Abstract
Trans-sodium crocetinate (TSC) is a novel synthetic carotenoid compound that improves diffusion of small molecules including oxygen in solutions. TSC provides neuroprotection in healthy rats and rabbits. We determine whether TSC is neuroprotective in obese mice. Sixteen-week old CD-1 male mice fed with high fat diet for the last 10 weeks were subjected to a 90-min middle cerebral arterial occlusion (MCAO). They received TSC by two boluses through a tail vein at 10 min after the onset of MCAO and reperfusion, respectively, with a total dose of 0.14, 0.28 and 0.7 mg/kg or by a bolus-infusion-bolus strategy with a total dose of 0.14 mg/kg during MCAO. Neurological outcome was evaluated 72 h after MCAO. Brain tissues were harvested at 24 h after MCAO to measure nitrotyrosine containing proteins, 4-hydroxy-2-nonenal, matrix metalloproteinase (MMP)-2 and -9 activity and expression, and inflammatory cytokines. TSC given by the two-bolus strategy did not improve neurological outcome. The bolus-infusion-bolus strategy significantly reduced brain edema, infarct volume and hemorrhagic transformation and improved neurological functions. TSC reduced nitrotyrosine containing proteins, MMP-9 activity and expression, and inflammatory cytokines in the ischemic brain tissues. Our results indicate that TSC delivered by the bolus-infusion-bolus strategy provides neuroprotection in obese mice. This protection may be through reduction of oxidative stress, MMP-9 activity and inflammatory cytokines in the ischemic brain tissues.
Keywords: focal brain ischemia, hemorrhagic transformation, matrix metalloproteinase-9, neuroprotection, obesity, trans-sodium crocetinate
Introduction
Stroke is a common disease. It is one of the leading causes of death and long-term neurological morbidity (Martin et al. 1999). However, up till now, effective neuroprotective strategies for clinical use have not been established and are urgently needed to improve neurological outcome after stroke.
Ischemic brain injury is the underlying pathophysiology for stroke. One potential way to reduce ischemic brain injury is to attenuate the degree of ischemia. Trans-sodium crocetinate (TSC) has been shown to facilitate the diffusivity of oxygen and other small molecules in aqueous solution (Stennett et al. 2006). It can reduce the degree of ischemia by facilitating the diffusion of oxygen or nutrients into ischemic brain tissues. This “metabolic reflow” during ischemia has been shown to reduce focal brain ischemia-induced injury in young adult animals (Manabe et al. 2010) and has been proposed to use in patients with hemorrhagic shock (Stennett et al. 2007). However, it is not known yet whether this neuroprotective effect occurs in animals with diseases or pathological conditions that are often associated with stroke.
Obesity is a major health problem in the U.S.A. and the world. Obesity and its associated metabolic disturbances including hyperlipidemia have been identified as risk factors for cardiovascular diseases and many other diseases (Bhatnagar et al. 2008). Hyperlipidemia has been consistently identified as a risk factor for stroke in patients including young patients (Balci et al. 2011; Iso et al. 1989). Statins, cholesterol lowering agents, reduce patients’ risk for stroke (Sacco and Liao 2005). Hyperlipidemia has also been shown to increase stroke severity (Mikdashi et al. 2007), although opposite findings have been reported (Jimenez-Conde et al. 2010; Olsen et al. 2007). On the other hand, obesity can induce neuroinflammation (Buckman et al. 2014; Purkayastha and Cai 2013) and worsen neuroinflammation and neurological outcome after brain ischemia (Dhungana et al. 2013; Tu et al. 2011). Neuroinflammation is a significant pathological process to lead to cell injury (Li et al. 2013; Lipton 1999). Since hyperlipidemia can alter the rheology of blood and extracellular fluids (Katayama et al. 2010), it can affect the effectiveness of TSC-enhanced diffusivity of small molecules. Thus, it is necessary to know whether TSC still provides neuroprotection in animals with obesity and hyperlipidemia. To determine possible mechanisms for this TSC effect, various parameters including those to reflect neuroinflammation were measured.
Materials and Methods
Animals
All experimental protocols used in this study were approved by the institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA). All surgical and experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80-23) revised in 2011. Our manuscript was written up in accordance with the Animal Research: Reporting in vivo Experiments.
Six-week old CD-1 male mice from Charles River (Wilmington, MA) were fed with high fat diet (45% calorie supplied by fat; Research Diets Inc, New Brunswick, NJ) for 10 weeks before they were used in experiments.
Animal groups and TSC delivery
In the first experiment, animals were randomly divided into 4 groups to receive normal saline (10 mice), 0.14 mg/kg TSC (8 mice), 0.28 mg/kg TSC (7 mice) or 0.7 mg/kg TSC (7 mice). The needed TSC was diluted in saline to a volume of 2 µl/g body weight and given as two boluses at 10 min after the onset of ischemia and reperfusion, respectively, via a tail vein.
In the second experiment, mice were randomized to receive saline (15 mice) or 0.14 mg/kg TSC (16 mice) by a bolus-infusion-bolus strategy via a tail vein as described before (Manabe et al. 2010). TSC was diluted in saline to a volume of 2 µl/g body weight. Ten minutes after the onset of ischemia, a bolus of 1/8 dosage of the needed saline or TSC was administered, followed by continuous infusion for 60 min to give 6/8 dosage of saline or TSC. At the end of infusion, the final 1/8 dosage was injected as a bolus.
These TSC dosages were selected based on previous studies (Lapchak 2010; Manabe et al. 2010; Okonkwo et al. 2003).
Transient focal cerebral ischemia
Focal cerebral ischemia was induced by middle cerebral artery occlusion (MCAO) in mice using an intraluminal filament technique as described previously (Li and Zuo 2011). Briefly, mice were anesthetized with ~1.5% isoflurane carried by pure oxygen. A small mid-incision was made in the neck to expose right common carotid artery and external carotid artery. The external carotid artery was gently cut open and a suture with a rounded 0.22 mm width tip (#1622; Beijing Sunbio Biotechnology Co. Ltd., Beijing, China) was put through the cut into the right internal carotid artery until a slight resistance was felt. Mice were awaked immediately after the MCAO was achieved in the first set of experiment but were kept under isoflurane anesthesia for the second experiment to facilitate TSC infusion. The filament was removed at 90 min after the onset of MCAO.
Neurological outcome evaluation
Three days after the MCAO, neurological deficit scores were evaluated by a blinded observer (Li and Zuo 2011). Mice were then anesthetized by 5% isoflurane and perfused with normal saline through the left cardiac ventricle until clear fluid ran out from incision of the right atrium. Brain was cut into 1-mm thick coronal slices. Each slice was observed carefully and blindly for hemorrhagic transformation (HT), which is defined as gross blood staining in the ischemic brain tissues. An animal was considered to have HT if any of the animal’s brain slices had evidence of HT. The slices were then stained with 1% 2,3,5-triphenyltet-razolium chloride solution to evaluate edema severity and infarct volume. Edema index = right hemisphere volume/left hemisphere volume. Corrected infarct volume in percentage = [left hemisphere volume − (right hemisphere volume − right infarction volume)] × 100 /left hemisphere volume. The infarct area in each brain slice was quantified using Image J (National Institutes of Health, Bethesda, MD).
Motor coordination was evaluated just before and 3 days after the MCAO as we described before (Li and Zuo 2011). Mice were placed on a Rota-rod with the speed accelerates from 4 rpm to 40 rpm in 5 min. The latency and speed at which a tested mouse fell off the rod was recorded. The speed-latency index, which is latency (s) × speed (rpm), was calculated. The ratio of this index obtained at 3 day after the MCAO over that before MCAO was calculated to reflect the change of coordinate function of the mouse after the MCAO.
Brain tissue harvesting
Mice were treated with saline (7 mice) or TSC (5 mice) in the same way as described above for the second experiment. They were anesthetized by 5% isoflurane and perfused with refrigerated normal saline through the left cardiac ventricle. The brains were rapidly removed and dissected into left and right hemisphere. Cerebral cortex, frontal cortex area 1 (Fr1) and striatum were dissected and immediately frozen and kept at −80 °C until use. Fr1 ipsilateral to the ischemia side is considered an ischemic penumbral region after MCAO (Li and Zuo 2011; Zheng and Zuo 2004).
Matrix metalloproteinase (MMP) activity assessment
MMP activity was assessed by gelatin zymography. Brain tissues were homogenized in phosphate buffer saline (PBS) containing protease inhibitor cocktail and 0.005% butylated hydroxytoluene and then incubated on ice for 30 min. The homogenate was centrifuged at 13,000 rpm for 10 min at 4°C. Thirty microliter supernatants were mixed with 2 × sample buffer for gelatin zymographical analysis as described previously (Wang et al. 2000). Briefly, 30 µg proteins per lane were loaded onto 10% polyacrylamide gels containing 0.1% gelatin. After electrophoresis, the gelatinolytic activity in the gel was revealed by incubating in a sequence in a renaturing buffer, a developing buffer, 0.5% coomassie blue G-250 in 30% methanol and 10% acetic acid, and a destaining solution. The MMP activity was presented as clear bands at the appropriate molecular weights in the gel.
Western Blotting
Approximately 30 µg proteins per lane were loaded on 10% polyacrylamide gels. After electrophoresis, proteins were transferred onto PVDF membrane that was incubated with primary antibodies overnight at 4°C. The primary antibodies were rabbit polyclonal anti-MMP-9 antibody (1:500; Aviva Systems Biology, San Diego, CA), goat polyclonal anti-tumor necrosis factor (TNF)-α antibody, rabbit polyclonal anti-interleukin (IL)-1β antibody and mouse monoclonal anti-intercellular adhesion molecule 1 (ICAM-1) antibody (1:100; Santa Cruz Biotechnology Inc., Santa Cruz, CA). After being washed with Tween PBS, appropriate secondary antibodies were used (1:5000; Santa Cruz Biotechnology Inc.). Protein bands were revealed using enhanced chemiluminescence method, photographed and analyzed with a gel imaging system (G-Box, Syngene, Frederic, MD).
Measurement of nitrotyrosine-containing proteins and 4-hydroxy-2-nonenal (HNE)
The contents of nitrotyrosine-containing proteins and HNE in the brain tissues were assessed using the OxiSelect Nitrotyrosine ELISA kit and OxiSelect HNE-His Adduct ELISA kit (Cell Biolabs Inc., San Diego, CA), respectively, according to the manufacture’s protocols and as we described before (Li and Zuo 2011). The results were normalized by protein content.
Immunofluorescent staining
One day after the MCAO, animals without TSC treatment were sacrificed under 5% isoflurane and perfused with ice-cold normal saline followed by 4% paraformaldehyde in PBS. Brain tissues were further fixed in 4% paraformaldehyde for 24 h, dehydrated and paraffin embedded. Five-micrometer thick coronal sections at Bregma −0.58 mm were acquired for immunofluorescent staining. Sections were incubated with primary antibodies in tris-buffered saline containing 1% bovine serum albumin at 4°C overnight and then incubated in fluorescence-conjugated secondary antibody for 1 h at room temperature in the dark. Primary antibodies were rabbit polyclonal anti-MMP-9 antibody (1:200; Aviva Systems Biology), mouse monoclonal anti-vascular endothelial growth factor (VEGF) antibody (1:100; Santa Cruz Biotechnology Inc.), mouse monoclonal anti-glial fibrillary acidic protein (GFAP) antibody (1:500; Millipore Cor., Billerica, MA), rabbit polyclonal anti-cluster of differentiation (CD)-11b (1:100; Abcam, Cambridge, MA) and mouse monoclonal anti-NeuN antibody (1:500; Millipore). Images were examined and taken in the ischemic Fr1 area with an Olympus BX51 fluorescence microscope.
Statistical Analysis
Data from all animals that completed this study are reported. Parametric results are presented as means ± S.E.M. (n ≥ 4) and were analyzed by Student’s t test or one way analysis of variance followed by the Tukey test after confirmation of normal distribution of the data. Neurological deficit scores or non-normally distributed results were analyzed by Kruskal-Wallis analysis of variance on ranks followed by the Dunn’s test. A P ≤ 0.05 was accepted as significant.
Results
Sixteen-week old male CD-1 mice fed with high fat diet for the last 10 weeks were significantly heavier than the age-matched male CD-1 mice fed with regular diet (50.4 ± 1.8 g vs. 39.8 ± 0.9 g, n = 9, P < 0.05), suggesting that the mice fed with high fat diet are obese. As we reported before, these mice also had hyperlipidemia and hyperglycemia (Deng et al. 2014). The following results were obtained with using the 16-week old male CD-1 mice fed with high fat diet for the last 10 weeks. As shown in table 1, there was no difference in physiological parameters among the groups at the time when MCAO was achieved.
Table 1.
Physiological parameters during brain ischemia and hemorrhagic transformation (HT) and mortality within 72 h after brain ischemia
| Temperature (°C) |
Heart rate (beats/min) |
Blood glucose (mg/dl) |
HT rate (%) |
Mortality (%) |
|
|---|---|---|---|---|---|
| Two bolus experiments | |||||
| Saline | 37.0 ± 0.1 | 475 ± 22 | 232 ± 32 | 70 | 40 |
| TSC 0.14 mg/kg | 37.1 ± 0.1 | 460 ± 16 | 263 ± 37 | 62.5 | 0 |
| TSC 0.28 mg/kg | 37.1 ± 0.1 | 465 ± 11 | 266 ± 78 | 71.4 | 0 |
| TSC 0.70 mg/kg | 37.2 ± 0.1 | 462 ± 37 | 249 ± 46 | 85.7 | 14 |
| Bolus-infusion-bolus experiment | |||||
| Saline | 37.2 ± 0.0 | 462 ± 12 | 255 ± 27 | 86.7 | 60 |
| TSC 0.14 mg/kg | 37.1 ± 0.0 | 464 ± 10 | 211 ± 31 | 43.8* | 25 |
The temperature, heart rate and blood glucose data were obtained at the time when the middle cerebral arterial occlusion was achieved. Results are mean ± S.E.M. (n = 7 – 16).
P < 0.05 compared with the corresponding saline group.
TSC administered by two boluses did not provide neuroprotection
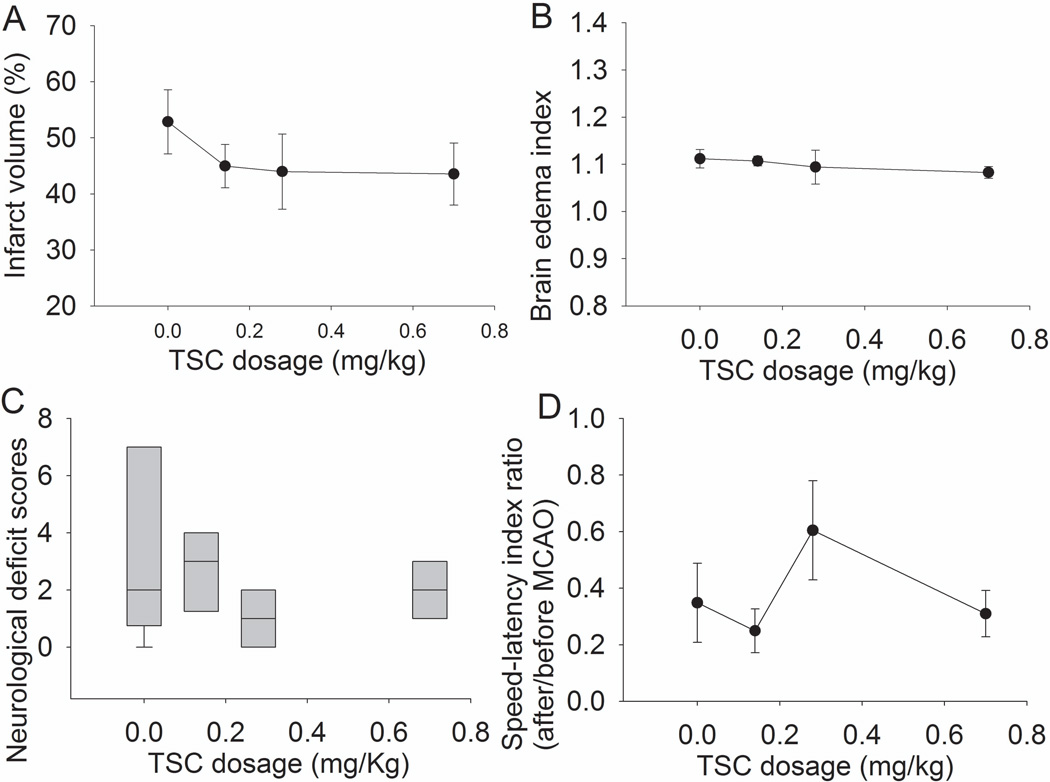
It is obviously helpful to establish metabolic reflow during brain ischemia. Also, blood flow interruption may induce microclot formation in the circulation distal to the arterial occlusion site. Some brain tissues may still be ischemic due to these microclots after the occluded artery is reopened. Thus, we designed the two bolus regimen for TSC. Total 10, 8, 7 and 7 mice were used for saline, 0.14, 0.28 and 0.7 mg/kg TSC groups. The mortality rate in these groups is listed in table 1. These deaths occurred in a time window from 2 h to 72 h after the onset of reperfusion. The difference in mortality among the four groups did not yet reach statistical significance. Also, the HT rates were not different among the groups (Table 1). The three TSC doses tested did not reduce brain edema and infarct volume, and did not improve neurological functions (Fig. 1). These results suggest that TSC at the chosen doses given by two boluses did not provide neuroprotection.
Fig. 1. Failure to induce neuroprotection by two-bolus TSC.
Mice had a 90-min middle cerebral arterial occlusion and were treated with various doses of TSC or saline. The results were evaluated 3 days after the MCAO. Infarct volume, edema index, neurological deficit scores and performance on rotarod are presented in panels A, B, C and D. Results are the means ± S.E.M. (n = 7 – 10).
TSC given during brain ischemia provided neuroprotection
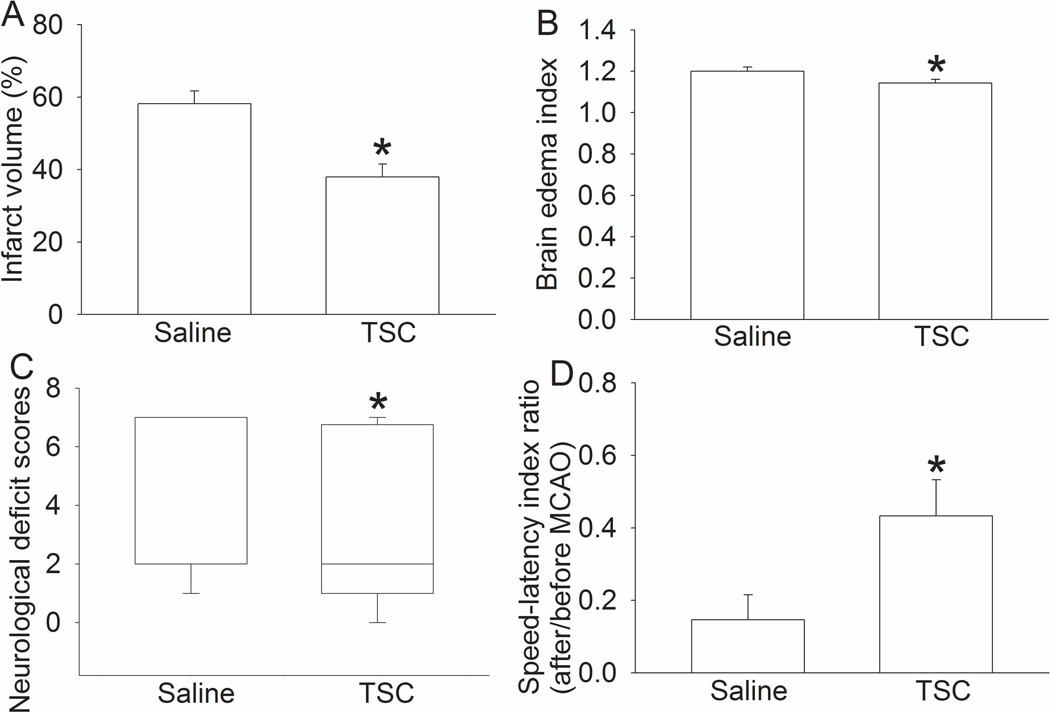
In this study, TSC was given by bolus-infusion-bolus regimen. Total 15 and 16 mice were used in the saline and 0.14 mg/kg TSC group. The mortality rate within the period from 2 h to 72 h after the MCAO in the saline and TSC groups was 60% and 25%, respectively (P = 0.073). Mice treated with TSC also had less severe brain edema, smaller brain infarct volume and better neurological functions (Fig. 2). TSC also significantly reduced the HT rate (Table 1). These results suggest that TSC administered by bolus-infusion-bolus provides neuroprotection.
Fig. 2. Neuroprotection induced by TSC administered by bolus-infusion-bolus strategy.
Mice had a 90-min middle cerebral arterial occlusion and were treated with 0.14 mg/kg TSC or saline. The results were evaluated 3 days after the MCAO. Infarct volume, edema index, neurological deficit scores and performance on rotarod are presented in panels A, B, C and D. Results are the means ± S.E.M. (n = 15 – 16). * P < 0.05 compared with mice subjected to MCAO and treated with saline.
TSC given during brain ischemia reduced oxidative stress, inflammatory cytokine expression and MMP-9 activity in the ischemic brain tissues
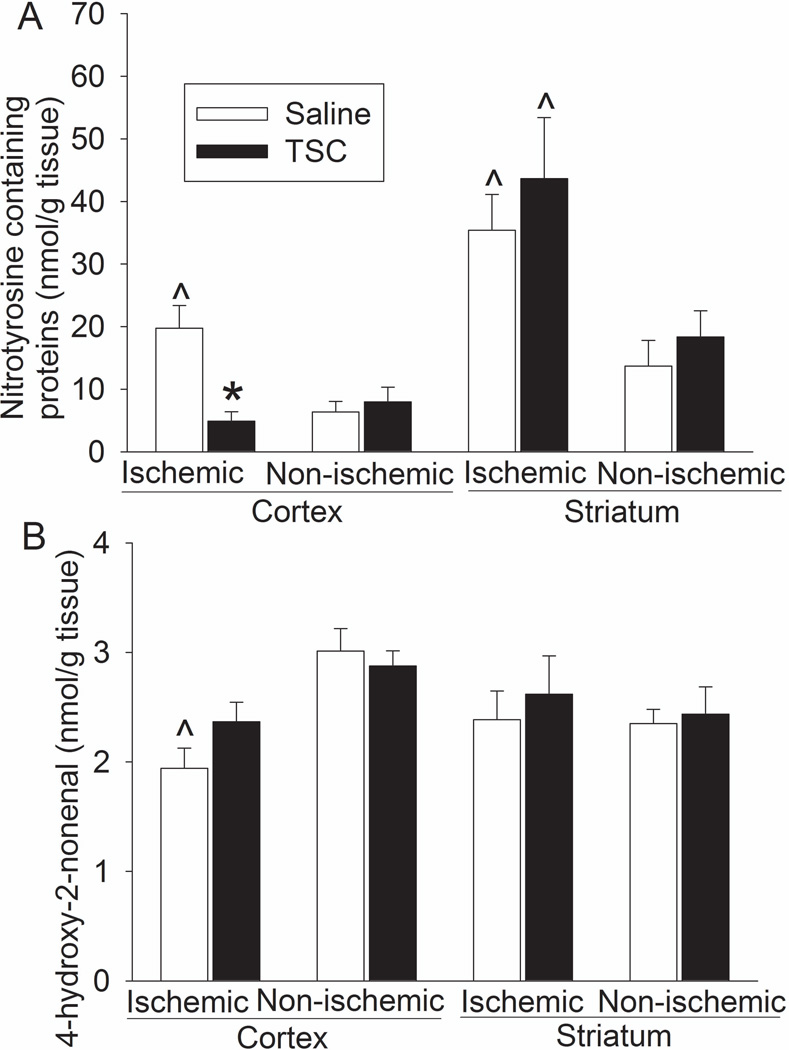
The ischemic striatum and cerebral cortex had a higher level of nitrotyrosine containing proteins than did the non-ischemic striatum and cortex. The increase of nitrotyrosine containing proteins in the ischemic cerebral cortex but not in the ischemic striatum was attenuated by TSC. The HNE level in the non-ischemic cerebral cortex was higher than that in the ischemic cortex. TSC did not affect the HNE levels in the ischemic and non-ischemic brain tissues (Fig. 3).
Fig. 3. TSC inhibition of nitrotyrosine-containing proteins in the ischemic brain tissues.
Brain tissues were harvested 24 h after the MCAO for measuring nitrotyrosine-containing proteins (panel A) and HNE (panel B). Results are the means ± S.E.M. (n = 5 – 8). * P < 0.05 compared with mice subjected to MCAO and treated with saline; ^ P < 0.05 compared to the corresponding non-ischemic tissues.
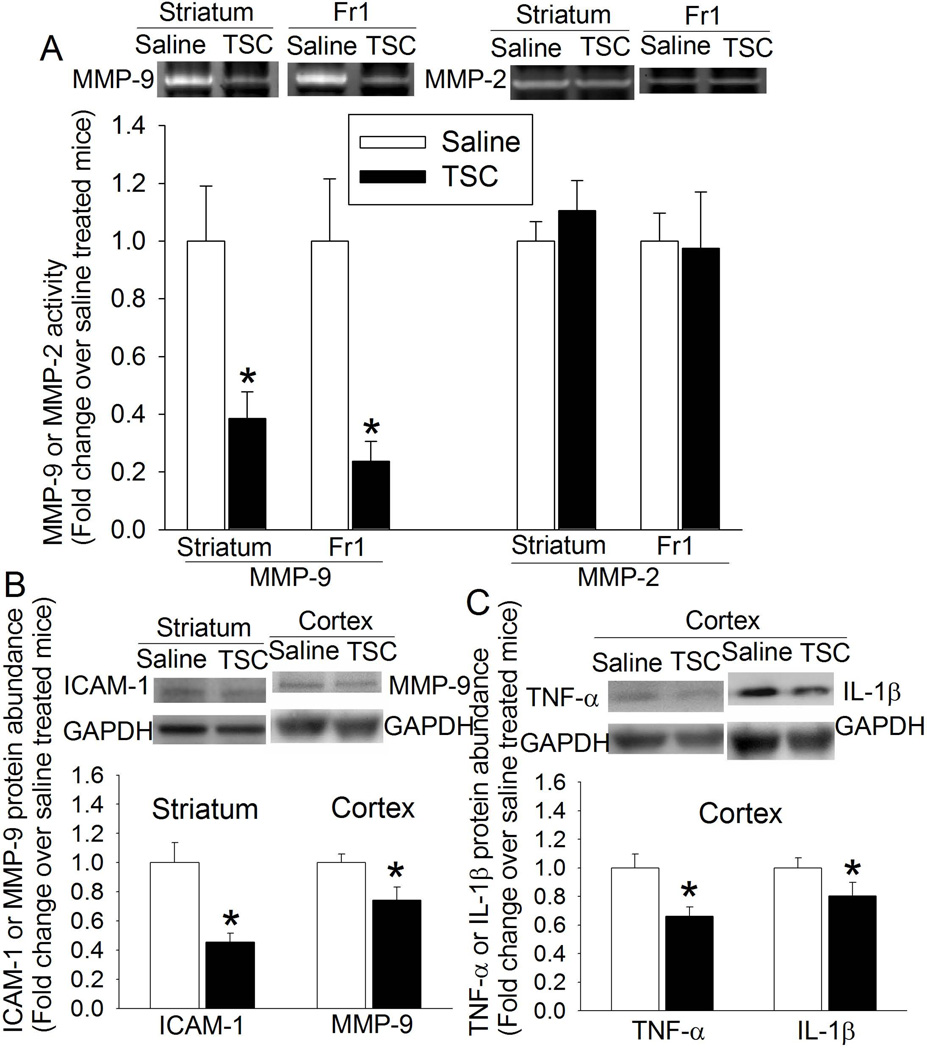
TSC significantly reduced the levels of ICAM1, TNFα and IL-1β in the ischemic brain tissues, suggesting that TSC reduces inflammatory cytokines in these tissues. TSC also reduced MMP-9 activity but did not affect MMP-2 activity in the ischemic striatum and penumbral cortex. TSC decreased MMP-9 expression in the ischemic cerebral cortex (Fig. 4).
Fig. 4. TSC inhibition of MMP-9 activity (panel A) and expression (panel B) and the expression of ICAM-1 (panel B) and inflammatory cytokines (panel C) in ischemic brain tissues.
Right cerebral tissues (striatum or cerebral cortex) were harvested 24 h after right MCAO. Results are the means ± S.E.M. (n = 5 – 8). * P < 0.05 compared with mice subjected to MCAO and treated with saline. Fr1: frontal cortex area 1; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
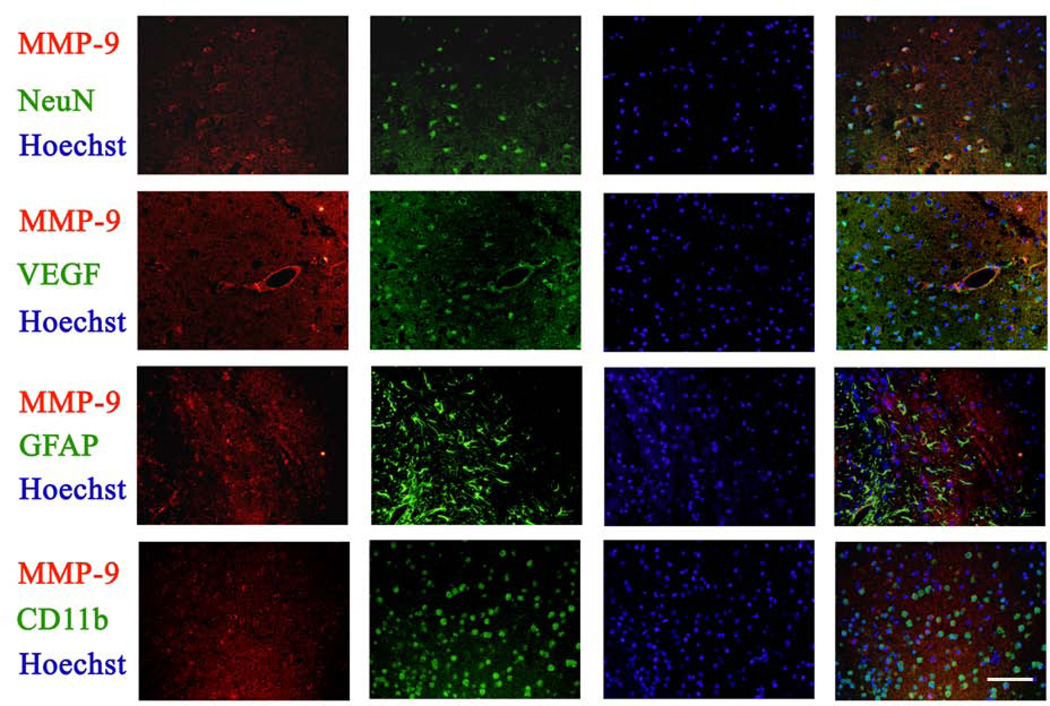
The expression of MMP-9 in the ischemic Fr1 areas was co-localized with NeuN, VEGF and CD11b, markers for neurons, endothelial cells and microglia, respectively, but was not co-localized with GFAP, a marker for astrocytes, suggesting that MMP-9 is expressed in the neurons, microglia and cerebral vessels (Fig. 5).
Fig. 5. Expression of MMP-9 in the brain tissues.
Coronal sections at Bregma −0.58 mm were obtained from animals subjected to brain ischemia only for immunofluorescent staining of MMP-9 (red), NeuN (green), VEGF (green), GFAP (green) and CD-11b (green). Images were taken in the ischemic Fr1 areas. The merged panels also include Hoechst staining (blue) to show cell nuclei. Bar = 100 µm.
Discussion
We used high fat diet feeding to induce obesity in mice. This model should closely simulate human obesity because intake of excessive high fat diet is a major cause for obesity and hyperlipidemia in humans (Kopelman 2000). Our results clearly showed that 0.14 mg/kg TSC given by bolus-infusion-bolus regimen improved neurological outcome after transient focal brain ischemia. TSC has been shown to improve neurological outcome after ischemic brain injury in young adult rats and rabbits (Lapchak 2010; Manabe et al. 2010). Our study provides initial evidence that TSC provides neuroprotection in animals with pathological conditions, such as obesity and hyperlipidemia, which often exist in patients suffering from ischemic stroke (Balci et al. 2011; Iso et al. 1989).
TSC can enhance hydrogen bonding among water molecules. This effect reduces chaos in aqueous solutions, which facilitates the diffusivity of small molecules, such as oxygen, in those solutions including plasma and interstitial fluids (Gainer 2008). This process creates a “metabolic reflow” phenomenon: oxygen and other small nutrients are diffused to the ischemic tissues from neighboring tissues with blood flow. Consistent with this idea, ischemic penumbral brain tissue oxygenation during transient focal brain ischemia is much improved by TSC in rats in a previous study (Manabe et al. 2010). This metabolic reflow mechanism is considered to be the major mechanism for TSC-induced neuroprotection (Manabe et al. 2010). However, the biochemical changes underlying the neuroprotection have not been studied.
We showed here that TSC inhibited TNF-α, IL-1β and ICAM-1 expression in the ischemic brain tissues. TNF-α and IL-1β are important proinflammatory cytokines. ICAM-1 whose expression can be increased by TNF-α and IL-1β (Stanimirovic and Satoh 2000) is an adhesion molecule to facilitate the migration of leukocytes into tissues. Inflammation after brain ischemia and reperfusion can cause cell death and injury (Lipton 1999). Leukocyte infiltration to tissues is part of inflammation and contributes to tissue damage. Thus, reducing inflammatory responses in the ischemic brain tissues may be a mechanism for TSC-induced neuroprotection. Consistent with this possibility, enhancing neuroinflammation after brain ischemia may contribute to the worsened neurological outcome in rodents with obesity (Dhungana et al. 2013; Tu et al. 2011)
Oxidative stress-induced cell injury is an important pathophysiological process after brain ischemia and reperfusion (Lipton 1999). TSC is an anti-oxidant but it may require a high TSC concentration for this effect (Stennett et al. 2007). Our study showed that nitrotyrosine containing proteins, an indicator of oxidative stress in protein, were increased in the ischemic brain tissues. This increase in the cerebral cortex ipsilateral to the ischemic side but not in the ischemic striatum was attenuated by TSC. The cerebral cortical tissues are a mixture of cortical tissues in the ischemic core, penumbral regions and normal tissues. The striatum is in the ischemic core after MCAO. Our results suggest that it may be difficult for TSC to reduce the oxidative stress in the ischemic core tissues. On the other hand, HNE, an indicator for oxidative stress in lipid, was not increased in the ischemic brain tissues. TSC did not affect the HNE levels in the ischemic or non-ischemic HNE tissues. These results suggest that HNE may not be a good indicator for oxidative stress in the ischemic tissues at 24 h after brain ischemia and reperfusion.
Our results showed that TSC significantly reduced HT. HT is a complication after ischemic stroke and can worsen the outcome of patients. Our results also showed that TSC reduced the MMP-9 expression and activity but not MMP-2 activity in the ischemic brain tissues. To maximize the possibility of detecting the changes in MMP activity, we used ischemic core and penumbral tissues for the study because HT usually only occurs in these tissues. MMP is involved in the breakdown of extracellular matrix. MMP2 and MMP9 are collagenases and gelatinase and exist in the blood vessels (Olszynski and Zimowska 2009). These MMPs have been implicated in HT after brain ischemia (Montaner et al. 2001; Montaner et al. 2003; Sumii and Lo 2002). Increased MMP-9 activity is considered an important factor for HT after thrombolytic therapy. In fact, increased MMP-9 expression may be a biomarker for HT in patients after thrombolytic therapy (Montaner et al. 2003). Consistent with a possible role of MMP-9 in HT, our results showed that MMP-9 is expressed in the cerebral vessels of obese mice after brain ischemia.
We showed here that TSC reduced inflammation, oxidative stress and MMP-9 expression and activity in the ischemic brain tissues, which may be the underlying biochemical changes for the TSC-induced neuroprotection including reduced HT. However, it is not known whether these biochemical changes are the direct effects of TSC or indirect effect from the metabolic reflow caused by TSC. It requires millimolar level of TSC in solution to have significant anti-oxidative effect (Stennett et al. 2007). It would be almost impossible to reach this concentration in the ischemic brain tissues with the used dose (0.14 mg/kg). Thus, it is possible that TSC via its metabolic reflow effect reduces the severity of brain ischemia, which leads to the favorable biochemical changes. These favorable changes then further reduce brain injury after brain ischemia and reperfusion.
Interestingly, it has been shown that TSC does not increase blood flow to ischemic tissues, oxygen solubility in the blood and oxidative phosphorylation process (Gainer et al. 1993; Holloway and Gainer 1988). Thus, TSC may not affect the cellular metabolism or carrying capacity of oxygen by blood to the ischemic brain tissues to provide neuroprotection.
The two-bolus regimen with 3 different doses did not provide neuroprotection. Interestingly, 0.14 mg/kg TSC given by bolus-infusion-bolus was neuroprotective but the same dose and two other higher doses given by two boluses was not neuroprotective. The reasons for this failure to induce neuroprotection by two-bolus regimen are not clear. A single bolus of 0.25 mg/kg TSC within 1 h after the brain embolization is neuroprotective in rabbits (Lapchak 2010). Also, 0.023 to 0.229 mg/kg TSC given as a bolus-infusion-bolus started at 10 min after the onset of brain ischemia significantly reduced brain infarct size in rats (Manabe et al. 2010). These results suggest that it is necessary to carefully perform dose-response study to identify the dosing strategy for TSC to be neuroprotective in conditions, such as obesity and hyperlipidemia, which are often associated with stroke.
Since an obvious mechanism for TSC to provide neuroprotection is by facilitating oxygen diffusion to the ischemic brain tissues, TSC needed to be administered soon after the onset of brain ischemia. This requirement may limit its use to the patients whose brain ischemia is a planned event during surgery or procedures, such as intracranial vascular surgery, if its neuroprotective effect is confirmed in humans. Patients whose ischemic stroke occurs in the hospital may also benefit from this therapy.
In summary, our results suggest that 0.14 mg/kg TSC given by the bolus-infusion-bolus regimen provides neuroprotection in obese mice. This effect may involve reduced oxidative stress, inflammation and MMP-9 expression and activity in the ischemic brain tissues.
Acknowledgement
Trans-sodium crocetinate was generously provided by the Diffusion Pharmaceuticals LLC (Charlottesville, VA).
Funding: This study was supported by grants (R01 GM065211 and R01 GM098308 to Z Zuo) from the National Institutes of Health, Bethesda, Maryland, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, Ohio, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z Zuo), Baltimore, Maryland, and the Robert M. Epstein Professorship endowment, University of Virginia.
Abbreviations
- CD-11b
cluster of differentiation-11b
- GFAP
glial fibrillary acidic protein
- ICAM-1
intercellular adhesion molecule 1
- IL-1β
interleukin-1β
- MCAO
middle cerebral artery occlusion
- MMP
matrix metalloproteinase
- PBS
phosphate buffer saline
- TSC
trans-sodium crocetinate
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
Footnotes
The research work was performed in and should be attributed to the Department of Anesthesiology, University of Virginia, Charlottesville, VA22908, U.S.A.
Conflict of interest: None.
References
- Balci K, Utku U, Asil T, Celik Y. Ischemic stroke in young adults: risk factors, subtypes, and prognosis. Neurologist. 2011;17(1):16–20. doi: 10.1097/NRL.0b013e3181f954a7. [DOI] [PubMed] [Google Scholar]
- Bhatnagar D, Soran H, Durrington PN. Hypercholesterolaemia and its management. BMJ. 2008;337:a993. doi: 10.1136/bmj.a993. [DOI] [PubMed] [Google Scholar]
- Buckman LB, Hasty AH, Flaherty DK, Buckman CT, Thompson MM, Matlock BK, Weller K, Ellacott KL. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav Immun. 2014;35:33–42. doi: 10.1016/j.bbi.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Feng J, Feng C, Xiong L, Zuo Z. Critical role of matrix metalloprotease-9 in chronic high fat diet-induced cerebral vascular remodeling and increase of ischemic brain injury in mice. Cardiovasc Res. 2014 doi: 10.1093/cvr/cvu154. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhungana H, Rolova T, Savchenko E, Wojciechowski S, Savolainen K, Ruotsalainen AK, Sullivan PM, Koistinaho J, Malm T. Western-type diet modulates inflammatory responses and impairs functional outcome following permanent middle cerebral artery occlusion in aged mice expressing the human apolipoprotein E4 allele. J Neuroinflammation. 2013;10:102. doi: 10.1186/1742-2094-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainer JL. Trans-sodium crocetinate for treating hypoxia/ischemia. Expert Opin Investig Drugs. 2008;17(6):917–924. doi: 10.1517/13543784.17.6.917. [DOI] [PubMed] [Google Scholar]
- Gainer JL, Rudolph DB, Caraway DL. The effect of crocetin on hemorrhagic shock in rats. Circ Shock. 1993;41(1):1–7. [PubMed] [Google Scholar]
- Holloway GM, Gainer JL. The carotenoid crocetin enhances pulmonary oxygenation. J Appl Physiol. 1988;65(2):683–686. doi: 10.1152/jappl.1988.65.2.683. [DOI] [PubMed] [Google Scholar]
- Iso H, Jacobs DR, Jr, Wentworth D, Neaton JD, Cohen JD. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med. 1989;320(14):904–910. doi: 10.1056/NEJM198904063201405. [DOI] [PubMed] [Google Scholar]
- Jimenez-Conde J, Biffi A, Rahman R, Kanakis A, Butler C, Sonni S, Massasa E, Cloonan L, Gilson A, Capozzo K, Cortellini L, Ois A, Cuadrado-Godia E, Rodriguez-Campello A, Furie KL, Roquer J, Rosand J, Rost NS. Hyperlipidemia and reduced white matter hyperintensity volume in patients with ischemic stroke. Stroke. 2010;41(3):437–442. doi: 10.1161/STROKEAHA.109.563502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Horigome H, Takahashi H, Tanaka K, Yoshinaga M. Determinants of blood rheology in healthy adults and children using the microchannel array flow analyzer. Clin Appl Thromb Hemost. 2010;16(4):414–421. doi: 10.1177/1076029609339745. [DOI] [PubMed] [Google Scholar]
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- Lapchak PA. Efficacy and safety profile of the carotenoid trans sodium crocetinate administered to rabbits following multiple infarct ischemic strokes: a combination therapy study with tissue plasminogen activator. Brain Res. 2010;1309:136–145. doi: 10.1016/j.brainres.2009.10.067. [DOI] [PubMed] [Google Scholar]
- Li H, Yin J, Li L, Deng J, Feng C, Zuo Z. Isoflurane postconditioning reduces ischemia-induced nuclear factor-kappaB activation and interleukin 1beta production to provide neuroprotection in rats and mice. Neurobiol Dis. 2013;54:216–224. doi: 10.1016/j.nbd.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zuo Z. Glutamate transporter type 3 knockout reduces brain tolerance to focal brain ischemia in mice. J Cereb Blood Flow Metab. 2011;31(5):1283–1292. doi: 10.1038/jcbfm.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79(4):1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Manabe H, Okonkwo DO, Gainer JL, Clarke RH, Lee KS. Protection against focal ischemic injury to the brain by trans-sodium crocetinate. Laboratory investigation. J Neurosurg. 2010;113(4):802–809. doi: 10.3171/2009.10.JNS09562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Smith BL, Matthews TJ, Ventura SJ. Births and Deaths: Preliminary Data for 1998. Natl Vital Stat Rep. 1999;47(25):1–45. [PubMed] [Google Scholar]
- Mikdashi J, Handwerger B, Langenberg P, Miller M, Kittner S. Baseline disease activity, hyperlipidemia, and hypertension are predictive factors for ischemic stroke and stroke severity in systemic lupus erythematosus. Stroke. 2007;38(2):281–285. doi: 10.1161/01.STR.0000254476.05620.14. [DOI] [PubMed] [Google Scholar]
- Montaner J, Alvarez-Sabin J, Molina CA, Angles A, Abilleira S, Arenillas J, Monasterio J. Matrix metalloproteinase expression is related to hemorrhagic transformation after cardioembolic stroke. Stroke. 2001;32(12):2762–2767. doi: 10.1161/hs1201.99512. [DOI] [PubMed] [Google Scholar]
- Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribo M, Quintana M, Alvarez-Sabin J. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107(4):598–603. doi: 10.1161/01.cir.0000046451.38849.90. [DOI] [PubMed] [Google Scholar]
- Okonkwo DO, Wagner J, Melon DE, Alden T, Stone JR, Helm GA, Jane JA., Sr Trans-sodium crocetinate increases oxygen delivery to brain parenchyma in rats on oxygen supplementation. Neurosci Lett. 2003;352(2):97–100. doi: 10.1016/j.neulet.2003.08.044. [DOI] [PubMed] [Google Scholar]
- Olsen TS, Christensen RH, Kammersgaard LP, Andersen KK. Higher total serum cholesterol levels are associated with less severe strokes and lower all-cause mortality: ten-year follow-up of ischemic strokes in the Copenhagen Stroke Study. Stroke. 2007;38(10):2646–2651. doi: 10.1161/STROKEAHA.107.490292. [DOI] [PubMed] [Google Scholar]
- Olszynski K, Zimowska M. Structure and function of matrix metalloproteinases. Postepy Biochem. 2009;55(1):76–84. [PubMed] [Google Scholar]
- Purkayastha S, Cai D. Neuroinflammatory basis of metabolic syndrome. Molecular metabolism. 2013;2(4):356–363. doi: 10.1016/j.molmet.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco RL, Liao JK. Drug Insight: statins and stroke. Nat Clin Pract Cardiovasc Med. 2005;2(11):576–584. doi: 10.1038/ncpcardio0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanimirovic D, Satoh K. Inflammatory mediators of cerebral endothelium: a role in ischemic brain inflammation. Brain Pathol. 2000;10(1):113–126. doi: 10.1111/j.1750-3639.2000.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stennett AK, Dempsey GL, Gainer JL. trans-Sodium crocetinate and diffusion enhancement. J Phys Chem B. 2006;110(37):18078–18080. doi: 10.1021/jp064308+. [DOI] [PubMed] [Google Scholar]
- Stennett AK, Murray RJ, Roy JW, Gainer JL. Trans-sodium crocetinate and hemorrhagic shock. Shock. 2007;28(3):339–344. doi: 10.1097/shk.0b013e3180487b2d. [DOI] [PubMed] [Google Scholar]
- Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33(3):831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- Tu YF, Tsai YS, Wang LW, Wu HC, Huang CC, Ho CJ. Overweight worsens apoptosis, neuroinflammation and blood-brain barrier damage after hypoxic ischemia in neonatal brain through JNK hyperactivation. J Neuroinflammation. 2011;8:40. doi: 10.1186/1742-2094-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20(18):7037–7042. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Zuo Z. Isoflurane preconditioning induces neuroprotection against ischemia via activation of p38 mitogen-activated protein kinase. Mol Pharmacol. 2004;65:1172–1180. doi: 10.1124/mol.65.5.1172. [DOI] [PubMed] [Google Scholar]