Abstract
Fibroblast growth factor (FGF) 9 is essential for lung development and is highly expressed in a subset of human lung adenocarcinomas. We recently described a mouse model in which FGF9 expression in the lung epithelium caused proliferation of the airway epithelium at the terminal bronchioles and led to rapid development of adenocarcinoma. Here, we used this model to characterize the effects of prolonged FGF9 induction on the proximal and distal lung epithelia, and examined the propagation potential of FGF9-induced lung tumors. We show that prolonged FGF9 overexpression in the lung resulted in the development of adenocarcinomas arising from both alveolar type II and airway secretory cells in the lung parenchyma and airways, respectively. We found that tumor cells harbored tumor-propagating cells that were able to form secondary tumors in recipient mice regardless of FGF9 expression. However, the highest degree of tumor propagation was observed when unfractionated tumor cells were coadministered with autologous, tumor-associated mesenchymal cells. Although the initiation of lung adenocarcinomas was dependent on activation of the FGF9/FGF receptor (FGFR) 3 signaling axis, maintenance and propagation of the tumor was independent of this signaling. Activation of an alternative FGF/FGFR and the interaction with tumor stromal cells is likely to be responsible for the development of this independence. This study demonstrates the complex role of FGF/FGFR signaling in the initiation, growth, and propagation of lung cancer. Our findings suggest that analyzing the expressions of FGFs/FGFRs in human lung cancer will be a useful tool for guiding customized therapy.
Keywords: FGF9, FGFR, Adenocarcinoma, Tumor propagation, Lung cancer, Tumor-associated lung fibroblasts
Introduction
The detection of large numbers of mutations that affect key signaling pathways in non-small cell lung cancer (NSCLC) led to the development of drugs that specifically target these pathways[1,2]. The earliest example is the activating mutation in the epidermal growth factor receptor (EGFR) that led to the development of EGFR tyrosine kinase inhibitors (TKIs)[3]. However, despite favorable initial responses, patients inevitably acquire resistance to EGFR TKI[4]. In addition, EGFR mutations were detected in only 5% and 40% of Caucasian and Asian NSCLC patients, respectively [5,6]. This probably reflects the existence of additional receptor tyrosine kinase systems, distinct from EGFR.
Fibroblast growth factors (FGF) and their tyrosine kinase receptors (FGFRs) signaling pathways are expressed in 5–10% of human lung adenocarcinomas and thus comprise an attractive therapeutic targets[1,2]. They regulate cell proliferation, differentiation, migration, and survival, and are important for embryonic development and angiogenesis[7]. FGF/FGFR signaling alterations are linked to several cancers, including lung[8]. There is ample evidence for the involvement of distinct FGFs and FGFRs in lung cancer[9] and that molecular silencing of FGF or FGFR inhibition with drugs can interfere with NSCLC growth[10]. Activation of the FGF2/FGFR1, FGFR2, and/or FGFR3 signaling could induce EGFR-TKI resistance[11-13].
Our previous data indicated that FGF9 expression in NSCLC specimens was associated with poor prognosis[14]. We used the doxycycline-inducible surfactant protein C-reverse tetracycline activator (Sftpc-rtTA) and tetracycline-responsive Tre-Fgf9-ires-eGfp double-transgenic (DT) mouse to induce FGF9 and EGFP expressions in cells that express surfactant protein-C (Sftpc) and found that FGF9 expression in adult lungs resulted in the rapid development of multiple adenocarcinoma-like tumor nodules, with small epithelial nodules already visible within 24 hours after FGF9 induction[15]. The very rapid response of adult lung tissues to FGF9 prompted us to perform most tumor analyses on days 4 and 8. At these early time points, most nodules and proliferating cells were in the distal bronchiolar epithelium near the bronchioalveolar duct junction (BADJ)[15]. In the current study, we aimed to examine the effects of prolonged FGF9 exposure on lung epithelial cells. We also investigated whether cancer stem cells (CSCs) were present within the tumor by comparing the propagation potential of several cellular subpopulations. Finally, we used a three-dimensional (3-D) in vitro colony formation assay to examine the mechanism by which tumor cells become FGF9-independent.
Methods
Mice
DT mice were maintained on FVB background as described[15]. Mice used for the propagation study were FVB wild-type (wt) and athymic nude Foxn1nu (hereafter, nude)(Charles River, Wilmington, MA). Doxycycline chow was from PMI Nutrition International (Modified Lab 5TP7). Animal experiments were approved by the Institutional Animal Care and Use Committee of Keio University.
MicroCT
DT and recipient mice from the propagation experiments were examined using the in vivo micro-X-ray-computed tomography (CT) system R_mCT2 (Rigaku, Tokyo, Japan) before doxycycline administration and monthly thereafter. Instrument settings are described in the online supplementary information.
Lung collection and histological processing
The DT and recipient mice from the propagation experiments were anesthetized and exsanguinated (at the indicated timepoints) as described[15]. The thoracic cavity was opened, and the lungs were exposed. The trachea was cannulated (21G), inflated with 4% paraformaldehyde, resected en-bloc, and examined for GFP-expressing nodules by using a fluorescent stereo-microscope (Leica M205FA, Mannheim, Germany). Paraffin-embedded lungs were sectioned (thickness = 6 μm). The whole lung thickness was examined by collecting 15–20 100 μm-spaced-apart sections that were stained with hematoxylin and eosin to identify tumor nodules/abnormalities under microscopy (Olympus BX53, Olympus, Tokyo). A pathologist with experience in rodent lung cancer was regularly consulted. To examine extrapulmonary seeding/metastasis, the brain, heart, liver, spleen, kidneys and mediastinum were analyzed.
Histology, immunofluorescence, and quantification of marker expression
The paraffin sections were stained with cell-type specific antibody as previously described[15]. Marker expression was quantified by counting the positively stained cells as described in the online supplementary information.
Lung digestion, fluorescence-activated cell sorting, and tumor propagation
The lungs of doxycycline-fed DT mice were digested into single-cell suspension. Cells were used as such (WLCs) or further stained with EpCAM antibody or Sca1 microbeads for sorting. Cells (103–105 cells/100 μL) were injected intratracheally, subcutaneously, or intravenously as described previously[15], in Supplementary Table 1 and the online supplementary information.
PCR and Quantitative real-time PCR
Total RNA was extracted from fibroblasts using the RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. FGF/FGFR gene expression levels were analyzed using TaqMan® assays on the StepOnePlusTM Real-Time PCR System (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. The sequences of the Taqman primers/probes and primers used to detect the GFP insert in nodules’ DNA are described in the online supplementary information.
Air-liquid interface (ALI) and in vitro 3D-organoid sphere cultures and treatments
The protocols for ALI and 3D sphere cultures were described previously [16, 17] and are described in the online supplementary information.
Statistical analysis
Data from ≥3 independent samples/experiments were averaged and either presented as percentage of total; or were compared using the two-tailed Student t test (p < 0.05, considered statistically significant).
Results
Characterization of the cells of origin and phenotypic differences of adenocarcinomas arising in the DT mouse lungs caused by prolonged FGF9 overexpression
Although SFTPC expression in the lung was thought to be localized to alveolar type II (AT II) cells[18-20], it was recently also detected in Club (Clara) cells at the BADJ and terminal bronchioles (TBs), and these cells were proposed to be the cells of origin of lung adenocarcinoma[21]. We observed FGF9 and GFP expressions primarily in the AT-II and TB epithelial cells of DT mice 4 hours after doxycycline administration (Figure S1A and B). However, most nodules and proliferating cells that were observed within 1 week of doxycycline administration were in the TBs rather than the lung parenchyma[15]. We hypothesized that prolonged FGF9 expression would promote adenocarcinoma development in other lung compartments. Therefore, we examined lung sections from mice that were fed doxycycline chow for up to 6-months (n=6). Large nodules were detected in all the lung compartments, including the proximal bronchi, mid-level bronchioles, and alveolar parenchyma (Figure 1A). Although nodule development in the airways, which lack SFTPC expression, could have been induced result from paracrine effects mediated by FGF9 secreted from alveoli and TBs, we consistently observed SFTPC+/GFP+ airway cells both early (FigureS1C) and late after induction (Figure s1B). We hypothesized that paracrine exposure of airway epithelial cells to FGF9 secreted by the nearby AT-II and TB cells could induce SFTPC expression and thus further activating and expanding the domain of expression of the Tre-Fgf9-ires-eGfp transgene. To prove this, recombinant FGF9 or PBS were administered intratracheally to wt mice (n=2 per group), and the mice were killed after 2 days. SFTPC+ cells in the control mice were only detected at the TBs near the BADJ, but not in the bronchi or bronchioles. In contrast, in the mice treated with FGF9, 10–20% of CC10+ cells were SFTPC+ (Figure 1C–E). To further confirm the ability of FGF9 to induce airway epithelium differentiation into SFTPC-expressing cells, mouse tracheal epithelial cells (MTEC) were seeded in the ALI assay for 7 days and then treated with recombinant FGF9 for 5 days starting from ALI day-7. In this model, a pseudostratified epithelium containing basal, Club, ciliated and goblet cells is observed from day-5 onward[16]. Immunostaining of the ALI membrane on day-12 revealed that 5– 15% of the cells on the membrane were SFTPC+ (Figure S1D).
Figure 1. The effects of prolonged FGF9 overexpression on the lungs.
DT mice were induced with doxycycline for 6 months. Representative lung sections show (A) the presence of tumor nodules in bronchi, in mid-level bronchioles, near BADJ and in the lung parenchyma. (B) FGF9 overexpression in the lung caused many of the epithelial cells lining the airways (primarily Club cells) to upregulate Sftpc expression and thus activated the FGF9/GFP transgene in them. (C–E) Examination of the lungs of wt mice 2-days after intratracheal administration of recombinant FGF9 revealed that many Club cells in the proximal bronchi, in mid-level bronchioles, and near the BADJ expressed Sftpc. (F) Both airways and lung parenchymal nodules expressed the pan-epithelial marker E-cadherin. (G) Phenotypic heterogeneity, with many GFP negative cells are observed within each nodule along with PCNA-positive (proliferating) cells that were both GFP-positive and negative. Scale bars: (A) 200 μm; (B, F, G) 100 μm; (C–E) 50 μm. See also Supplementary Figures S1–S3.
The ability of FGF9 to induce adenocarcinoma development from epithelial cells in both the airway and lung parenchyma prompted us to compare the molecular characteristics of tumors in both sites. E-cadherin immunostaining showed that both airway and lung parenchymal tumors were derived from the lung epithelium (Figure 1F). To characterize the type of cells present within each tumor, we stained for cytokeratin-5, CC10, lysozyme, calcitonin gene-related peptide (CGRP), acetylated alpha-tubulin, aquaporin-5 (AQP5) and Sftpc, which are markers of basal, Club, serous, neuroendocrine, ciliated, AT-I, and AT-II cells, respectively. No cytokeratin-5 or acetylated alpha-tubulin expression was detected (data not shown). Approximately 5–20% of the cells within the airway nodules, but none in the parenchymal nodules, were positive for CC10 and CGRP (Figure S2A-D). Interestingly, AQP5, Sftpc and lysozyme were extensively expressed in both airway and parenchymal nodules (40–70%). Lysozyme was brighter in the airway nodules, while AQP5 and Sftpc were similar in both (Figure S2E–H).
Phenotypic heterogeneity, caused by differentiation, genetic changes, and changes in the tumor microenvironment, can arise in cancer cells within a single tumor[22]. In our model, Sftpc-expressing cells that form the bulk of early tumors were induced to express GFP and FGF9[15]. Phenotypic heterogeneity was observed after 6 months as evidenced by GFP− cells within nodules and nodules with heterogeneous GFP expression (30–70%; Figure 1G). There were some Sftpc+/GFP− and GFP+/Sftpc− cells in the tumor nodules (Figure S3A and B). With respect to AQP5, 10–20% of the cells were also GFP+, suggesting that they were derived from Sftpc+ AT-II tumor cells (Figure S2G–H). The presence of proliferating cell nuclear antigen (PCNA)+ cells that were GFP−/Sftpc− within nodules indicated tha some cells, which were FGF9−, responded to FGF9 secreted by nearby cells and proliferated (Figure 1G). We found that 15–40% of PCNA+ cells within older tumor nodules were GFP− and 50–75% of GFP+ cells were PCNA−. This suggests that FGF9 has a paracrine effect on various cell types in tumor nodules, including epithelial, parenchymal, and hematopoietic cells. Many PCNA+ cells were located in areas with few GFP+ cells (airways and alveoli; Figure 1G). A tumor-associated immune response similar to that which occurs in human tumors [23] was visible, with large macrophage-like and small lymphocyte-like cells surrounding/infiltrating the nodules (Figure S3C and D). Both airway and lung parenchymal nodules were Mucin+ by AB/PAS staining (Figure S3E and F).
FGF9-induced adenocarcinomas contain tumor-propagating cells
Increasing evidence suggests that the ability of a tumor to grow is dependent on a subset of cells within it, termed cancer stem cells (CSC). The CSC model proposes that tumors have a hierarchical organization in which only some cells indefinitely self-renew and thereby sustain tumor growth[24]. Tumor-propagating cells (TPC) are identified based on their ability to form/propagate tumors after transplantation into a suitable host[25]. Because FGF9-induced adenocarcinoma yields tumors that recapitulate key aspects of human lung cancer, we studied it for the presence of TPCs (Figure 1A). We used microCT to confirm that lungs were tumor-free prior to doxycycline induction and to monitor nodule development in DT mice after doxycycline administration and in recipient mice after cells transplantation (Figure 2B–E). Lungs from doxycycline-fed DT mice with tumor nodules confirmed by microCT (Figure 2D) were dissociated into single cell suspensions. Hematopoietic and endothelial cells were depleted, and the cells were either used directly (i.e., WLCs containing all epithelial and mesenchymal cells of the lungs and tumor) or stained for sorting. GFP, EpCAM and Sca1 were used to fractionate cells into various cellular subtypes. Lung fibroblasts isolated from wt and DT mice were used as controls. Recipient mice (syngeneic FVB wt or nude mice) were fed doxycycline, and secondary tumor development was examined monthly by microCT.
Figure 2. Propagation potential of the FGF9-induced adenocarcinoma.
(A) Schematic diagram summarizing the design and main findings of the tumor propagation study. (B–E) MicroCT is a sensitive tool for the early detection of tumor shadows in mouse lungs. CT images from wt syngeneic (n=3) (B) and all DT mice before the start of the doxycycline feeding (C) showed nodule-free lungs. (D) Four weeks after the start of doxycycline feeding, the lungs of the DT mice that were intended for use as a cell source for the tumor propagation experiment exhibited several tumor shadows (red arrows, n=20). (E) A wt syngeneic mouse that was subcutaneously injected with WLCs from the doxycycline-fed DT mice had a large nodular shadow in its lung 1-month after the subcutaneous injection (red arrow, n=1). (F) Secondary tumors stained with H&E showed histological characteristics that recapitulated those of primary lung adenocarcinoma. (G-H) Immunofluorescent staining of the secondary tumor nodules confirmed its similarity to primary lung parenchymal nodules (expressions of Sftpc and AQP5, but not that of CC10 or CGRP). (I) Lungs of recipient mice had accumulation of inflammatory cells around blood vessels and bronchi/bronchioles,; BALT and perivascular cuffing. (J-K) BALT and perivascular cuffs stained positive for the pan-hematopoietic marker CD45 and the T-lymphocytic marker CD3, confirming their identity. Scale bars: (D) 200 μm; (E–I) 100 μm. See also Supplementary Figure S4.
To verify the ability of primary lung tumor cells to form secondary tumors, we subcutaneously injected WLCs from DT mice into syngeneic wt recipient mice. Surprisingly, no subcutaneous nodules developed. However, microCT revealed a secondary tumor shadow in one recipient mouse lung (Figure 2E) without evidence of tumor growth in any other organ/tissue. This suggests that the lung has unique properties that make it particularly permissive for tumor-specific cell localization and proliferation. We also tried to propagate tumor cells directly to the lungs by transferring them to the lung epithelial or vascular compartments (i.e., intratracheal or intravenous administration)[26-27]. Although microCT revealed the development of primary and secondary lung nodules when the cells were administered subcutaneously or intravenously, the intratracheally injected mice developed a prolonged inflammatory reaction to the injected cells, which resulted in hazy shadows that obscured detection secondary nodules in the lungs (Figure S4A). Therefore, all the mice that underwent intratracheal administration were killed after 4 months, and then histologically assessed for the presence of secondary tumors.
Because the primary tumors in the DT mice were FGF9 induced, we hypothesized that GFP+ cells (i.e. FGF9+) would be the most efficient in propagating secondary tumors compared to GFP− or other mixed populations. However, examination of recipient mouse lungs revealed secondary tumor nodules in all the recipient groups, except the Sca1− group. The propagation rate was low (5.6–25%; Supplementary Table 1 and Figure 2A).
Curtis et al. used Sca1 as the sole enrichment factor for identifying TPCs in three mouse models of lung adenocarcinoma[26]. However, Sca1 is also expressed in lung mesenchymal cells (mainly fibroblasts) and in distal airway epithelial cells[28,29]. Thus, the contributions of non-tumor Sca1+ (and Sca1−) mesenchymal, epithelial, and stromal cells to the sorted/transplanted populations could not be ruled out/assessed. Examination of lung cells sorted based on Sca1 expression revealed that the Sca1− population had many GFP+ cells (15%) that were larger and mostly epithelial, while the Sca1+ population had few GFP+ cells (3%), which were smaller and mostly mesenchymal (Figure S4B–E). In our model, putative oncogenic cells expressed GFP and thus could be purely sorted. We sorted WLCs into GFP+ (enriched for tumor cells), GFP−, EpCAM+ (contained both tumor and non-tumor cells), and GFP−/EpCAM− (mesenchymal, non-tumor cells) populations (Figure S4F). Interestingly, both the GFP+ and GFP− epithelial cells could propagate secondary tumors, but WLCs (a mixture of GFP+ and GFP− epithelial cells and various mesenchymal cells) had a higher propagation potential than all other fractionated populations, even though the proportion of tumor cells within this cell mixture would have been considerably lower than pure GFP+ or GFP− epithelial cells due to dilution with mesenchymal cells. Similarly, Sca1+ cells had 10% propagation ability while Sca1− cells could not propagate secondary tumors, although Sca1+ cells mainly consisted of mesenchymal cells and a few tumor cells (Figure S4B–E). Taken together, these data indicate that both GFP+ and GFP− epithelial populations contain TPCs and that their coexistence with lung tumor stromal cells facilitates and potentiates their propagation compared to transplanting them alone.
Interestingly, the secondary tumors that developed in mice injected with GFP+ cells expressed GFP and FGF9, while the nodules in the mice injected with GFP− cells did not. In contrast, secondary tumors resulting from injection of WLCs had both GFP/FGF9-expressing (Figure S4G) and non-expressing nodules. We confirmed that the GFP/FGF9 negative secondary nodules were of donor origin by demonstrating the presence of the GFP insert through PCR by using GFP-specific primers (Figure S4H).
Notably, all the secondary tumors detected were located in the lung parenchyma of the recipient mice and not in the airways (Figure 2F and S4I). Histological analysis of the secondary tumors recapitulated the histopathology of the parenchymal primary tumors as they expressed Sftpc and AQP5 but not CC10 or CGRP (Figure 2G–H).
We observed the presence of bronchus-associated lymphoid tissue (BALT) and perivascular cuffing with inflammatory cells in the syngeneic recipient mice (Figure 2I). The accumulation of lymphocytes, plasma cells, and other inflammatory cells to form a dense mass around the pulmonary vessels and bronchi is a known sign of inflammation and/or an immune reaction in the lung[30]. BALT is also known to be a preferential site for metastatic tumor cell growth in the lungs[31]. They were observed in 60% of the recipient syngeneic mice, including the mice injected with wt fibroblasts, but not in the vehicle-only or nude mice, indicating that their development is a nonspecific immunological reaction to the presence of donor cells in the lung, regardless of tumor status. The GFP insert could not be detected by using PCR in the DNA collected from the BALT and perivascular cuffs by PCR. Staining for the pan hematopoietic marker CD45 and T-lymphocyte marker CD3 showed that almost all the cells within them were of hematopoietic origin and that many were T-lymphocytes (Figure 2J and K).
Lung fibroblasts from mice with FGF9-induced adenocarcinomas support lung epithelial proliferation and colony formation in vitro
Recent studies have demonstrated several critical roles for stromal cells, called cancer-associated fibroblasts (CAFs), in promoting primary tumor growth and metastasis by serving as a critical component of the CSC niche[32]. We observed that mixing of tumor cells with autologous lung mesenchymal cells markedly increased the tumor propagation potential. To determine whether autologous, “tumor-associated lung fibroblasts” (TALFs) could promote proliferation of lung epithelial and tumor cells, we used an in vitro 3D colony formation assay in which TALFs or fibroblasts isolated from the lungs of syngeneic wt mice were co-cultured with the wt lung epithelium. Fibroblasts are a known essential component of the lung epithelial stem cell niche, and lung epithelial stem/progenitor cells form clonal spheres in vitro only when co-cultured with fibroblasts[17]. We found that the wt lung epithelium formed spheres when co-cultured with TALFs, which appeared earlier and were of larger size and number than spheres observed in the co-culture with wt fibroblasts (Figure 3A, B, and E).
Figure 3. TALF-induced colony/sphere formation by epithelial cells possibly through FGF2/FGFR signaling.
Fibroblasts isolated from the lungs of a wt (A) or tumor-bearing DT mice lungs (i.e.TALF) (B) were co-cultured with wt lung epithelium in the 3D-sphere/colony formation organoid assay and were observed for 7-days (n=3, independent experiments). TALF co-culture produced epithelial spheres that were of larger size and number than the spheres observed in the co-culture with wt fibroblasts. (C) Quantitative real-time PCR analysis comparing the gene expressions of FGF2, FGF9, FGF10, FGFR1, FGFR2, and FGFR3 between TALFs, wt fibroblasts, and a mouse lung epithelial cell line. FGF2 and FGFR2 expression levels were significantly higher in TALFs than in the wt fibroblasts (* p <0.001). (D) Wt lung epithelial cells were co-cultured with TALFs and treated with an FGFR inhibitor. Blocking of FGFRs resulted in formation of smaller and fewer colonies. (E) Quantification of sphere number and diameter. The results confirmed that TALFs significantly induced clonal growth of epithelial progenitors cells compared to wt fibroblasts. (*,# p<0.01 compared to A and B, respectively).
During lung development and morphogenesis, FGF10 is dynamically expressed in the lung mesenchyme surrounding the distal buds and functions to control the directional outgrowth of the lung epithelial cells through FGF receptor signaling[33]. To explore the role of FGF signaling in TALF-mediated induction of epithelial tumor and stem cell growth, we examined the expressions of FGF2, FGF9, FGF10, and FGFR1-3 in wt fibroblasts, TALFs, and lung epithelial cells. Both types of fibroblasts expressed FGF2, FGF10, FGFR1, and FGFR2. Notably, the expressions of FGF2 and FGFR2 were increased (by 4.3- and 7-fold, respectively; p < 0.001) in TALFs compared to wt fibroblasts (Figure 3C). To determine whether signaling through the FGF/FGFR axis was critical for the preferential support provided by TALFs, lung epithelial cells co-cultured with TALFs were treated with the FGFR-inhibitor PD173074 (10 nM) or a vehicle control. Blocking FGFR signaling resulted in the loss of the TALF-induced increase in epithelial sphere number and size (Figure 3D and E). Collectively, these data suggest that TALFs have a critical role in supporting tumor growth and propagation, possibly through potentiation of components in the FGF/FGFR signaling pathway.
Evidence that FGF9-induced tumors become FGF9 independent through activation of an alternative FGF/FGFR signaling pathway
To examine if tumors regress, remain stable, or continue to grow after withdrawal of FGF9, tumor growth was monitored monthly by using microCT after withdrawal of doxycycline. Tumor nodules remained unchanged, grew slowly, or appeared de novo after doxycycline withdrawal (Figure 4A–I). When the lungs from these mice were examined by using a stereo-microscope (Figure S5A–D) after 4 months, GFP/FGF9 expression was absent in most nodules. These data along with the ability of GFP− (i.e. FGF9−) tumor cells to form secondary tumors in recipient mice (Figure 2) indicate that the established tumors have acquired FGF9 independence.
Figure 4. Effect of FGF9 withdrawal on established lung tumors.
DT mice were fed doxycycline chow for 1-month (n=4), and the presence of tumor nodules in their lungs was confirmed by microCT (A, D and G). Then, they were switched into dox-free chow, and the status of tumor nodules in their lungs was examined monthly. Many of the previously identified nodules persisted, and their size remained the same or increased (compare B and C to A, and E and F to D). Some nodules appeared de novo after doxycycline withdrawal and increased in size between months 3 and 4 (compare H and I to G). The role of signaling through the FGF/FGFR pathway. (J-K) EpCAM+ epithelial cells were sorted from dox-fed, DT mouse lungs and then co-cultured with wt fibroblasts in the 3D-sphere/colony formation assay. Culture wells were treated with the FGFR inhibitor or a vehicle control (n=3 well replicates, two independent experiments). FGFR inhibitor-treated cells formed spheres that were of smaller size and fewer number than those in the control group. (L) Quantification of sphere number and diameter. (*p<0.01, compared to J). Scale bar: (J) 100 μm. See also Supplementary Figures S5–S7.
To determine whether FGF9 independence is due to activation of an alternative FGF/FGFR pathway, we examined the expressions of FGFR1, FGFR2, FGFR3, and FGFR4 in lungs from naive mice, mice that received short- (2 days) and long-term (6 months) doxycycline administration, and mice that received 2 months of doxycycline followed by 4 months of doxycycline deprivation. We also examined the expression of pFRS2, the intracellular docking protein phosphorylated during the transmission of FGFR signals. Our findings suggest a dynamic profile of FGF receptors expression and activation. Although FGFR3 is expressed in the naive lung epithelium and is required for initiation of FGF9-induced adenocarcinoma[15], it undergoes a marked negative feedback/downregulation in response to FGF9 (Figure S5E). On the other hand, FGFR1 was not expressed in naive lungs before doxycycline administration but was upregulated shortly after doxycycline administration in the airways, lung parenchyma, and nodules developing within them in response to FGF9 (Figure S5F). FGFR2 was expressed in airways and in tumor nodules regardless of FGF9 status[15]. FGFR4 was not expressed in the naive lungs, but some expression was detected in the bronchial but not parenchymal nodules (Figure S5G). Interestingly, pFRS2 was strongly expressed in the airways of the naive mice (Figure S6A). It was also detected in lungs in response to short- and long-term FGF9 induction, and after long-term FGF9 deprivation, suggesting a continuous role for FGFR downstream activation (Figure S6B–D). Notably, pFRS2 expression was observed in peripheral (i.e. expanding/invasive) areas of the nodules (Figure S6C and D).
To further confirm that FGF/FGFR signaling is essential for continuing tumor cell proliferation, we co-cultured EpCAM+ primary tumor cells isolated from DT mice with wt fibroblasts. Cells were treated with the FGFR-inhibitor PD173074 or vehicle control. The cells treated with the FGFR-inhibitor formed colonies that appeared later, were of smaller size, and fewer in number than untreated spheres (Figure 4J-L). To further confirm that differential sphere growth was independent of FGF9 secreted by tumor epithelial cells within these colonies, an additional set of matrigel cultures were treated with doxycycline or vehicle to induce FGF9/GFP transgene activation. Induction of FGF9 in culture did not affect sphere size, number, or growth rate, although spheres in the doxycycline-treated wells showed GFP+ spheres while untreated wells showed no GFP expression(Figure S7A–E). The cartoon in figure 5 summarizes the main findings of this study.
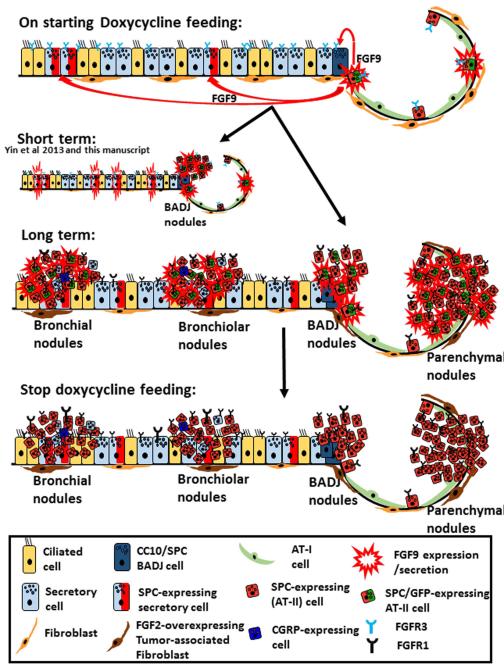
Figure 5. Summary of the response of various respiratory cells to FGF9 induction and withdrawal.
On feeding doxycycline to the Sftpc-rtTA and Tre-Fgf9-ires-eGfp double-transgenic mice, Sftpc-expressing cells started to express FGF9 and GFP. Secreted FGF9 binds to its receptor, FGFR3, which is constitutively expressed in all lung epithelial cells. Within hours to days, adenomas and adenocarcinomas develop, mostly as a result of proliferation of epithelial cells at BADJ (15). Furthermore, FGF9 induces airway secretory cells to overexpress Sftpc and thus the FGF9/GFP transgene is switched in these cells. Within weeks to months, tumor nodules develop in bronchi, bronchioles, and lung parenchyma. FGFR1 and FGF2 expressions were upregulated in tumor epithelial cells and lung fibroblasts, respectively. FGFR3 expression gradually diminishes. Tumor nodules contained both GFP-positive and GFP-negative cells, and bronchial nodules expressed CC10 and CGRP. After withdrawal of doxycycline feeding for 3 months, FGF9/GFP expression was lost, but tumor nodules persisted or continued to grow. Tumor epithelial cells continued to express FGFR1.
Discussion
The Sftpc-rtTA mouse line (rtTA controlled by the Sftpc promoter) is the first mouse model to assess the effects of FGF9 overexpression on lung epithelial cell transformation[15]. Lung cancers derived from different cell types may have distinct mutation profiles leading to different characteristics and behavior. Several studies have used mice carrying oncogenic Kras with or without p53 loss-of-function mutations to identify the cell of origin of lung adenocarcinoma and achieved varied results, describing cells at the BADJ[21], AT-II cells[34-36], or both Club and AT-II cells as the cells of origin[37]. In another model carrying a mutant EGFR gene, tumors develop both peripherally in alveoli as well as proximally in bronchioles[38]. In contrast, in the FGF9-induced model, we found multiple cells of origin for the tumors, with the earliest responders being cells close to the BADJ. However, continued exposure to FGF9 resulted in tumor nodule development both in airways and alveolar spaces. We speculate that overexpression of FGF9 (or its receptors) is capable of inducing adenocarcinoma development from several different cells of origin, including cells lining the TBs near the BADJ, more proximal airway secretory cells, and AT-II cells.
In addition to Club and goblet cells, serous cells are the third well-known type of secretory cells in the airway and they also line the serous tubules of submucosal glands[39]. While they are proposed to be a cell of origin for serous adenocarcinoma of the ovary and uterus[40-41], airway serous cells have never been discussed as a possible cell of origin for lung adenocarcinoma. We demonstrated that both parenchymal and bronchial nodules express lysozyme, a serous cell marker, and that its expression was higher in bronchial nodules. Furthermore, within airway nodules, lysozyme+ cells were more abundant than CC10+ cells suggesting that serous cells warrant further investigation as a possible cell of origin.
Cho et al. compared the propagation potential of airway and alveolar tumors and showed that secondary tumors initiated in the airways had a higher ability to propagate/form secondary nodules in the airways and lung parenchyma of recipient mice[37]. We found, however, that transplantation of an unfractionated mixture of bronchial and parenchymal tumor cells produced secondary tumor nodules that were located mostly in lung parenchyma. This difference is probably caused by differences in the tumor driver (Kras mutation vs. overexpression of FGF9) and/or in the transplanted tumor cells (site-based and pure epithelial vs. whole-lung epithelium mixed with mesenchymal cells). The variability of the results of studies that examined the cell of origin and propagation potential of histologically similar lung adenocarcinomas indicates the different initiating mutations, and genotypes of lung adenocarcinomas are producing tumors with different cells of origin and propagation potentials. Therefore, identification of functionally important cells within the tumor (i.e., CSCs or TPCs) requires a strategy that considers the tumor genotype and microenvironment.
Tissue resident mesenchymal stromal cells contribute to organ/tissue maintenance and regeneration through various mechanisms including secretion of trophic factors that act directly on epithelial stem cells. Lung fibroblasts are essential for distal lung progenitor/stem cell growth and regeneration[17,42] and FGF10 has been suggested to be a major regulator of these processes[17,43]. We showed that although both wt and TALF expressed FGF10, only FGF2 was differentially expressed and was likely responsible for the ability of TALFs to induce lung stem/progenitor cell proliferation. Recently, a role for CAF-secreted FGF2 in adenocarcinoma of the breast was described[44]. It is possible that the CSC supporting role of fibroblasts is mediated by FGF10 during homeostasis and FGF2 during carcinogenesis. Additional studies are necessary to explore the interactions between TALFs, FGF2, and tumor cells.
Withdrawal of FGF9 after FGF9-induced tumor development did not result in tumor regression. GFP− (i.e. FGF9−) tumor cells propagated secondary tumors in recipient mice. We speculate that FGF9 independence involves activation of an alternative FGF/FGFR signaling system because FRS2, which lies downstream of activated FGFRs, was continually phosphorylated after FGF9 withdrawal and in secondary tumors. In addition, an FGFR inhibitor could block in vitro colony formation by doxycycline-deprived primary tumor cells. Our data suggest two possible mechanisms. First, FGFRs on tumor cells may be activated by another FGF ligand secreted by the tumor cells themselves or by nearby stromal (niche) cells. TALFs overexpress FGF2 and are one candidate. Second, there may be ligand-independent activation of an FGFR. We observed FGFR1 and FGFR2 expressions in the lung epithelium and tumor nodules. Supraphysiological receptor expression -on its own- has been described as a mechanism for ligand-independent signaling of FGFRs in cancer[13,45]. Additional studies are necessary to elucidate the mechanism, level of regulation, and potential other contributing pathways.
In conclusion, we have provided evidence for the roles of FGFs/FGFRs in lung cancer. Molecular therapeutics targeting FGF/FGFR signaling might provide new primary or adjuvant therapies for a select lung cancer patients in which FGF signaling is activated (i.e., patients without EGFR mutations or that are resistant to EGFR TKIs).
Supplementary Material
Acknowledgments
We thank Yuichiro Hayashi for performing the pathological assessments, Miyuki Yamamoto, and Mikiko Shibuya for the technical support, and Steven Brody for the help with the ALI.
Financial support This work was supported by a JSPS Grant-in-Aid for Scientific Research C and B (Nos. 25461196 and 23390218 for A.E.H. and T.B., respectively). NIH grant (No. HL111190 for D.M.O).
Footnotes
The authors declare that there are no conflicts of interest.
Statement of author contributions D.A.: study conception and design, collection and/or assembly of data, and data analysis and interpretation; A.E.H.: study conception and design, collection and/or assembly of data, data analysis and interpretation, financial support, manuscript writing; A.K., K.I., K.S., J.H., and Y.Y.: collection and/or assembly of data, data analysis and interpretation; K.S., H.Y., and K.N.: study conception and design and data analysis and interpretation, D.M.O.: study conception and design, provision of study material, data analysis and interpretation, financial support, and manuscript writing; T.B.: study conception and design, data analysis and interpretation, financial support, administrative support, manuscript writing, and final manuscript approval.
References
- 1.Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taron M, Ichinose Y, Rosell R, et al. Activating mutations in the tyrosine kinase domain of the epidermal growth factor receptor are associated with improved survival in gefitinib-treated chemorefractory lung adenocarcinomas. Clin Cancer Res. 2005;11:5878–5885. doi: 10.1158/1078-0432.CCR-04-2618. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer. 2009;10:281–289. doi: 10.3816/CLC.2009.n.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boch C, Kollmeier J, Roth A, et al. The frequency of EGFR and KRAS mutations in non-small cell lung cancer (NSCLC): routine screening data for central Europe from a cohort study. BMJ Open. 2013;3:e002560. doi: 10.1136/bmjopen-2013-002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kosaka T, Yatabe Y, Endoh H, et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 7.Thisse B, Thisse C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev Biol. 2005;287:390–402. doi: 10.1016/j.ydbio.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Brooks AN, Kilgour E, Smith PD. Molecular pathways: fibroblast growth factor signaling: a new therapeutic opportunity in cancer. Clin Cancer Res. 2012;18:1855–1862. doi: 10.1158/1078-0432.CCR-11-0699. [DOI] [PubMed] [Google Scholar]
- 9.Wynes MW, Hinz TK, Gao D, et al. FGFR1 mRNA and protein expression, not gene copy number, predict FGFR TKI sensitivity across all lung cancer histologies. Clin Cancer Res. 2014;20:3299–3309. doi: 10.1158/1078-0432.CCR-13-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marek L, Ware KE, Fritzsche A, et al. Fibroblast growth factor (FGF) and FGF receptor-mediated autocrine signaling in non-small-cell lung cancer cells. Mol Pharmacol. 2009;75:196–207. doi: 10.1124/mol.108.049544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terai H, Soejima K, Yasuda H, et al. Activation of the FGF2-FGFR1 autocrine pathway: a novel mechanism of acquired resistance to gefitinib in NSCLC. Mol Cancer Res. 2013;11:759–767. doi: 10.1158/1541-7786.MCR-12-0652. [DOI] [PubMed] [Google Scholar]
- 12.Ware KE, Hinz TK, Kleczko E, et al. A mechanism of resistance to gefitinib mediated by cellular reprogramming and the acquisition of an FGF2-FGFR1 autocrine growth loop. Oncogenesis. 2013;2:e39. doi: 10.1038/oncsis.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ware KE, Marshall ME, Heasley LR, et al. Rapidly acquired resistance to EGFR tyrosine kinase inhibitors in NSCLC cell lines through de-repression of FGFR2 and FGFR3 expression. PLoS One. 2010;5:e14117. doi: 10.1371/journal.pone.0014117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohgino K, Soejima K, Yasuda H, et al. Expression of fibroblast growth factor 9 is associated with poor prognosis in patients with resected non-small cell lung cancer. Lung Cancer. 2014;83:90–96. doi: 10.1016/j.lungcan.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Yin Y, Betsuyaku T, Garbow JR, et al. Rapid induction of lung adenocarcinoma by fibroblast growth factor 9 signaling through FGF receptor 3. Cancer Res. 2013;73:5730–5741. doi: 10.1158/0008-5472.CAN-13-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You Y, Richer EJ, Huang T, et al. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1315–1321. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
- 17.McQualter JL, Yuen K, Williams B, et al. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci U S A. 2010;107:1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalina M, Mason RJ, Shannon JM. Surfactant protein C is expressed in alveolar type II cells but not in Clara cells of rat lung. Am J Respir Cell Mol Biol. 1992;6:594–600. doi: 10.1165/ajrcmb/6.6.594. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland KM, Combs TJ, Edwards PC, et al. Site-specific differences in gene expression of secreted proteins in the mouse lung: comparison of methods to show differences by location. J Histochem Cytochem. 2010;58:1107–1119. doi: 10.1369/jhc.2010.956052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoor A, Stahlman MT, Gray ME, et al. Temporal-spatial distribution of SP-B and SP-C proteins and mRNAs in developing respiratory epithelium of human lung. J Histochem Cytochem. 1994;42:1187–1199. doi: 10.1177/42.9.8064126. [DOI] [PubMed] [Google Scholar]
- 21.Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 22.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butts CA. Anti-tumor immune response in early stage non small cell lung cancer (NSCLC): implications for adjuvant therapy. Transl Lung Cancer. 2013;2:415–422. doi: 10.3978/j.issn.2218-6751.2013.10.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 25.Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–728. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Curtis SJ, Sinkevicius KW, Li D, et al. Primary tumor genotype is an important determinant in identification of lung cancer propagating cells. Cell Stem Cell. 2010;7:127–133. doi: 10.1016/j.stem.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Podsypanina K, Du YC, Jechlinger M, et al. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321:1841–1844. doi: 10.1126/science.1161621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McQualter JL, Brouard N, Williams B, et al. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the sca-1 positive cell fraction. Stem Cells. 2009;27:623–633. doi: 10.1634/stemcells.2008-0866. [DOI] [PubMed] [Google Scholar]
- 29.Teisanu RM, Lagasse E, Whitesides JF, et al. Prospective isolation of bronchiolar stem cells based upon immunophenotypic and autofluorescence characteristics. Stem Cells. 2009;27:612–622. doi: 10.1634/stemcells.2008-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fagarasan S, Cerutti A. Advances in Immunology (Volume 107) Academic Press; 2010. [DOI] [PubMed] [Google Scholar]
- 31.Geldof AA, Rao BR. Metastasis of prostate tumor cells to bronchus associated lymphoid tissue. In Vivo. 1989;3:87–91. [PubMed] [Google Scholar]
- 32.Togo S, Polanska UM, Horimoto Y, et al. Carcinoma-associated fibroblasts are a promising therapeutic target. Cancers (Basel) 2013;5:149–169. doi: 10.3390/cancers5010149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ornitz DM, Yin Y. Signaling networks regulating development of the lower respiratory tract. Cold Spring Harb Perspect Biol. 2012;4:a008318. doi: 10.1101/cshperspect.a008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu X, Rock JR, Lu Y, et al. Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. Proc Natl Acad Sci U S A. 2012;109:4910–4915. doi: 10.1073/pnas.1112499109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin C, Song H, Huang C, et al. Alveolar type II cells possess the capability of initiating lung tumor development. PLoS One. 2012;7:e53817. doi: 10.1371/journal.pone.0053817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mainardi S, Mijimolle N, Francoz S, et al. Identification of cancer initiating cells in K-Ras driven lung adenocarcinoma. Proc Natl Acad Sci U S A. 2014;111:255–260. doi: 10.1073/pnas.1320383110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho HC, Lai CY, Shao LE, et al. Identification of tumorigenic cells in Kras(G12D)-induced lung adenocarcinoma. Cancer Res. 2011;71:7250–7258. doi: 10.1158/0008-5472.CAN-11-0903. [DOI] [PubMed] [Google Scholar]
- 38.Li D, Shimamura T, Ji H, et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell. 2007;12:81–93. doi: 10.1016/j.ccr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Jeffery PK, Li D. Airway mucosa: secretory cells, mucus and mucin genes. Eur Respir J. 1997;10:1655–1662. doi: 10.1183/09031936.97.10071655. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Fadare O, Xiang L, et al. Ovarian serous carcinoma: recent concepts on its origin and carcinogenesis. J Hematol Oncol. 2012;5:8. doi: 10.1186/1756-8722-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flesken-Nikitin A, Hwang CI, Cheng CY, et al. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 2013;495:241–245. doi: 10.1038/nature11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barkauskas CE, Cronce MJ, Rackley CR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McQualter JL, McCarty RC, Van der Velden J, et al. TGF-β signaling in stromal cells acts upstream of FGF-10 to regulate epithelial stem cell growth in the adult lung. Stem Cell Res. 2013;11:1222–1233. doi: 10.1016/j.scr.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Giulianelli S, Cerliani JP, Lamb CA, et al. Carcinoma-associated fibroblasts activate progesterone receptors and induce hormone independent mammary tumor growth: A role for the FGF-2/FGFR-2 axis. Int J Cancer. 2008;123:2518–2531. doi: 10.1002/ijc.23802. [DOI] [PubMed] [Google Scholar]
- 45.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.