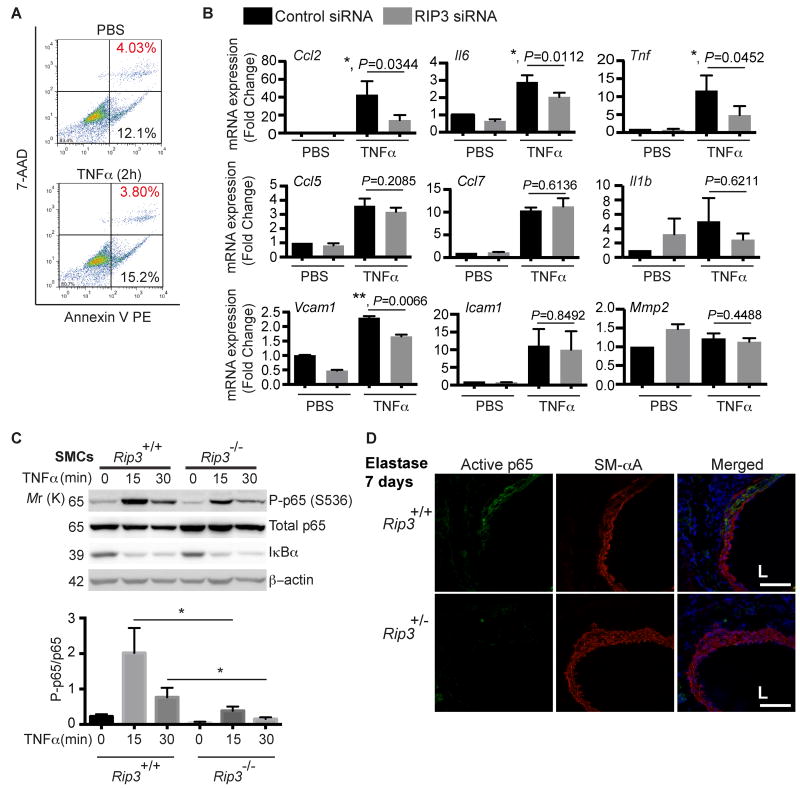
Vascular SMCs in injured or diseased vessels are a major source of pro-inflammatory chemokines.30-32 We next examined whether RIP3 plays a role in the inflammatory response of SMCs. TNFα is elevated in human AAA 33 and contributes to both CaCl2-induced and elastase-induced AAAs.34, 35 Here, TNFα was used as a trigger for inflammation. Aortic SMCs isolated from C57BL/6 mice were treated with 10ng/ml TNFα for 2 hours. At this condition, TNFα itself did not induce apoptosis or necroptosis (Figure 5A). We then examined the inflammatory gene and adhesion molecule expression by real-time PCR. As shown in Figure 5B, compared to control siRNA, RIP3 siRNA significantly reduced Ccl2 expression by 66.2%, Il6 expression by 29.8%, Tnf expression by 58.2% and Vcam1 expression by 27.9%. In contrast, Ccl5, Ccl7, Il1b, Icam1 and Mmp2 mRNA levels were not significantly affected by Rip3 knockdown (Figure 5B). Since TNFα is known to stimulate gene expression of chemokines through the nuclear factor-κB (NFκB) pathway, we compared NFκB signaling in Rip3+/+ and Rip3-/- SMCs. While both genotypes responded to TNFα with comparable IκB degradation, phosphorylation of p65 on serine536 was profoundly diminished by Rip3 gene knockout (Figure 5C). In contrast, RIP3 deletion didn't alter the phosphorylation status of p65 at Ser276 (Online Figure VIII) or nuclear retention of p65 following TNFα treatment (Online Figure IX). Furthermore, using an antibody that recognizes an epitope that overlaps with the nuclear localization signal of the p65 subunit, we examined level of the activated form of NFκB. As shown in Figure 5D and Online Figure X, aneurysm induction caused a robust increase in activation of p65 in aortic SMCs of Rip3+/+ mice. The NFκB activation was significantly reduced in Rip3 deficient mice.
Figure 5. RIP3 is involved in TNFα-induced cytokine expression in aortic SMCs.
(A) Aortic SMCs isolated from C57BL/6 mice were treated with TNFα (10ng/ml) for 2 h. The absence of cell death induced by TNFα at this concentration was confirmed by flow cytometry. n=2. (B) Aortic SMCs were transfected with control or RIP3-specific siRNA. After 48 hours, cells were stimulated with 10ng/ml TNFα for 2 hours for cytokines and Mmp2 or 2ng/ml TNFα for 4 hours for Vcam1 and Icam1. mRNA levels were determined by Real-time PCR. Data are mean±SEM. n=4. (C) Aortic SMCs isolated form Rip3+/+ and Rip3-/- mice were treated with 10ng/mL TNFα for indicated time. Cell lysates were subjected to Western blot analysis with indicated antibodies. *P<0.05. Data represent mean±SEM. n=4. (D) Representative photographs of aortic cross-sections harvested from mice on Day 7 post-perfusion with elastase. Sections were co-stained for active p65 and SM-αA. L indicates lumen. n=3. Scale bars=50μm.
PKCδ is required for SMC necroptosis
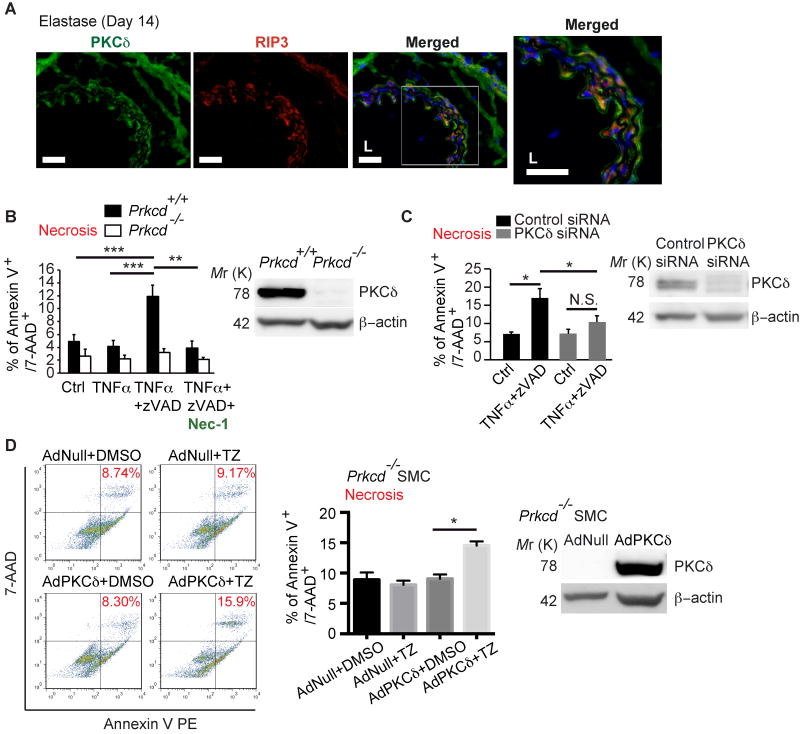
Protein Kinase C-delta (PKCδ) is endogenously expressed by vascular SMCs36 and becomes upregulated in injured arteries and aneurysmal tissues.10, 37 We have previously demonstrated that PKCδ plays an important role in SMC apoptosis38 and chemokine expression.31 Prkcd-/- mice are resistant to aneurysm inductions in both calcium chloride induced- and elastase-induced mouse models of AAA.10 Interestingly, co-immunostaining revealed that the RIP3 and PKCδ are co-expressed in the SMC-rich medial layer of mouse aneurysmal tissues (Figure 6A). To delineate functional interactions between RIP3 and PKCδ, we subjected aortic SMCs isolated from Prkcd-/- mice and their WT littermates to necroptosis induction with TNα/zVAD. Prkcd+/+ SMCs responded to the TNα/zVAD treatment with necrosis that was inhibited by necrostatin-1. However, Prkcd-/- SMCs failed to undergo necrostatin-1 sensitive-necroptosis (Figure 6B). Similar necroptosis resistances in both primary cultured aortic SMCs from C57BL/6 mice and MOVAS cells (a mouse aortic SMC line) were also produced by siRNA-mediated transient knockdown of Prkcd (Figure 6C; Online Figure VII B and XI A). Restoration of PKCδ expression with a PKCδ-expressing adenovirus (AdPKCδ) rescued sensitivity to necroptosis induction (Figure 6D). Notably, knocking down Prkcd in L929, a murine aneuploid fibrosarcoma cell line widely used in necroptosis studies39, did not protect cells from necroptosis (Supplemental Figure XI B), indicating that the involvement of PKCδ in necroptosis might be cell-type specific.
Figure 6. Prkcd -/- SMCs are resistant to necroptosis induction.
(A) Representative photographs of double immunostaining with PKCδ (green) and RIP3 (red) in elastase-perfused aortae harvested 14 days after surgery. Higher magnifications of highlighted regions are shown on the right. n=3. Scale bars=50μm. (B) Prkcd+/+ and Prkcd-/- SMCs were treated with DMSO (vehicle control), TNFα, TNFα plus zVAD with or without necrostatin-1 for 24 hours. Cell necrosis was determined by flow cytometric analysis following PE Annexin V and 7-AAD staining. Necrotic cells were identified as dual positive for both PE Annexin V and 7-AAD. Quantification of Necrosis (% of PE Annexin-V+/7-AAD+) is shown. The Western blot confirms the lack of PKCδ protein in Prkcd-/- SMCs. (C) Aortic SMCs were transfected with control siRNA or PKCδ-specific siRNA for 24 hours followed by treatment with DMSO (vehicle control) or TNFα plus zVAD for additional 24 hours. Cell necrosis was determined as described in (B). The efficacy of PKCδ knockdown was confirmed by Western blot. (D) Prkcd-/- aortic SMCs were infected with AdNull or AdPKCδ at M.O.I. of 5×104. Expression of PKCδ was analyzed by Western blot (rightmost panel). 36 hours post-infection, cells were treated with DMSO or TNFα plus zVAD (TZ). Cell necrosis was determined as described in (B). *P<0.05; **P<0.005; ***P<0.0005. Data represent mean±SEM. n=3 (B and D), n=5 (C).
PKCδ regulates RIP3 expression in SMCs
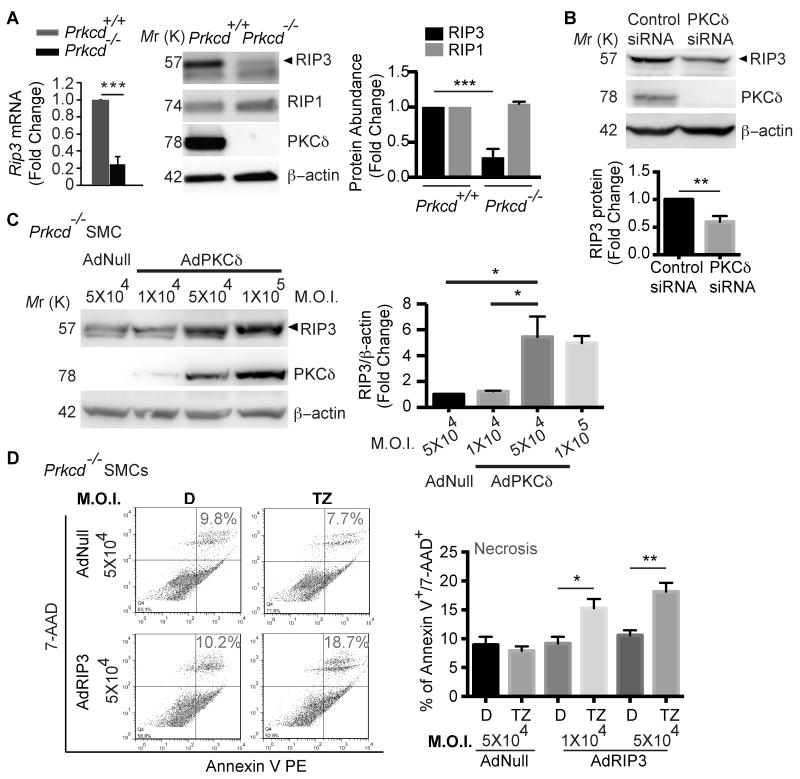
Given the importance of cellular RIP3 level in necroptosis, we next analyzed RIP3 expression in aortic SMCs isolated from Prkcd-/- mice and their WT littermates. As shown in Figure. 7A, levels of RIP3 mRNA and protein were markedly reduced in Prkcd-/- SMCs as compared to WT SMCs. RIP1 protein level was not significantly changed by PKCδ deficiency. Transient knockdown of Prkcd in SMCs isolated from C57BL/6 mice also produced a significant downregulation of RIP3 (Figure. 7B). AdPKCδ-mediated restoration of PKCδ expression in Prkcd-/- SMCs dose-dependently corrected RIP3 protein contents (Figure 7C). Moreover, analysis of Rip3 expression in tissue extracts from our prior PKCδ study showed a significantly lower level of aneurysm-associated RIP3 expression in the aortic wall of Prkcd-/- mice as compared to the wildtype mice (Online Figure XII). Collectively, these data demonstrate an essential role of PKCδ in controlling RIP3 expression in aortic SMCs under both resting and stressed conditions.
Figure 7. PKCδ regulates necroptosis through controlling RIP3 expression.
(A) Aortic SMCs isolated from Prkcd+/+ and Prkcd-/- mice were analyzed for Rip3 expression by real-time PCR and Western blot. ***P<0.0005. Data represent mean±SEM. n=3. (B) Aortic SMCs isolated from C57BL/6 mice were transfected with control siRNA or PKCδ siRNA. Cell lysates were collected 48 hours post-transfection and aliquots of 30μg protein were subjected for Western blot analysis of RIP3, PKCδ, and β-actin. Data represent mean±SEM. n=6. (C) Prkcd-/- aortic SMCs were infected with AdNull or AdPKCδ at indicated M.O.I. units. Cell lysates were collected 48 hours post-infection and aliquots of 30μg protein were analyzed by Western blot with indicated antibodies. Data represent mean±SEM. n=3. (D) Prkcd-/- aortic SMCs were infected with AdNull or AdRIP3. 48 hours post-infection, cells were treated with DMSO (D, vehicle control) or TNFα plus zVAD (TZ) for 24 hours. Cell necrosis was determined by flow cytometric analysis following PE Annexin V and 7-AAD staining. Necrotic cells were identified as PE Annexin V+/7-AAD+. Data represent mean±SEM. n=6 (D). *P<0.05; **P<0.005; ***P<0.0005.
To further delineate the functional relationship between PKCδ and RIP3 in necroptosis, we ectopically expressed RIP3 in Prkcd-/- SMCs using AdRIP3 (Figure 7D). AdRIP3 successfully rescued the necroptosis response in the absence of PKCδ (Figure 7D). AdPKCδ failed to rescue the necroptosis defect of Rip3-/- SMCs (Online Figure XIII).
Discussion
The aneurysm resistant phenotype rendered by Rip3 heterozygous and homozygous gene deletion, along with elevated RIP3 expression detected in human and experimental aneurysmal tissues, highlights the importance of RIP3 in aneurysm pathophysiology. The finding that aortic segments from Rip3+/- mice were resistant to elastase-induced AAA even when allograft to the aortae of Rip3+/+ mice indicates that RIP3 in cells resident to the aortic wall, most likely SMCs, is critical for the development of aneurysm.
Due to the lack of specific histological marker of necroptosis, we were unable to determine in vivo to what degree the lack of RIP3 inhibits necroptosis. Despite this, we found that the number of PI-positive necrotic cells, which include necroptotic cells, was markedly reduced in Rip3-/- aortae as compared to Rip3+/+ aortae following elastase-perfusion. Necrosis is considered as a pro-inflammatory event, and thus suppression of vascular inflammation by Rip3 deficiency is not unexpected. However, our data suggest that in aortic SMCs RIP3 may directly modulate inflammation. The necroptosis-independent function of RIP3 in inflammation is supported by: (1) in isolated aortic SMCs, knockdown or knockout Rip3 attenuated TNFα-induced phosphorylation of p65 serine536 and expression of several pro-inflammatory cytokines; (2) following elastase-perfusion, we observed reduced p65 activation in Rip3 deficient aortae as compared to Rip3+/+ aortae.
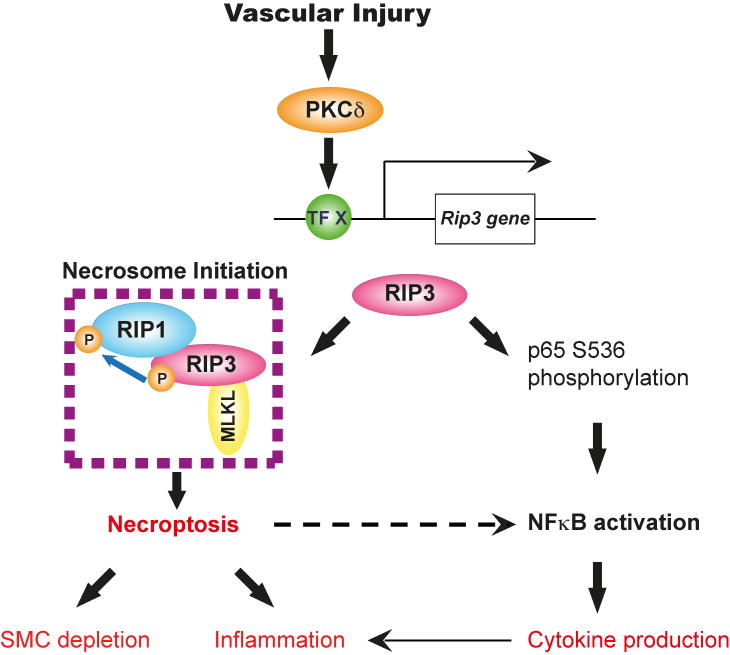
We therefore propose a model that elevated RIP3 in the aortic wall contribute to aneurysm pathogenesis through both necroptosis-dependent and independent mechanisms (Figure 8). In this model, RIP3 mediates SMC necroptosis by initiating necrosome formation and also actively involves in inflammation by stimulating NFκB. It is unclear whether crosstalks occur between these two RIP3-mediated pathways. Clarke et al shows that primary necrotic SMCs release IL1α, which could subsequently cause the neighboring viable aortic SMCs to produce IL6 and MCP-1.40 Since necroptosis is a form of primary necrosis, a similar mechanism is likely to exist downstream from necrosome formation, as indicated in Figure 8 by a dotted arrow.
Figure 8. Proposed roles of RIP3 in pathophysiology of abdominal aortic aneurysm.
RIP3 is upregulated in the injured aneurysmal wall via a PKCδ-mediated mechanism (TF X: Transcription factor X). In the presence of elevated RIP3, SMCs respond to TNFα or other cytokines by undergoing necroptosis or NFκB-mediated chemokine/cytokine production. The precise determinants that switch RIP3 between necroptosis and NFκB in SMCs remain unclear, however, both processes may contribute to inflammation in aortic wall during AAA development and progression.
Our data of the direct involvement of RIP3 in NFκB-mediated vascular inflammation adds support to the notion of necroptosis-independent function of RIP3, which has only been reported in a few studies thus far.41, 42 Interestingly, we found in aortic SMCs that Rip3 knockdown significantly attenuated TNFα-induced expression of Ccl2 (MCP-1), Il6, Tnf and Vcam1 but not Ccl5, Ccl7, Il1b, Icam1or Mmp2. Since all of these molecules are known to be regulated by NFκB, we speculate that the selective requirement of RIP3 might be related to their sensitivity to serine536 phosphorylation of p65, which was attenuated in Rip3-/- SMCs treated with TNFα. Among the multiple phosphorylation sites within p65, serine536 is critical for enhancing the transcriptional activity of NFκB by recruitment of TATA-binding protein-associated factor II31.43 It is possible that not all cytokine genes require this fine-tuning mechanism for optimal expression.
Apoptosis and necrosis are two major mechanisms through which cell death occurs. Recent findings suggest that apoptosis and necroptosis are not mutually exclusive mechanisms, perhaps taking place in the same disease process.14, 22 Multiple factors identified in aneurismal tissues such as TNFα 13, 34, 35 and Fas ligand 13, 44 could trigger apoptosis as well as necroptosis. The molecular network regulating necroptosis is known to intertwine with apoptotic regulators13, 14, one such example being the involvement of RIP1 in both apoptosis and necroptosis.44, 45 Indeed, we found that many RIP1 positive cells were also apoptotic, evidenced by double positive for TUNEL. In comparison, none of elastase-treated aortic sections we examined contained RIP3 and TUNEL double-positive cells. The lack of direct involvement of RIP3 in the apoptotic pathway is supported by the published observations that Rip3 deficiency inhibits necroptosis but not apoptosis.16, 22
Atherosclerosis and AAA share many common risk factors including aging and smoking. Mechanistically, chronic inflammation underlies both types of vascular disease. Data generated by this study and by the previous work implicate that RIP3 contribute to disease progression of both AAA and atherosclerosis16, though, through discrete mechanisms. We show here that loss of Rip3 in the aortic wall was sufficient to protect mice from AAA, while Lin et al. found that Rip3 deficiency in vascular wall is not responsible for the diminished atherosclerotic lesion burden.16 While our arterial transplant experiments support the importance of RIP3 in the vascular wall, we cannot exclude a potential role for RIP3 in circulating inflammatory cells. Generation of cell-specific knockouts will be beneficial to our understanding of cell-specific functions of RIP3 in aneurysm as well as other diseases. Another interesting divergent result from these two disease models is the phenotype of Rip3 heterozygous mutants. Rip3+/- ;Apoe -/- mice develop atherosclerotic plaques that are smaller than those in Rip3+/+; Apoe-/- mice but larger compared to Rip3-/-; Apoe-/- mice.16 Our data demonstrated that Rip3+/- mice developed significantly smaller aortic dilatations in response to the AAA induction compared to their wildtype littermates. However, statistical analysis showed no difference in aortic dilatations between Rip3+/- and Rip3-/- mice.
Prkcd gene deficient mice are highly resistant to aneurysm development triggered by two different experimental methods, and vascular SMC death is attenuated in Prkcd-/- mice.10 At the time, we attributed this observation to the stimulatory role of PKCδ in apoptosis and chemokine expression. The current findings indicate a new role of PKCδ in regulating necroptosis in vascular SMCs, which is at least in part through the regulation of Rip3 gene expression. RIP3 expression is essential for cells to undergo necroptosis.15, 25, 45 In support of this notion, we showed that overexpression of RIP3 in Rip3-/- SMCs via an adenoviral vector was sufficient to elicit necroptosis in the absence of exogenous triggers. Of note, adenovirus-mediated expression of RIP3 in Prkcd-/- SMCs failed to induce necroptosis in the absence of TNFα and zVAD as it did in Rip3-/- SMCs. The exact causes of this discrepancy is unclear, however, several factors might be involved. First, Prkcd-/- mice are on a mixed background of C57BL/6 and 129/Sv while Rip3-/- mice are on a C57BL/6J background. These different genetic backgrounds can very well affect the necroptosis response. Second, PKCδ is a critical mediator of SMC apoptosis.31, 38 Overexpression of PKCδ may activate several downstream signaling pathways in addition to RIP3. Finally, it is also possible that PKCδ may be required for optimal activation of RIP3.
Although PKC is reported to be required for a pan-caspase inhibitor zVAD-fmk (zVAD) induced necroptosis in L929 cells by regulating autocrine TNFα production via the PKC-MAPKs-AP-1 pathway 46, knocking down PKCδ in L929 appeared to have little effects on necroptosis, suggesting that the involvement of PKCδ in necroptosis is likely to be cell-type specific.
Several mouse models have been created for AAA, each with unique strengths and weaknesses.26 The elastase-induced mouse model of AAAs used here is a widely used model that recapitulates the major pathological features of human AAA including SMC depletion, inflammation, and elastin degradation.26, 27 There are several limitations associated with this model. For example, AAA rupture, a major complication in human AAA7, is rare in this model. Mechanical effects of elastase perfusion and variability from different commercial elastase preparation are also noted in this model.26 It is important to recognize that none of current animal models reproduces all aspects of human AAAs. In our study we did not detect significant changes in RIP1 in the elastase-induced aneurysms while this protein, which is known for its role in apoptosis, necroptosis as well as cell survival, was found to be elevated in human aneurysmal tissues. Whether this discrepancy reflects different regulation of RIP1 in human versus mouse or a limitation of the particular aneurysm model used in our studies remains to be tested.
To summarize, through the use of Rip3 gene deficient mice and an aorta transplant model, we demonstrate for the first time that enhanced RIP3 signaling in arterial wall is a critical pathological process during aneurysm progression, at least in elastase-induced mouse model of AAA. Mechanistic studies demonstrate a critical role for RIP3 in mediating SMC necroptosis as well as NF-κB–mediated inflammatory response in SMCs. Notably, overexpression of RIP3 is sufficient to induce necroptosis in SMCs, suggesting the importance of RIP3 expression level in determining cell necroptosis. . Interestingly, PKCδ is necessary for RIP3 expression and necroptosis in SMCs, though this role for PKCδ may be cell type specific.
Supplementary Material
Novelty and Significance.
What Is Known?
Depletion of medial smooth muscle cells (SMCs), along with inflammation and elastin degradation, is a major histological characteristic of human abdominal aortic aneurysms (AAAs), which is replicated in a number of murine AAA models including the elastase perfusion model.
Receptor-interacting protein kinase 3 (RIP3) is a critical mediator of a regulated form of necrosis called necroptosis.
Protein kinase C delta (PKCδ) is elevated in human aneurysmal tissues and contributes to AAA formation in murine models.
What New Information Does This Article Contribute?
RIP3 is elevated in human and mouse aneurysmal tissues and is necessary for SMC depletion and aneurysm formation in the murine elastase AAA model.
In isolated aortic SMCs, RIP3 is required for TNFα-induced necroptosis as well as NF-κB-mediated inflammatory response.
PKCδ is involved in RIP3-mediated necroptosis of aortic SMCs, at least in part through the regulation of Rip3 gene expression.
AAA is a common and highly lethal vascular disease with no effective pharmacological therapies available. The rising prevalence of AAA and the lack of approved drug treatments highlight the need for a detailed understanding of the disease mechanism. Necroptosis is a recently described form of regulated necrosis. Whether necroptosis contributes to the paucity of aortic SMCs associated with AAAs has not yet been documented. Our work demonstrates that human aneurysmal tissues contain elevated levels of the necroptosis mediators RIP1 and RIP3. In the elastase-induced mouse model of AAA, deletion of one or both copies of the Rip3 gene protects mice from developing AAA and aneurysm-associated pathological features including SMC depletion and inflammation. In primary aortic SMCs, we found that RIP3 is required for both TNFα-induced necroptosis and NF-κB-mediated inflammatory response. We show that PKCδ, a known contributor to AAA disease, serves as an upstream regulator of RIP3 expression and necroptosis in SMCs. Our work implicates RIP3 in contributing to elastase-induced AAA development via regulation of SMC death and inflammation. RIP3 may therefore serve as a novel target for aneurysm treatment.
Acknowledgments
We thank Dr. Vishva M. Dixit of Genentech and Dr. Harris Perlman of Northwestern University for Rip3+/- mice, Dr. Keiichi I. Nakayama of Kyushu University in Japan for the generous gift of Prkcd+/- mice.
Sources of Funding: This study was supported by NIH R01HL088447 (B. Liu), American Heart Association predoctoral fellowship 14PRE18560035 (Q. Wang), and the Ruth L. Kirschstein National Research Service Award T32 HL 07936 and T32 HL110853 from the National Heart, Lung, and Blood Institute to the University of Wisconsin-Madison Cardiovascular Research Center and Department of Surgery (S. Morgan). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Nonstandard Abbreviations and Acronyms
- AAA
Abdominal Aortic Aneurysm
- ANOVA
Analysis of Variance
- CD68
Cluster of Differentiation 68
- DMSO
Dimethyl sulfoxide
- PI
Propidium iodide
- PKCδ
Protein Kinase C, delta
- RIP1
Receptor-interacting serine/threonine-protein kinase 1
- RIP3
Receptor-interacting serine/threonine-protein kinase 3
- RNA
Ribonucleic acid
- RT-PCR
Real time polymerase chain reaction
- TNF
Tumor necrosis factor
- zVAD
Carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone
Footnotes
Disclosures: None.
References
- 1.Baxter BT, Terrin MC, Dalman RL. Medical management of small abdominal aortic aneurysms. Circulation. 2008;117:1883–1889. doi: 10.1161/CIRCULATIONAHA.107.735274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weintraub NL. Understanding abdominal aortic aneurysm. N Engl J Med. 2009;361:1114–1116. doi: 10.1056/NEJMcibr0905244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. The Journal of Clinical Investigation. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase b) suppresses development of experimental abdominal aortic aneurysms. The Journal of Clinical Investigation. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, Sun C, Zhang Y, Liu J, Ennis TL, Knispel R, Xiong W, Thompson RW, Baxter BT, Shi G-P. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. The Journal of Clinical Investigation. 2007;117:3359–3368. doi: 10.1172/JCI31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 8.Curci JA. Digging in the “soil” of the aorta to understand the growth of abdominal aortic aneurysms. Vascular. 2009;17(Suppl 1):S21–29. doi: 10.2310/6670.2008.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Candales A, Holmes D, Liao S, Scott M, Wickline S, Trompson R. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997;150:993–1007. [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan S, Yamanouchi D, Harberg C, Wang Q, Keller M, Si Y, Burlingham W, Seedial S, Lengfeld J, Liu B. Elevated protein kinase c-delta contributes to aneurysm pathogenesis through stimulation of apoptosis and inflammatory signaling. Arterioscler Thromb Vasc Biol. 2012;32:2493–2502. doi: 10.1161/ATVBAHA.112.255661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamanouchi D, Morgan S, Kato K, Lengfeld J, Zhang F, Liu B. Effects of caspase inhibitor on angiotensin ii-induced abdominal aortic aneurysm in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2010;30:702–707. doi: 10.1161/ATVBAHA.109.200527. [DOI] [PubMed] [Google Scholar]
- 12.Edinger AL, Thompson CB. Death by design: Apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Han J, Zhong CQ, Zhang DW. Programmed necrosis: Backup to and competitor with apoptosis in the immune system. Nat Immunol. 2011;12:1143–1149. doi: 10.1038/ni.2159. [DOI] [PubMed] [Google Scholar]
- 14.Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to tnf-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Lin J, Li H, Yang M, Ren J, Huang Z, Han F, Huang J, Ma J, Zhang D, Zhang Z, Wu J, Huang D, Qiao M, Jin G, Wu Q, Huang Y, Du J, Han J. A role of rip3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 2013;3:200–210. doi: 10.1016/j.celrep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Newton K, Sun X, Dixit VM. Kinase rip3 is dispensable for normal nf-kappa bs, signaling by the b-cell and t-cell receptors, tumor necrosis factor receptor 1, and toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 19.Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of rip1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase rip mediates the tnf-induced nf-kappab signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 21.Teng X, Degterev A, Jagtap P, Xing X, Choi S, Denu R, Yuan J, Cuny GD. Structure-activity relationship study of novel necroptosis inhibitors. Bioorg Med Chem Lett. 2005;15:5039–5044. doi: 10.1016/j.bmcl.2005.07.077. [DOI] [PubMed] [Google Scholar]
- 22.Trichonas G, Murakami Y, Thanos A, Morizane Y, Kayama M, Debouck CM, Hisatomi T, Miller JW, Vavvas DG. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc Natl Acad Sci U S A. 2010;107:21695–21700. doi: 10.1073/pnas.1009179107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luedde M, Lutz M, Carter N, Sosna J, Jacoby C, Vucur M, Gautheron J, Roderburg C, Borg N, Reisinger F, Hippe HJ, Linkermann A, Wolf MJ, Rose-John S, Lullmann-Rauch R, Adam D, Flogel U, Heikenwalder M, Luedde T, Frey N. Rip3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc Res. 2014;103:206–216. doi: 10.1093/cvr/cvu146. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi N, Duprez L, Grootjans S, Cauwels A, Nerinckx W, DuHadaway JB, Goossens V, Roelandt R, Van Hauwermeiren F, Libert C, Declercq W, Callewaert N, Prendergast GC, Degterev A, Yuan J, Vandenabeele P. Necrostatin-1 analogues: Critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 2012;3:e437. doi: 10.1038/cddis.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the rip1-rip3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 27.Thompson RW, Curci JA, Ennis TL, Mao D, Pagano MB, Pham CT. Pathophysiology of abdominal aortic aneurysms: Insights from the elastase-induced model in mice with different genetic backgrounds. Ann N Y Acad Sci. 2006;1085:59–73. doi: 10.1196/annals.1383.029. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Wang Q, Ren J, Assa CR, Morgan S, Giles J, Han Q, Liu B. Murine abdominal aortic aneurysm model by orthotopic allograft transplantation of elastase-treated abdominal aorta. J Vasc Surg. 2014 doi: 10.1016/j.jvs.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han JH. Rip3, an energy metabolism regulator that switches tnf-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 30.Si Y, Ren J, Wang P, Rateri DL, Daugherty A, Shi XD, Kent KC, Liu B. Protein kinase c-delta mediates adventitial cell migration through regulation of monocyte chemoattractant protein-1 expression in a rat angioplasty model. Arterioscler Thromb Vasc Biol. 2012;32:943–954. doi: 10.1161/ATVBAHA.111.244921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren J, Wang Q, Morgan S, Si Y, Ravichander A, Dou C, Kent KC, Liu B. Protein kinase c-delta (pkcdelta) regulates proinflammatory chemokine expression through cytosolic interaction with the nf-kappab subunit p65 in vascular smooth muscle cells. J Biol Chem. 2014;289:9013–9026. doi: 10.1074/jbc.M113.515957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexander MR, Murgai M, Moehle CW, Owens GK. Interleukin-1beta modulates smooth muscle cell phenotype to a distinct inflammatory state relative to pdgf-dd via nf-kappab-dependent mechanisms. Physiol Genomics. 2012;44:417–429. doi: 10.1152/physiolgenomics.00160.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juvonen J, Surcel HM, Satta J, Teppo AM, Bloigu A, Syrjala H, Airaksinen J, Leinonen M, Saikku P, Juvonen T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1997;17:2843–2847. doi: 10.1161/01.atv.17.11.2843. [DOI] [PubMed] [Google Scholar]
- 34.Xiong W, MacTaggart J, Knispel R, Worth J, Persidsky Y, Baxter BT. Blocking tnf-alpha attenuates aneurysm formation in a murine model. J Immunol. 2009;183:2741–2746. doi: 10.4049/jimmunol.0803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hingorani A, Ascher E, Scheinman M, Yorkovich W, DePippo P, Ladoulis CT, Salles-Cunha S. The effect of tumor necrosis factor binding protein and interleukin-1 receptor antagonist on the development of abdominal aortic aneurysms in a rat model. J Vasc Surg. 1998;28:522–526. doi: 10.1016/s0741-5214(98)70139-9. [DOI] [PubMed] [Google Scholar]
- 36.Itoh H, Yamamura S, Ware JA, Zhuang S, Mii S, Liu B, Kent KC. Differential effects of protein kinase c on human vascular smooth muscle cell proliferation and migration. Am J Physiol Heart Circ Physiol. 2001;281:H359–370. doi: 10.1152/ajpheart.2001.281.1.H359. [DOI] [PubMed] [Google Scholar]
- 37.Yamanouchi D, Kato K, Ryer EJ, Zhang F, Liu B. Protein kinase c delta mediates arterial injury responses through regulation of vascular smooth muscle cell apoptosis. Cardiovasc Res. 2010;85:434–443. doi: 10.1093/cvr/cvp328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato K, Yamanouchi D, Esbona K, Kamiya K, Zhang F, Kent KC, Liu B. Caspase-mediated protein kinase c-{delta} cleavage is necessary for apoptosis of vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2009;297:H2253–2261. doi: 10.1152/ajpheart.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen W, Zhou Z, Li L, Zhong CQ, Zheng X, Wu X, Zhang Y, Ma H, Huang D, Li W, Xia Z, Han J. Diverse sequence determinants control human and mouse receptor interacting protein 3 (rip3) and mixed lineage kinase domain-like (mlkl) interaction in necroptotic signaling. J Biol Chem. 2013;288:16247–16261. doi: 10.1074/jbc.M112.435545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke MC, Talib S, Figg NL, Bennett MR. Vascular smooth muscle cell apoptosis induces interleukin-1-directed inflammation: Effects of hyperlipidemia-mediated inhibition of phagocytosis. Circ Res. 2010;106:363–372. doi: 10.1161/CIRCRESAHA.109.208389. [DOI] [PubMed] [Google Scholar]
- 41.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase ripk3-mediated activation of the nlrp3 inflammasome. Immunity. 2013;38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Moriwaki K, Balaji S, McQuade T, Malhotra N, Kang J, Chan FK. The necroptosis adaptor ripk3 promotes injury-induced cytokine expression and tissue repair. Immunity. 2014;41:567–578. doi: 10.1016/j.immuni.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buss H, Dorrie A, Schmitz ML, Hoffmann E, Resch K, Kracht M. Constitutive and interleukin-1-inducible phosphorylation of p65 nf-{kappa}b at serine 536 is mediated by multiple protein kinases including i{kappa}b kinase (ikk)-{alpha}, ikk{beta}, ikk{epsilon}, traf family member-associated (tank)-binding kinase 1 (tbk1), and an unknown kinase and couples p65 to tata-binding protein-associated factor ii31-mediated interleukin-8 transcription. J Biol Chem. 2004;279:55633–55643. doi: 10.1074/jbc.M409825200. [DOI] [PubMed] [Google Scholar]
- 44.Wang Q, Ren J, Morgan S, Liu Z, Dou C, Liu B. Monocyte chemoattractant protein-1 (mcp-1) regulates macrophage cytotoxicity in abdominal aortic aneurysm. PLoS One. 2014;9:e92053. doi: 10.1371/journal.pone.0092053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 46.Wu YT, Tan HL, Huang Q, Sun XJ, Zhu X, Shen HM. Zvad-induced necroptosis in l929 cells depends on autocrine production of tnfalpha mediated by the pkc-mapks-ap-1 pathway. Cell Death Differ. 2011;18:26–37. doi: 10.1038/cdd.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, Tsukiyama T, Nagahama H, Ohno S, Hatakeyama S, Nakayama KI. Increased proliferation of b cells and auto-immunity in mice lacking protein kinase cdelta. Nature. 2002;416:865–869. doi: 10.1038/416865a. [DOI] [PubMed] [Google Scholar]
- 48.Clowes A, Clowes M, Fringerle J, Reidy M. Kinetics of cellular poliferation after arterial injury: Role of acute distension in the induciton of smooth muscle proliferation. Lab Invest. 1989;60:360–364. [PubMed] [Google Scholar]
- 49.Clowes MM, Lynch CM, Miller AD, Miller DG, Osborne WR, Clowes AW. Long-term biological response of injured rat carotid artery seeded with smooth muscle cells expressing retrovirally introduced human genes. J Clin Invest. 1994;93:644–651. doi: 10.1172/JCI117016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.