Abstract
Rationale
Despite four decades of intense effort and substantial financial investment, the cardioprotection field has failed to deliver a single drug that effectively reduces myocardial infarct size in patients. A major reason is insufficient rigor and reproducibility in preclinical studies.
Objective
To develop a multicenter randomized controlled trial (RCT)-like infrastructure to conduct rigorous and reproducible preclinical evaluation of cardioprotective therapies.
Methods and Results
With NHLBI support, we established the Consortium for preclinicAl assESsment of cARdioprotective therapies (CAESAR), based on the principles of randomization, investigator blinding, a priori sample size determination and exclusion criteria, appropriate statistical analyses, and assessment of reproducibility. To validate CAESAR, we tested the ability of ischemic preconditioning (IPC) to reduce infarct size in three species (at two sites/species): mice (n=22-25/group), rabbits (n=11-12/group), and pigs (n=13/group). During this validation phase, i) we established protocols that gave similar results between Centers and confirmed that IPC significantly reduced infarct size in all species, and ii) we successfully established a multi-center structure to support CAESAR’s operations, including two surgical Centers for each species, a Pathology Core (to assess infarct size), a Biomarker Core (to measure plasma cardiac troponin levels), and a Data Coordinating Center – all with the oversight of an external Protocol Review and Monitoring Committee.
Conclusions
CAESAR is operational, generates reproducible results, can detect cardioprotection, and provides a mechanism for assessing potential infarct-sparing therapies with a level of rigor analogous to multicenter RCTs. This is a revolutionary new approach to cardioprotection. Importantly, we provide state-of-the-art, detailed protocols (“CAESAR protocols”) for measuring infarct size in mice, rabbits, and pigs in a manner that is rigorous, accurate, and reproducible.
Keywords: Heart, ischemia-reperfusion injury, ischemic preconditioning, cardioprotection, infarct size, randomized controlled trial
INTRODUCTION
Heart disease is the leading cause of death in the United States and acute myocardial infarction is the primary culprit. In the U.S. alone, more than 750,000 patients experience a new myocardial infarction every year1. Because the amount of tissue destroyed during infarction is a major determinant of prognosis2-4, protecting the ischemic myocardium remains an urgent, yet unmet, need. Over the past 40 years, innumerable therapies have been claimed to be efficacious in limiting the size of a myocardial infarction in animal models5; based upon these “positive” preclinical data, many of these interventions have been tried in patients but the results have been disappointing. Although several agents including metoprolol6, cyclosporine7, 8, ANP9, and exenatide10 show promise in early clinical trials, to date, there is no accepted method to reduce infarct size in the clinical arena beyond timely reperfusion5.
While there are a number of reasons to explain such failures11, 12, probably the most important is that many preclinical studies have lacked sufficient rigor5. For example, in most studies published to date, animals have not been randomized, investigators have not been blinded, exclusion criteria and sample sizes have not been established a priori, and details of protocols (which may impact outcome) have not been thoroughly standardized. Not surprisingly, the results have frequently not been reproducible from one laboratory to another5. If a therapy is not reproducibly effective in the experimental setting, it is unlikely that it will be effective in the much more complex clinical setting, in which a large number of confounding variables affect the results5,13, 14. The need for a new strategy is obvious5,15-18. Given that the approach used in the past 40 years has failed to produce a single therapy that is approved for infarct size reduction in humans, it seems implausible that continuing with this same failed approach will be fruitful.
Accordingly, we adopted a new paradigm by establishing a functional consortium (CAESAR)19, analogous to a multicenter, randomized clinical trial, in which promising therapies are subjected to a rigorous test of efficacy and reproducibility with respect to infarct size reduction (Figure 1). Our rationale is that therapies that survive the “CAESAR test” have the best chance of clinical success and should be considered for human studies. This approach represents a dramatic departure from the modus operandi of the past 40 years. Performing preclinical studies with the rigor of multicenter, randomized clinical trials has rarely, if ever, been done in basic research, particularly in studies of cardioprotection, and thus constitutes a veritable paradigm shift for the field. The NIH Director, Dr. Francis Collins, recently lamented the lack of reproducibility of scientific work and effectively issued a call to action20.
Figure 1. Overview of CAESAR structure and data flow.
The locations of the Surgical Centers are indicated in the figure. The Pathology Core is located at Johns Hopkins (Dr. Steenbergen). The Biomarker Core (Dr. Li) and Data Coordinating Center (Drs. Jones and Kong) are located at the University of Louisville. The members of the Surgical Centers do not participate in any of the activities of any of the Cores (including the Data Coordinating Center). Only the Data Coordinating Center has access to data prior to a study being reported to the Protocol Review and Monitoring Committee (composed of cardioprotection experts from non-CAESAR member institutions).
The goal of CAESAR is to serve as a public resource for the rigorous assessment of candidate infarct-sparing drugs; however, before CAESAR can be used to screen cardioprotective therapies, it is necessary to validate it by testing the ability of the consortium to i) detect cardioprotection in all three species used (mice, rabbits, and pigs), and ii) generate results that are comparable and reproducible between two different centers that use the same protocol without knowledge of each other’s data. For these essential validation studies, we selected ischemic preconditioning (IPC)21, which activates the largest number of protective pathways and is the most robust and reproducible cardioprotective intervention known thus far. Here we report the development and validation of CAESAR and our initial experience with this novel approach to preclinical investigation. Perhaps more importantly, we provide detailed protocols for testing potential infarct-sparing therapies in mice, rabbits, and pigs; these protocols, which are based on extensive studies and thoughtful discussions among CAESAR investigators and the Protocol Review and Monitoring Committee, should be useful to many investigators working in the field of cardioprotection.
METHODS
One of the main goals of CAESAR was to develop protocols that enable rigorous assessment of potential infarct-sparing therapies in mice, rabbits and pigs. To make these protocols available to the entire scientific community and to facilitate their use by others, the protocols are described herein in great detail.
All studies were approved by the Animal Care and Use Committees of the component Universities of CAESAR and were performed in accordance with the Guide for the Care and Use of Laboratory Animals [DHHS Publication No. (NIH) 86–23]. Ultimately, both Centers for each species used the same ACUC protocol. In addition, a group of extramural scientists serve on the Protocol Review and Monitoring Committee (PRMC) (Figure 1).
Mouse model of myocardial ischemia/reperfusion injury
Male ICR (Institute of Cancer Research) mice were obtained from Harlan Laboratories (Houston, TX) and housed under specific pathogen-free conditions in a room (22-24°C temperature, 55–65% relative humidity, and a 12:12-h light-dark cycle). The mouse diet was Rodent Diet 5010 (www.LabDiet.com). At approximately 13 weeks of age and 42 grams of body weight, mice were pre-medicated with atropine sulfate (0.04 mg/kg i.m.) and anesthetized 5 min later with pentobarbital sodium (60 mg/kg i.p). Additional doses of pentobarbital (5 mg/kg, i.p.) were given during the protocol as needed to maintain anesthesia, which was determined by the presence of the toe pinch reflex and leg movements during the procedures. The animals were placed in a supine position with the paws taped to the operating table. Surface leads were placed subcutaneously to obtain the electrocardiogram (ECG), which was recorded throughout the experiments. Before surgery started, mice were given gentamicin (0.7 mg/kg i.m.).
A midline cervical skin incision was performed, and the muscles overlying the trachea were retracted to allow visualization of the endotracheal tube (PE-60 tubing) as it was placed in the trachea. To facilitate intubation, a rubber band was placed behind the upper incisors and fastened to the operating table so that the neck was slightly extended. To place the endotracheal tube, the tongue was slightly retracted, and the beveled end of the tube (which was marked with a black marker) was inserted through the larynx and into the trachea with care taken not to puncture the trachea or other structures in the pharyngeal region. The tube was advanced 8–10 mm from the larynx and taped in place to prevent dislodgment. The animals were ventilated with room air supplemented with oxygen (1 L/min) at a rate of 105 breaths/min and with a tidal volume of 10.3 μL/g using a mouse ventilator (MiniVent 845, Hugo Sachs Elektronik-Harvard Apparatus, Germany). The endotracheal tube was inserted loosely into the tube connected to the ventilator to avoid lung overexpansion. To prevent blood pressure drops due to artificial ventilation, the loss of negative intrathoracic pressure during the open-chest state, and blood losses, blood from a donor mouse was given intravenously at a dose of 20 mL/kg (approximately 0.5 mL/mouse) divided into three equal boluses (first bolus, after the endotracheal tube was connected to the ventilator; second bolus, after the chest was opened; third bolus, after the chest was closed22). Body temperature was carefully monitored with a rectal probe connected to a digital thermometer (Cole-Parmer Instrument, Vernon Hill, IL) and was strictly maintained between 36.7-37.3°C throughout the experiment using an external heating system (i.e., heating pad and/or lamps).
Aseptic techniques were used for surgery, which was performed in a dedicated clean room. With the aid of a dissecting microscope or surgical loupes (Care Optical Industrial Co., Ltd., China) and a microcoagulator (ASSI Polar-Mate Isolator, San Diego, CA), the chest was opened through a midline sternotomy. An 8-0 nylon suture was passed with a tapered needle under the left coronary artery 2-3 mm from the tip of the left auricle, and an atraumatic balloon occluder was applied on the artery and inflated to occlude the artery. Successful performance of coronary occlusion and reperfusion was verified by visual inspection (i.e., by noting the development of a pale color in the distal myocardium on inflation of the balloon and the return of a bright red color due to hyperemia after deflation), observing S-T segment elevation, and widening of the QRS on the ECG during ischemia and their resolution after reperfusion. After the coronary occlusion-reperfusion protocol was completed, the chest was closed in layers, and a small catheter was left in the thorax for 10-20 min to evacuate air and fluids. The mice were removed from the ventilator, kept warm, given fluids (1.0-1.5 mL of 5% dextrose in water intraperitoneally), and allowed 100% oxygen via nasal cone (or the mouse cage was kept in an oxygen tent). A ~70 μL blood sample was obtained from the tail vein at 2 h of reperfusion for measurement of the cardio-specific injury marker, cardiac troponin I (cTnI). We have found that plasma cTnI levels peak at 2 h in this model, and that the 2-h values of cTnI correlate well with pathologist-based measurements of infarct size.
For analgesia, we administered buprenorphine (0.1 mg/kg s.c. right after closing the chest) and ketoprofen (5 mg/kg i.m. once a day for 48 h, starting immediately after closing the chest). After surgery, mice were observed every hour for the first six hours. Animals were placed in cages with heating pads in an enriched oxygen tent for at least 24 h.
Ischemic preconditioning (IPC)
IPC was produced with a sequence of six cycles of 4-min coronary occlusion and 4-min reperfusion (Figure 2A). This protocol was selected because it is highly effective in inducing early and late PC in mice 22-32. Myocardial infarction (i.e., index ischemia) was produced by a 30-min coronary occlusion followed by 48 h of reperfusion. A 30-min occlusion was selected because in our previous studies it produced infarcts averaging 50% of the region at risk in control animals 22-32, which enabled us to detect either a detrimental or a beneficial effect of the intervention examined. Mice were subjected to either IPC or sham IPC.
Figure 2. Ischemic preconditioning protocol for each species used in CAESAR.
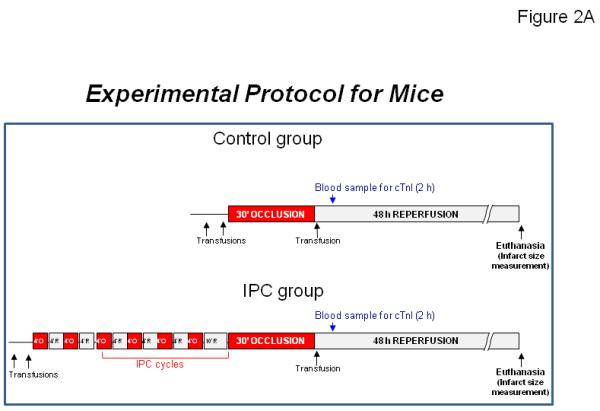


(A) Mice. (B) Rabbits. (C) Pigs.
At the conclusion of the study, mice were given heparin (1 U/g i.p), after which they were anesthetized with pentobarbital sodium (60 mg/kg i.p.) and euthanized with an intravenous bolus of KCl. The heart was excised and perfused with Krebs-Henseleit solution through an aortic cannula (22- or 23-gauge needle) using a Langendorff apparatus. To delineate infarcted from viable myocardium, the heart was then perfused with a 1% solution of 2,3,5-triphenyltetrazolium chloride in phosphate buffer (pH 7.4, 37°C) at a pressure of 60 mmHg (approximately 3 mL over 3 min). To delineate the ischemic zone, the coronary artery was then tied at the site of the previous occlusion and the aortic root was perfused with a 5% solution of phthalo blue dye (Heucotech, Fairless Hill, PA) in normal saline (2 mL over 3 min). As a result of this procedure, the portion of the left ventricle (LV) supplied by the previously occluded coronary artery (region at risk) was identified by the absence of blue dye, whereas the rest of the LV was stained dark blue 22. All atrial and right ventricular tissues were excised and the heart was frozen in a −20°C freezer. The LV was cut into 5-7 transverse slices, which were fixed in 10% neutral buffered formaldehyde, weighed, and photographed. The images were uploaded to the secure CAESAR data site and fixed heart sections were sent to the CAESAR Pathology Core to measure infarct size.
Exclusion criteria
Mice with major technical problems including failure of intubation, severe bleeding, lung damage, coronary artery damage, or balloon malfunction; any signs of hypoxia or hypotension before the ischemia/reperfusion injury procedures; outside the weight range of 32-48 g; outside the age range of 12-15 wk; or with a region at risk <20% of LV weight were excluded from the final analysis.
Rabbit model of myocardial ischemia/reperfusion injury
Pathogen-free, healthy, New Zealand White male rabbits were used at approximately 90 days of age and 2.2 kg of body weight. After establishment of a venous access by placement of a butterfly needle catheter (23G × ¾”) on a marginal ear vein, rabbits were anesthetized with sodium pentobarbital (0.5 mL of 5% solution i.v.), intubated with an endotracheal tube, and mechanically ventilated with air enriched with oxygen. The ventilation rate was maintained between 28-30 breaths per minute. Anesthesia was maintained with sodium pentobarbital (total dose: 35 mg/kg i.v.). Body temperature was monitored continuously with a rectal probe attached to a thermocouple. A heating blanket was used to maintain body temperature between 36.0-39.0°C. The rabbits were shaved and the surgical area washed three times with isopropyl alcohol. Betadine was applied with sterile gauze to the surgical area and allowed to dry. A transparent, sterile, adhesive drape (3M) was used as a barrier on the surgical site. Under sterile conditions, the heart was exposed through a left thoracotomy in the fourth intercostal space. After opening the pericardium, a 3-0 tapered needle silk suture was passed beneath a major branch of the left coronary artery perpendicularly to the vessel. Lifting the suture against a cotton-tip applicator performed a brief test of coronary occlusion to estimate the size of the region at risk. The site of the balloon inflation was chosen in an effort to obtain a risk area of ~25% (not less than 15% nor greater than 35%) of the LV.
A balloon occluder (fashioned from 18-gauge Tygon tubing) was placed along the top of the coronary artery and secured with 3-0 silk on the anterior LV wall. A piece of aluminum sheet [0.2-0.3 mm thick] was placed on top of the balloon as a backing material for holding a silk suture knot and for aligning the balloon occluder parallel to the coronary artery. Careful placement of the balloon is critical for a later successful conscious study, i.e. the suture knot should be neither too tight (which would cause blood flow restriction after balloon deflation) nor too loose (which would fail to induce complete coronary occlusion during balloon inflation). After balloon placement, a brief test (10-s balloon inflation) was performed and proper function of the occluder was confirmed by observing cyanosis of the distal myocardium upon inflation of the balloon and hyperemia after deflation. The tail of the balloon was anchored to the surface of the LV wall with a 6-0 suture to avoid twisting of the balloon occluder. A small piece of plastic film (~0.1 mm thick) was placed between the surface of the LV wall and the balloon occluder to help distribute pressure evenly on top of the coronary artery during balloon inflation. A bipolar lead was anchored to the chest wall to record the ECG. The wires and the occluder tubing were tunneled under the skin and exteriorized through small incisions between the scapulae. The chest wound was closed in layers, and a small tube was left in the thorax for three days to evacuate air and fluids. After surgery, rabbits recovered in a dedicated recovery area where they received supplemental oxygen and were kept warm. Buprenorphine (0.1 mg/kg i.m.) was administered pre-operatively for pain and repeated every 12 h for the first 48 h postoperatively. Cefazolin (15 mg/kg, s.c.) was administered pre-operatively and repeated every 24 h for the first 48 h post-operatively. Rabbits were allowed to recover for a minimum of 7 d after surgery.
Throughout the coronary occlusion-reperfusion sequence, rabbits were kept in a cage in a quiet, dimly lit room. A venous line was established by placing a 24-gauge angiocatheter on a marginal ear vein for blood sample draw and for medication. Under local anesthesia (benzocaine spray), the ear dorsal artery was cannulated with a 22-gauge angiocatheter to monitor arterial pressure. Arterial pressure and ECG were continuously recorded. Rabbits received ketoprofen (3.0 mg/kg, s.c.) 2 h before and diazepam (4 mg/kg, i.p.) 20 min before the onset of ischemia; in addition, they received heparin (50 U/kg, i.v.) before occlusion to prevent coronary arterial thrombosis. All rabbits were subjected to a 45-min coronary artery occlusion and 3 days of reperfusion by inflating/deflating the hydraulic balloon occluder. The performance of successful coronary occlusions was verified by observing the development of ST-segment elevation and changes in the QRS complex on the ECG. Rabbits that were assigned to the IPC group received three 5-min coronary occlusions interspersed with 10 min of reperfusion prior to the 45 min coronary occlusion (Figure 2B). When VF occurred, defibrillation was performed immediately (50 J) by placing a pediatric defibrillation paddle on both sides of chest. Serial 0.4 mL blood samples were collected at baseline and 1, 2, 4, 24, and 48 h after reperfusion for measurement of cTnI (both peak levels and area-under-the-curve).
At the end of the experiment, rabbits were given heparin (100 U/kg i.v.), after which they were anesthetized with pentobarbital (50 mg/kg i.v.) and euthanized with KCl. The heart was quickly mounted onto a Langendorff apparatus. The aortic root was perfused with Krebs-Henseleit solution (~30 mL, 37°C) at a pressure of ~60 mmHg to remove all blood. The coronary artery was then tied at the site of the previous occlusion and the aortic root was perfused with a 5% solution of phthalo blue dye in normal saline (~10 mL over 3 min). The heart was cut into 5-6 transverse slices (each ~2-mm-thick), which were incubated for 15 min at 37°C in a 1% solution of triphenyltetrazolium chloride in phosphate buffer (pH 7.4) to delineate infarcted from viable myocardium. The heart slices were fixed in 10% neutral buffered formaldehyde for 4 to 24 h with a weight on top to keep the heart slice flat for the initial 30 min. The atrial and RV tissue were removed, and the LV slices were numbered from apex to base as 1 through 5 or 6, weighed, and photographed on both sides of the slices together with a ruler. The images together with the tissue slice weight information were uploaded to the secure CAESAR data site for measurement of the sizes of the nonischemic, ischemic/reperfused, and infarcted areas. After photographs were taken, each slice was placed in the cassette for paraffin embedding. All formalin-fixed tissue sections were sent to the Infarct Size Core for histology assessment. The images and tissue slice weight information were uploaded to the appropriate folder on the secure, CAESAR data site. The Pathology Core subsequently accessed these files for measurement of the sizes of the non-ischemic, ischemic, and infarcted areas.
Exclusion criteria
Rabbits with major technical problems including failure of intubation, severe bleeding, lung damage, coronary artery damage, or occluder malfunction; any signs of hypoxia or hypotension before the ischemia/reperfusion injury procedures; outside the 2.5-3.0 kg weight range; or with a region at risk <20% of LV weight were excluded from the final analysis.
Pig model of myocardial ischemia/reperfusion injury
Experimental myocardial infarction was performed in approximately 90-day-old female Yorkshire pigs weighing 30-45 kg (Palmetto Research Swine, Reevesville, SC). The animals were acclimated for 7-10 d after arrival and maintained on Lab Diet 5084 (Cincinnati Lab Supply). Pigs were assigned randomly to the control or IPC group and fasted for at least 12 h prior to sedation for the closed-chest myocardial infarct procedure. On the day of the coronary artery occlusion, the pigs were sedated using a cocktail of ketamine hydrochloride (20 mg/kg, i.m.) and xylazine (2 mg/kg, i.m.). Following adequate sedation (~15 min), they were moved to the pre-operative care room. An intravenous catheter was placed in a marginal ear vein for the administration of fluids and drugs. Diazepam (1 mg/kg, i.v.) was administered to allow for endotracheal intubation. An appropriately sized endotracheal tube was inserted for mechanical ventilation. The chest and groin were shaved and prepared for cardiac catheterization procedure. The pig was then moved to the catheterization suite and placed on the operating table in the lateral position with the right side up. Body temperature was maintained at 36.5-39.0°C during the procedure with a water blanket and/or hot air veterinary blanket. Aspirin (300 mg, i.v.) and ceftiofur (3 mg/kg, s.c.) were administered and the pig was ventilated with 50/50 oxygen/room air or nitrogen at initial settings of 10-20 breaths per minute and 10-12 mL/kg tidal volume. Another ear vein catheter was placed for infusion of methohexital sodium (10 mg/kg/h, i.v.). Surface ECG leads (limb lead II) were placed and connected to a patient monitor for continuous electrocardiographic data. To induce a stable anesthetic plane, methohexital sodium infusion was initiated once the pig was on ventilation. Methohexital sodium dosing was adjusted between 5.5-12.0 mg/kg/h throughout the experimental procedures and actual dosing varied slightly based on the anesthetic state of each animal. Animals were monitored continuously to confirm adequate anesthesia.
The groin and right neck were scrubbed with chlorhexidine-based surgical disinfectant scrub, followed by alcohol-soaked gauze in a series of three cycles. Then, providone-iodine solution was sprayed on the surgical sites. The animal was fitted with sterile drapes and sterile instruments were handed off to the surgeon. All surgical procedures were performed using standard sterile technique, including sterile gowns, gloves, masks, and instruments. Arterial blood gases were measured routinely to ensure physiological stability. Ventilation rates were adjusted to maintain pCO2 between 35-45 mmHg, pO2 between 100-200 mmHg, and pH between 7.35-7.45. If metabolic acidosis (low pH with HCO3 deficit) occurred, sodium bicarbonate was given according to the formula: Sodium Bicarbonate (mEq) required = 0.3 × body weight (kg) × base deficit (mEq/L). A cut-down (local lidocaine injection) on the right side of the neck was performed and the right jugular was used for placement of a 7-9 F Chronic-Cath polyurethane catheter (Access Technologies). This catheter was implanted, secured, and tunneled to the back of the neck for serial blood withdrawals at the postoperative time points. The catheter dead-space was filled with heparin (1000 U/mL) after each withdrawal to maintain patency; care was taken to fill the dead-space only with no spillover into the systemic circulation.
After the jugular catheter was placed, the pig was moved to its supine position. External defibrillator pads were placed, fixed on the pig’s chest skin and connected to a “hands-free” defibrillator. Through a right femoral artery cut-down, a 7-8 F fast-cath sheath was introduced so that a 6-7 F Hockey-stick guide catheter could be advanced to the heart for the angiogram and balloon placement. A left femoral cut-down was performed and a 6-7 F fast-cath sheath was introduced and used for a 5-6 F pigtail catheter that was advanced to the LV and used to inject microspheres. Arterial blood pressure was measured from the side arm of the right arterial sheath, which was connected to a fluid filled pressure transducer. Heparin (300 U/kg, i.v.) was given to prevent clotting of the sheaths and catheters during the procedure.
A 6-7 F Hockey-stick catheter over a 0.038″ guide wire was fluoroscopically guided to the left main coronary ostium. A bolus of contrast dye was injected to obtain a coronary cineangiogram for determining the site of balloon placement and the size of the balloon catheter. After angiographic measurement of the mid-LAD diameter at the site to be occluded, a 0.014″ guide wire was advanced into the distal LAD and an appropriately sized (2.5 to 3.5 mm) balloon-tipped angioplasty catheter was telescoped over the wire and positioned in the mid-LAD distal to the second diagonal terminal branch (when the second diagonal branch was too distal, a first diagonal target was attempted.). Placement of the balloon was verified by intracoronary contrast dye injection, and documented by cineangiogram prior to inflation. The goal was to occlude the LAD coronary artery just distal to the second diagonal branch, producing a region at risk approximating 25-30% of the LV mass. If the region at risk (measured postmortem) was less than 20% of the LV, the animal was excluded. This region at risk is distributed primarily in the anterior free wall, but includes a portion of the anterior septum.
Following the instrumentation described above, baseline hemodynamics were collected and microspheres (Samarium [Sa], isotope #1) were given following stabilization. Following baseline hemodynamics and microsphere injection, a prophylactic dose of amiodarone (4 mg/kg, i.v.) was administered slowly to reduce arrhythmias. At reperfusion, amiodarone was infused at a rate of 2.4 mg/kg/h (0.04 mg/kg/min, i.v.) until 15 min into reperfusion. A lidocaine bolus (2 mg/kg, i.v.) was also given, followed by a lidocaine infusion at a rate of 3.0 mg/kg/h (0.05 mg/kg/min).
Pigs were randomly assigned to a control or an IPC group (Figure 2C). In control pigs, the LAD occlusion was maintained for 60 min (index ischemia) followed by reperfusion, targeting an infarct size of approximately 60% of the region at risk. In pigs undergoing IPC, three 5-min LAD occlusion/10-min reperfusion cycles were performed prior to the 60-min index ischemia. To achieve LAD occlusion, the angioplasty balloon was inflated at 8-10 Atm. After balloon inflation, a cineangiogram was obtained with contrast injection through the hockey-stick guiding catheter to confirm the balloon position and complete occlusion of the distal LAD. Both the inflation and the position of the balloon were confirmed by contrast angiogram again at the end of ischemia. When ventricular fibrillation occurred, defibrillation was immediately performed through the fixed skin electrodes using a 360 joules DC shock. At 45 min into occlusion, a second injection of microspheres (Europium [Eu], isotope #2) was given to quantify collateral blood flow to the region at risk, which is used as a covariate of infarct size. After the 60-min ischemic period, the intracoronary balloon was deflated to initiate reperfusion and the balloon catheter was withdrawn. After balloon withdrawal, an additional cineangiogram was made to verify vessel patency. A third injection of microspheres (Lutetium [Lu], isotope #3,) was given at 15 min of reperfusion to confirm vessel patency and early reperfusion. Hemodynamic data were collected at 15 min and 45 min of ischemia and at 15 min and 60 min of reperfusion.
Buprenorphine (0.025 mg/kg, i.m.) was given for post-procedural analgesia at 15 min into reperfusion and a transdermal fentanyl patch (2.5 μg/kg/h) was placed at the end of the procedure for the 72 h follow-up. The femoral catheters were removed and the groin access sites were closed in layers using 3-0 Vicryl for internal sutures and 3-0 PDS (polydioxanone suture) for the final subcutaneous layer. After hemodynamic readings were recorded through 60 min of reperfusion, pigs were weaned from anesthesia and moved to a postoperative care area when spontaneous breathing and hemodynamic stability were achieved. A pulse oximeter probe was placed on the ear or tail to measure oxygen saturation. Extubation was performed when the pig began to chew and exhibited a gag reflex. Pigs were monitored until they righted themselves without assistance. Ceftiofur (3 mg/kg, s.c.) was repeated on postoperative days 1 and 2. Blood samples (1 mL) were collected from the jugular venous catheter at 2 h, 4 h, 6 h, 24 h, and 48 h of reperfusion for cTnI assays.
At 72 h of reperfusion, the pig was sedated using a cocktail of ketamine hydrochloride (20 mg/kg, i.m.) and xylazine (2 mg/kg, i.m.). Following adequate sedation (~15 min), the pig was moved to the pre-operative care room, received propofol (1.5 mg/kg i.v.) via the indwelling venous catheter prior to intubation, and was intubated with an appropriately sized endotracheal tube. For final data collection, the pig was then transported to the catheterization laboratory, where it was placed on a heating blanket to maintain rectal temperature at 36.5-39.0°C. The pig was ventilated on 50/50 oxygen/nitrogen mixtures at 10-12 breaths/min and 10 - 12 mL/kg tidal volume. General anesthesia was maintained with isoflurane (1.0-1.5%). Surface ECG leads (limb lead II) were placed to record electrocardiographic data. Through a right carotid artery cut-down, a 7-8 F sheath was placed and a pressure transducer was connected to the side port of the sheath to record arterial pressure. A 5-6 F pigtail catheter with infusion lumen was guided into the LV for injection of the 72-h microsphere injection (Lanthanum [La], isotope #4). After the final microsphere injection, the pig was heparinized (100 U/kg, i.v.), and deeply anesthetized with 5% isoflurane. A bolus of 3-6 mL/kg of 3 M KCl solution was injected to the LV cavity via the pigtail catheter until the heart was completely arrested. Asystole was confirmed by cessation of cardiac electric activity from ECG monitoring. After cessation of vital signs, the heart was harvested for postmortem perfusion.
The heart was mounted onto a dual perfusion system. The LAD was cannulated at the site of the previous occlusion to perfuse the ischemic/reperfused myocardium (referred to as the region at risk), while the aortic root was cannulated to retrogradely perfuse the nonischemic myocardium. After perfusion with Krebs-Henseleit solution at 37°C (to remove any blood), the LAD cannula was perfused with a 1% (w/v) TTC solution in phosphate buffered saline at 37°C and the aortic cannula with a 5% (v/v) solution of phthalo blue dye in normal saline. The pressure was maintained at approximately 80 mmHg in both cannulas. After perfusion for ~15 min when a deep red in the epicardial surface of the risk zone was seen, the perfusion was stopped. The heart was then sectioned from apex to base into 6-7 transverse slices, each ~1 cm thick, until no region at risk tissue was noted. The LV slices were fixed in 10% neutral buffered formaldehyde for 4-24 h. This dual perfusion stains the nonischemic myocardium dark blue, the surviving tissue (TTC-positive region) brick red, and the infarcted tissue (TTC-negative region) pale tan/yellow. After formalin fixation, the atrial and right ventricular tissue were removed, the LV slices were numbered from apex to base as 1 through 6 (or 7, if applicable), weighed, and photographed on both sides of the slices together with a ruler. After photographs were taken, formalin-fixed tissue was sent to the Pathology Core for infarct size/histology assessment. The images and tissue slice weight information were uploaded to the appropriate folder on the secure CAESAR data site. The Pathology Core subsequently accessed these files for measurement of the sizes of the nonischemic, ischemic, and infarcted areas.
Measurement of regional myocardial blood flow (RMBF) using microspheres
for measurement of rmbf, biopal stable-isotope neutron-activated microspheres (15 ± 5 μm, BioPhysics Assay Laboratory, Inc., Worcester, MA) were used as described 33. As mentioned above, microspheres were injected at four time-points: baseline, during coronary occlusion (15 min before reperfusion), 15 min after reperfusion, and 72 h after reperfusion. At each time point, a total of 5 × 106 microspheres (2 mL) labeled with samarium (Sa), europium (Eu), lutetium (Lu), or lanthanum (La) were injected over 30 s into the LV cavity through the pigtail catheter while a reference blood sample was simultaneously drawn from the side arm of the arterial sheath catheter for calculation of absolute myocardial blood flow using a withdrawal pump at 7 mL/min for 90 s.
After the LV sections were photographed and weighed, four transmural tissue blocks (~1 g) were obtained from both the ischemic/reperfused and the nonischemic regions (to avoid admixture of ischemic and non-ischemic tissue, ischemic specimens were obtained at least 1 cm inside the boundaries of the occluded bed). Each specimen was divided into endocardial and epicardial halves, rinsed in a saline-free buffer, placed in tissue sample vials, and weighed. Tissue and reference blood samples were processed according to BioPAL’s General Protocol For Blood Flow Experiments and were sent to BioPAL for the analysis, which uses neutron activation technology for the measurement of microsphere content 33. Absolute RMBF was computed in each sample with the formula: RMBF = (counts in tissue sample × reference blood sample withdrawal rate) / (counts in reference blood sample × tissue weight in gram) and expressed as mL/min/g. These data were uploaded to the appropriate folder on the secure CAESAR data site for statistical analysis by the DCC.
Exclusion criteria
The following events represented grounds for excluding a pig from the study: 1) Body weight outside of the range of 30-45 kg; 2) body temperature outside of the range (36.5-39.0°C); 3) dose of anesthetic during ischemia outside of the range (7.0-8.0 mg/kg/h); 4) region at risk <20% of LV; 5) RMBF during ischemia >8.0% of the nonischemic zone RMBF; 6) absence of RMBF data during ischemia; 7) average heart rate during ischemia (average of all ischemic time points in a given animal) outside the range of 60-120 bpm; 8) average mean arterial pressure during ischemia (average of all ischemic time points in a given animal) outside the range of 50-110 mmHg; 9) a total of 15 unsuccessful electric shocks delivered during VF failing to convert to normal sinus rhythm.
Image analysis for infarct size determination
In all three species (mice, rabbits, and pigs) infarct size determination was performed from the image files. With our protocol, the non-ischemic myocardium is blue, the viable tissue within the ischemic zone is red, and the infarct is white, tannish, yellow, or brown, depending on whether there was hemorrhage and depending on how much organization of the infarct had occurred. For analysis, Photoshop was used to delineate the area of the entire LV slice; then the blue nonischemic tissue was erased to generate an image that included only the region at risk, and then the red viable myocardium was erased to generate an image that included only the infarct. These images were then analyzed using ImageJ to determine the number of pixels in each image, following which we calculated, for each image, the region at risk as a percentage of the LV and the infarct as a percentage of the LV. The data were entered into a spreadsheet that included the slice weight. For each slice, the mean value for infarct size and region at risk was determined by averaging the data for the apical and basal surfaces and by multiplying the average by the slice weight to calculate the amount of infarct, viable-ischemic tissue, and non-ischemic tissue. The weights from each slice were added to determine the overall amount of infarct and region at risk. At the end of the analysis, the spreadsheet was reviewed along with the corresponding histology to validate the data and eliminate data entry errors.
To confirm the results obtained with TTC staining and resolve any ambiguous situations, hematoxylin and eosin stained sections were examined by light microscopy and correlated with the TTC images. The histology sections were from the center of the slice whereas the TTC images were from both the apical and basal surfaces of the slice, so there was not a perfect match, but the histology was used to guide the analysis. Completed spreadsheets with data for individual slices as well as total values were then uploaded to the CAESAR data site, without knowledge of the experimental protocol for individual hearts. All of the images generated by Photoshop were saved on the server so that any discrepancies between the Core analysis and analysis by the animal Centers could be investigated by comparison of the images, which was only done post-hoc.
Cardiac Troponin I (cTnI) release
For all species, blood was collected in heparinized tubes and centrifuged to separate plasma. For mice, a ~70 μL blood sample was obtained from the tail vein at 2 h of reperfusion because we have found that cTnI levels peak at this time in this model. Moreover, the 2-h values of cTnI correlate well with TTC-based measurements of infarct size. For rabbits, serial blood samples (~0.4 mL) were obtained from the ear vein at baseline and 1, 2, 4, 24, and 48 h of reperfusion. For pigs, serial blood samples (~1.0 mL) were obtained at baseline and 2, 4, 6, 24, and 48 h of reperfusion. Plasma cTnI levels were measured with species-specific cTnI ELISA kits according to the manufacturer’s instructions (Life Diagnostics, West Chester, PA). Each assay was performed in duplicate without knowledge of the treatment group. All of the final cTnI results (ng/mL) were submitted to the DCC via the CAESAR ShareFile site for statistical analysis.
Endpoints for each species
The primary endpoint for the murine model was infarct size (expressed as a fraction of the region at risk [Inf/RR]); the secondary endpoints were age (in weeks), body weight (g), heart rate (bpm) at each time point (baseline, 5 min, 15 min, and 30 min occlusion, and 5 min and 15 min reperfusion), body temperature (°C) at each time point (baseline, 5 min, 15 min, and 30 min occlusion, and, 5 min and 15 min reperfusion), heart weight (mg), LV weight (mg), region at risk (mg), infarct size (mg), region at risk per LV (RR/LV), infarct size per LV (Inf/LV), cTnl levels at baseline, and the cTnI plasma level at 2 h of reperfusion.
In rabbits, the primary endpoint was infarct size (expressed as a fraction of the region at risk [Inf/RR]); the secondary endpoints were age (in days), body weight (kg), heart rate (bpm) at each time point (baseline, 15 min, 30 min, and 45 min occlusion, and 15 min reperfusion), heart weight (g), LV weight (g), region at risk (g), infarct (g), region at risk (% of LV), infarct (% of LV), cTnI (ng/mL) at each time point (0 h, 1 h, 2 h, 4 h, 24 h, and 48 h since reperfusion), and the cumulative cTnI release (ng/mL).
For pigs, the primary endpoint was infarct size (expressed as a fraction of the region at risk [Inf/RR]); the secondary endpoints were age (in days), body weight (kg), number of defibrillations, heart rate (bpm) and mean arterial pressure (mmHg) at each time point (baseline, 15 min, and 45 min occlusion, and, 15 min and 72 h reperfusion), heart weight (g), LV weight (g), region at risk (g), infarct (g), region at risk (% of LV), infarct (% of LV), cTnI (ng/mL) at each time point (0 h, 2 h, 4 h, 6 h, 24 h, and 48 h of reperfusion), the cumulative cTnI release (ng/mL ), and RMBF at four time points (baseline, occlusion, and 15 min and 72 h of reperfusion).
Statistical analyses
This study was designed to determine whether IPC reduces infarct size in each of the three species (mouse, rabbit, and pig), and whether the results from two laboratories using the same animal protocol were reproducible. The sample size was calculated and decided a priori (i.e., before the start of the experiments) to detect a 20% (for mice and rabbits) or a 25% (for pigs) reduction in infarct size normalized to the region at risk (Inf/RR) in the IPC groups, with a power 80% and a two-sided 5% Type I error rate34 (e.g., if infarct size was 50% of the region at risk in control pigs, the sample size would enable us to detect a reduction of infarct size to 25% of the region at risk in the IPC group with a power of 80% and a two-sided 5% Type I error rate). The required number of animals was adjusted to account for 20% attrition due to mortality and exclusions based on the investigators’ recent experience.
The animals from each laboratory were assigned to IPC or control groups using a blocked randomization scheme to ensure that the assignment was randomized and balanced34. The Pathology and Biomarker Cores measured infarct size and cTnl levels, respectively, while being blinded to the treatment group. Animal characteristics (e.g., age, weight, heart rate, and body temperature) were measured in each animal at each Center, according to the protocol. The DCC’s statistician (Dr. Kong) analyzed the data for significant differences.
To examine whether IPC produced a significant infarct size reduction in each lab, and to examine whether the control groups in the two labs that used the same model were similar, two-way analysis of variance (ANOVA) with t-tests was used35. Secondary endpoints were also analyzed using two-way ANOVA in the same fashion as the primary endpoint. The summarized mean and standard deviations (SD) for each endpoint for each lab and each treatment group, along with the P values to test whether the IPC and control groups from each lab were significantly different, the P values for comparing the control groups from the two labs, as well as the P values for comparing the IPC groups from the two labs, are reported. A test was deemed significant if the P value was <0.05. The correlation between the histologic measurements of infarct size and cTnI levels was assessed by linear regression, Pearson’s correlation coefficients, and graphical data analyses.
RESULTS
Overview
The goal of this first phase of CAESAR was to initiate the operations of the Consortium, to address logistical issues, to refine the protocols, to validate the work of the Consortium by demonstrating its ability to detect the protective effects of IPC, and to verify the reproducibility of the work between Centers. We worked diligently to reconcile technical aspects of the surgical models between Centers and to optimize the sample and data flow through the various Cores. Using two methods to assess myocardial injury (TTC staining and cTnI release), our results, detailed below, show that IPC reduces infarct size in all three species, that its cardioprotective effects are reproducible between the two Centers for each species, and that the measurements of myocardial injury in each species and in each group are also reproducible between two Centers.
Preliminary studies
In the initial iterations of the mouse model, the data (not shown) indicated that infarct size reduction in each lab was, indeed, significant in the IPC group; however, secondary endpoints, such as age and body weight, were also significantly different between the two Centers. Although both Centers used the same models, there were subtle differences in interpretation and execution that became apparent and required reconciliation for subsequent studies. Accordingly, we engaged in multiple conference calls and site visits to identify differences in protocol performance. These efforts required scrupulous auditing of detailed laboratory practices, animal care facility procedures (e.g. specific diet used), coordination between the Centers, and the most Herculean task of all: having two laboratories strictly adhere to a continuously refined set of practices. The initial phase of CAESAR’s operation was precisely designed to address such minor procedural differences, and such a sweeping evaluation was also performed for the rabbit and pig models. Establishing consistent protocols, with attention to the minutest details, represented a major hurdle to reproducibility in the early stages of CAESAR, and was an issue for all three models (mouse, rabbit, and pig). The experience accumulated in these preliminary studies demonstrates not only how difficult it is for different laboratories to adhere to the same protocol but also how important even subtle differences in execution can be in shaping the final outcome.
To illustrate the types of differences encountered, we provide examples of changes that were made in the early phases of CAESAR. For example, in the initial pilot studies in the mouse model, the region at risk normalized to the left ventricle (RR/LV) was significantly larger at the Emory Center than at the UofL Center because of slightly different practices in reporting tissue weights. The Centers subsequently normalized the procedural differences to achieve similar RR/LV; however, other differences were ascribed to small, seemingly inconsequential differences at each site. For example, in the pilot studies body weights were slightly but significantly greater at Emory than at UofL, despite identical diets and despite using the same mouse vendor. Such differences can be accounted for (as is done in clinical trials); because there was no statistically attributable influence of body weight on infarct size in the present study, this particular issue was moot.
Results in the mouse model
Table 1 contains the combined data from the two Centers (after reconciliation of differences in the pilot stage) with respect to group assignment. Thirty-two mice were enrolled in the control group; seven were excluded. Twenty-five mice were enrolled in the IPC group; three were excluded. There were no significant differences between the control and IPC groups with respect to the endpoints of exclusions, age, body weight, or region at risk. At 5 min of occlusion, body temperature was significantly lower (~0.2°C) in the IPC group than in controls. Although such a difference is statistically significant, it is difficult to assign physiologic significance to such a transient, nominal difference; there were no other significant differences in body temperature. Heart rate was significantly lower at 5 and 15 min of occlusion, and at 15 min of reperfusion, in the IPC group; however, post hoc analysis to determine whether heart rate was associated with infarct size indicated that no such relationship existed in this study, thereby ruling out heart rate differences as an explanation for differences in infarct size. Most importantly, the primary endpoints of infarct size and cTnI release (Figure 3) were significantly reduced in the IPC group compared with controls. Thus, CAESAR was able to successfully demonstrate the cardioprotective effects of the most widely reproduced intervention for infarct size reduction.
TABLE 1.
Data from the CAESAR mouse model, combined Centers.
| Control Mean ± SD (n=25) |
IPC Mean ± SD (n=22) |
P value | |
|---|---|---|---|
| Exclusion | 7/32(=21.9%) | 3/25(=12%) | 0.5025 |
| Age (weeks) | 12.79±1.81 | 13.49±2.36 | 0.2692 |
| Body Weight at Infarction (g) | 42.47±4.01 | 41.97±3.73 | 0.6632 |
| HR (bpm) at pre-Occlusion | 556.32±58.75 | 527.86±61.35 | 0.1128 |
| HR (bpm) at 5’ Occlusion | 601.12±61.49 | 539.73±78.68 | 0.0053 |
| HR (bpm) at 15’ Occlusion | 593.28±59.91 | 548.68±79.78 | 0.0384 |
| HR (bpm) at 30’ Occlusion | 575.28±62.96 | 553.68±84.39 | 0.3315 |
| HR (bpm) at 5’ Reperfusion | 586.64±63.22 | 552.77±75.11 | 0.1045 |
| HR (bpm) at 15’ Reperfusion | 591.44±65.28 | 550.64±61.51 | 0.0326 |
| Body Temp (°C) at pre-Occlusion | 37.06±0.42 | 36.89±0.36 | 0.1348 |
| Body Temp (°C) at 5’ Occlusion | 37.19±0.34 | 36.97±0.31 | 0.0243 |
| Body Temp (°C) at 15’ Occlusion | 37.09±0.33 | 37.06±0.32 | 0.7294 |
| Body Temp (°C) at 30’ Occlusion | 37.14±0.37 | 37.07±0.31 | 0.4737 |
| Body Temp (°C) at 5’ Reperfusion | 37.04±0.35 | 37.01±0.29 | 0.7754 |
| Body Temp (°C) at 15’ Reperfusion | 37.02±0.39 | 37±0.28 | 0.8052 |
| Heart Weight (mg) | 196.48±18.82 | 182.77±24.58 | 0.0400 |
| LV Weight (mg) | 143.85±20.55 | 130.6±19.51 | 0.0283 |
| Region at Risk (mg) | 65.47±23.47 | 56.11±16.3 | 0.1163 |
| Infarct (mg) | 35.74±14.16 | 9.46±9.29 | <0.0001 |
| Region at Risk (% of LV) | 44.94±12.5 | 42.48±9.16 | 0.4411 |
| Infarct (% of RR) | 54.84±10.08 | 15.1±11.67 | <0.0001 |
| Infarct (% of LV) | 24.64±8.47 | 6.84±5.94 | <0.0001 |
| cTnI (ng/ml) at baseline | 0.21±0.34(17) | 0.55±1.12(14) | 0.2865 |
| cTnI (ng/ml) at | |||
| 2 h Reperfusion | 112.69±51.99 | 34.93±47.20 | <0.0001 |
Note: The number of observations for baseline cTnl measurements is 17 for control and 14 for IPC. IPC – ischemic preconditioning; HR – heart rate; LV – left ventricle/ventricular; RR – region at risk; cTnI – cardiac troponin I.
Figure 3. Ischemic preconditioning reduces infarct size in mice.
(A) Region at Risk (RR), Infarct (Inf), and Left Ventricular (LV) areas were determined with a dual-stain technique as described in the Methods. There was no difference in RR/LV between Control and Ischemic Preconditioning (IPC); however, IPC significantly reduced Inf/RR. (B) To determine cardiac damage with a different method, plasma levels of cardiac troponin I (cTnI) were determined with a mouse-specific commercial ELISA kit. IPC significantly reduced cTnI levels at 2 h of reperfusion in mice. Results are expressed as means ± SD *, p< 0.05.
Online Table I lists the summarized values for all of the assessed endpoints in mice; the data are segregated by Center. Overall, these data indicate internal consistency within each Center (i.e., few statistically significant differences between the two groups, within a Center). Moreover, the experiences of the two Centers were very similar, inasmuch as there were few differences between the two Centers. These data indicate that the Mouse Centers can collectively demonstrate infarct size reduction and consistently achieve similar results in an anesthetized mouse model of myocardial ischemia/reperfusion injury.
Results in the rabbit model
Table 2 contains the combined data from the two Centers with respect to group assignment in the rabbit model. Sixteen rabbits were enrolled in the control group; four were excluded. Thirteen rabbits were enrolled in the IPC group; two were excluded. There were no significant differences between the control and IPC groups with respect to number of exclusions, age, body weight, number of defibrillations, heart rate (at any time), or region at risk. Because the rabbit model is a conscious model of myocardial infarction, body temperature was not measured. Most importantly, the primary endpoints of infarct size and cTnI release (Figure 4) were significantly reduced in the IPC group compared with control. Thus, CAESAR was able to successfully demonstrate the infarct-sparing actions of the most widely reproduced intervention for infarct size reduction in a conscious rabbit model of myocardial ischemia/reperfusion injury.
TABLE 2.
Data from the CAESAR rabbit model, combined Centers.
| Control Mean ± SD (n=12) |
IPC Mean ± SD (n=11) |
P value | |
|---|---|---|---|
| Exclusions | 4/16(=25%) | 2/13(=15.4) | 0.6804 |
| Number of defibrillations | 0.5±1.17 | 0±0 | 0.1661 |
| Age (days) | 94.08±1.24 | 93.82±1.4 | 0.6372 |
| Body weight (kg) | 2.23±0.27 | 2.15±0.19 | 0.4758 |
| HR (bpm) at Baseline | 224.75±22.34 | 244.64±30.94 | 0.0965 |
| HR (bpm) at 15’ Occlusion | 250.75±22.16 | 253.18±26.44 | 0.8144 |
| HR (bpm) at 30’ Occlusion | 242.17±23.78 | 251±33.16 | 0.4758 |
| HR (bpm) at 45’ Occlusion | 237.92±26.14 | 246.64±24.11 | 0.4146 |
| HR (bpm) at 15’ Rep. | 229.75±27.53 | 243.27±28.1 | 0.2576 |
| Heart Weight (g) | 7.41±1 | 7.47±0.76 | 0.8708 |
| LV Weight (g) | 3.42±0.41 | 3.19±0.4 | 0.1965 |
| Region at Risk (g) | 1.07±0.29 | 1.04±0.18 | 0.7595 |
| Infarct (g) | 0.49±0.15 | 0.24±0.08 | 0.0001 |
| Region at Risk (% of LV) | 31.01±5.96 | 32.56±3.7 | 0.4592 |
| Infarct (% of RR) | 45.54±8.09 | 23.45±7.75 | <0.0001 |
| Infarct (% of LV) | 14.11±3.46 | 7.51±2.28 | <0.0001 |
| cTnI (ng/ml) at baseline | 0±0 | 0.02±0.06 | 0.2946 |
| cTnI (ng/ml) at 1h Rep. | 84.96±73.31 | 21.06±26 | 0.0134 |
| cTnI (ng/ml) at 2h Rep. | 112.69±71.61 | 36.43±33.57 | 0.0044 |
| cTnI (ng/ml) at 4h Rep. | 88.63±54.89 | 31.14±16.6 | 0.0042 |
| cTnI (ng/ml) at 24h Rep. | 5.62±5.88 | 3.06±3.79 | 0.2267 |
| cTnI (ng/ml) at 48h Rep. | 0.64±1.04 | 0.72±2.05 | 0.9132 |
| cTnI (ng/ml) cumulative | 1360.22±787.08 | 493.24±251.33 | 0.0030 |
IPC – ischemic preconditioning; HR – heart rate; LV – left ventricle/ventricular; RR – region at risk; cTnI – cardiac troponin I.
Figure 4. Ischemic preconditioning reduces infarct size in rabbits.
(A) Region at Risk (RR), Infarct (Inf), and Left Ventricular (LV) areas were determined with a dual-stain technique as described in the Methods. There was no difference in RR/LV between Control and Ischemic Preconditioning (IPC); however, IPC significantly reduced Inf/RR. (B) To determine cardiac damage with a different method, plasma levels of cardiac troponin I (cTnI) were determined with a rabbit-specific commercial ELISA kit. IPC significantly reduced cTnI levels early during reperfusion. (C) IPC also significantly reduced total cTnI release, as estimated by the area under the curve. Results are expressed as means ± SD *, p< 0.05.
It is important to note that – similar to our experience in the mouse model – the pilot studies (not shown) in the rabbit model indicated that IPC significantly reduced infarct size in each Center; however, several secondary endpoints, such as age and body weight, were also significantly different between the two Centers. To achieve reproducibility between the two Centers, UofL and VCU scrupulously evaluated their detailed protocols and identified several small differences. The result of this audit led the two Centers to switch to the same vendor for the rabbits and better coordinate the age by synchronizing their Centers’ animal orders. Because the rabbit model involved survival surgery (for the instrumentation) there were also small differences in the type and route of analgesia that, although unlikely to contribute to Center differences, were reconciled through cooperation of the Centers’ respective IACUCs. Once the Center differences were addressed, the models were used for the IPC study and the data are reported in Table 2 and Online Table II.
Online Table II lists the summarized values for all of the assessed endpoints in rabbits; the data are segregated by Center. Overall, these data indicate internal consistency within each Center (i.e., few statistically significant differences between the two groups, within a Center). Moreover, the experiences of the two Centers were very similar, inasmuch as there were few differences between the Centers. These data indicate that the rabbit Centers can demonstrate the infarct-sparing effect of IPC and consistently achieve similar results.
Results in the pig model
Table 3 contains the combined data from the two Centers with respect to group assignment in the pig model. Fifteen pigs were enrolled in the control group; two were excluded. Nineteen pigs were enrolled in the IPC group; six were excluded. There were no significant differences between the control and IPC groups with respect to number of exclusions, age, body weight, number of defibrillations, heart rate (at any time), mean arterial pressure, region at risk, or regional myocardial blood flow. Most importantly, the primary endpoints of infarct size and cTnI release (Figure 5) were significantly reduced in the IPC group compared with control. Thus, CAESAR was able to successfully demonstrate the cardioprotective effects of the most widely reproduced intervention for infarct size reduction in the pig model of myocardial ischemia/reperfusion injury.
TABLE 3.
Data from the CAESAR pig model, combined Centers.
| Control Mean±SD (13) |
IPC Mean±SD (13) |
P value | ||
|---|---|---|---|---|
| Exclusions (# excluded/# assigned) | 2/15(=13%) | 6/19(=31%) | 0.4388 | |
| Age (days) | 92.54±7.86(13) | 96.31±8.44(13) | 0.2502 | |
| Body Weight (kg) | 34.83±3.48(13) | 35.16±3.68(13) | 0.8157 | |
| Number of defibrillations | 3.38±4.23(13) | 3.54±2.57(13) | 0.9119 | |
|
Heart Rate
(beats per minute) |
Baseline | 114.08±34.7(13) | 103.31±26.86(13) | 0.3856 |
| 15’ Occlusion | 99.54±16.78(13) | 91.23±24.25(13) | 0.3211 | |
| 45’ Occlusion | 97.08±18.17(13) | 90.77±23.18(13) | 0.448 | |
| 15’ Reperfusion | 100.31±28.72(13) | 92.92±25.49(13) | 0.4948 | |
| 72 h Reperfusion | 100.92±19.04(13) | 100.85±15.12(13) | 0.991 | |
|
Mean Arterial
Pressure (mmHg) |
Baseline | 102.92±14.94(13) | 107.31±15.35(13) | 0.4676 |
| 15’ Occlusion | 91±18.33(13) | 89.23±14.77(13) | 0.7888 | |
| 45’ Occlusion | 86.62±22.75(13) | 79.15±15.53(13) | 0.3398 | |
| 15’ Reperfusion | 83.62±17.83(13) | 80.92±14.3(13) | 0.6749 | |
| 72 h Reperfusion | 77.77±14.14(13) | 83.23±18.36(13) | 0.4044 | |
| Heart Weight (g) | 242.11±21.58(13) | 244.02±18.46(13) | 0.8107 | |
| LV Weight (g) | 118.16±21.27(13) | 117.86±19.64(13) | 0.971 | |
| Region at Risk (g) | 35.67±8.37(13) | 35.77±7.31(13) | 0.9734 | |
| Infarct (g) | 20.38±6.53(13) | 13.63±8.13(13) | 0.0287 | |
| Region at Risk (% of LV) | 30.25±5.41(13) | 30.27±4.02(13) | 0.9912 | |
| Infarct (% of Region at Risk) | 57.68±14.43(13) | 37.34±19.43(13) | 0.0061 | |
| Infarct (% of LV) | 17.29±5.05(13) | 11.32±6.1(13) | 0.0121 | |
| cTnI (ng/ml) | Baseline | 0.01±0.03(13) | 0.02±0.03(13) | 0.6864 |
| 2 h Reperfusion | 102.17±52.82(13) | 44.38±32.58(13) | 0.0031 | |
| 4 h Reperfusion | 107.88±35.87(13) | 55.69±38.32(13) | 0.0015 | |
| 6 h Reperfusion | 96.63±42.2(13) | 62.02±36.51(13) | 0.0351 | |
| 24 h Reperfusion | 34.72±19.67(13) | 25.06±15.32(13) | 0.1758 | |
| 48 h Reperfusion | 9.64±7.56(13) | 5.3±4.32(13) | 0.0879 | |
| Cumulative | 2230.7±902.47(13) | 1409.36±825.09(13) | 0.0234 | |
| Baseline | NIZ Endo RMBF (nl/min/g) | 0.77±0.3(13) | 0.86±0.32(13) | 0.4878 |
| NIZ Epi RMBF (nl/min/g) | 0.69±0.25(13) | 0.7±0.31(13) | 0.9621 | |
| NIZ Endo/Epi Ratio | 1.12±0.19(13) | 1.28±0.23(13) | 0.0708 | |
| RR Endo RMBF (nl/min/g) | 0.71±0.27(13) | 0.75±0.38(13) | 0.7448 | |
| RR Epi RMBF (nl/min/g) | 0.51±0.28(13) | 0.51±0.24(13) | 0.9384 | |
| RR Endo/Epi Ratio | 1.5±0.35(13) | 1.48±0.36(13) | 0.8491 | |
| BDZ Endo RMBF (nl/min/g) | 0.75±0.31(13) | 0.84±0.41(13) | 0.538 | |
|
BDZ Epi RMBF
(nl/min/g) |
0.77±0.28(13) | 0.76±0.27(13) | 0.9141 | |
| BDZ Endo/Epi Ratio | 0.99±0.17(13) | 1.12±0.42(13) | 0.3052 | |
| Occlusion |
NIZ Endo RMBF
(nl/min/g) |
0.92±0.66(13) | 0.76±0.26(13) | 0.4253 |
| NIZ Epi RMBF (nl/min/g) | 0.86±0.61(13) | 0.67±0.28(13) | 0.3405 | |
| NIZ Endo/Epi Ratio | 1.09±0.3(13) | 1.19±0.34(13) | 0.4413 | |
| RR Endo RMBF (nl/min/g) | 0±0.01(13) | 0±0.01(13) | 0.9576 | |
| RR Epi RMBF (nl/min/g) | 0.01±0.03(13) | 0±0.01(13) | 0.4459 | |
| RR Endo/Epi Ratio | 0±0(13) | 0.09±0.32(13) | 0.3370 | |
|
BDZ Endo RMBF
(nl/min/g) |
0.62±0.69(13) | 0.56±0.36(13) | 0.8142 | |
| BDZ Epi RMBF (nl/min/g) | 0.72±0.88(13) | 0.52±0.37(13) | 0.4713 | |
| BDZ Endo/Epi Ratio | 1.14±0.75(13) | 1.76±2(13) | 0.3085 | |
| 15' Rep |
NIZ Endo RMBF
(nl/min/g) |
0.79±0.55(13) | 1.08±1.61(13) | 0.5428 |
| NIZ Epi RMBF (nl/min/g) | 0.71±0.54(13) | 0.9±1.22(13) | 0.6274 | |
| NIZ Endo/Epi Ratio | 1.17±0.25(13) | 1.2±0.23(13) | 0.7275 | |
| RR Endo RMBF (nl/min/g) | 1.48±1.26(13) | 2.03±1.59(13) | 0.3425 | |
| RR Epi RMBF (nl/min/g) | 1.5±1.01(13) | 2.05±2.2(13) | 0.4234 | |
| RR Endo/Epi Ratio | 0.94±0.41(13) | 1.07±0.42(13) | 0.4167 | |
|
BDZ Endo RMBF
(nl/min/g) |
1.59±1.23(13) | 2.15±2.9(13) | 0.5307 | |
| BDZ Epi RMBF (nl/min/g) | 1.36±0.95(13) | 1.44±1.25(13) | 0.8596 | |
| BDZ Endo/Epi Ratio | 1.24±0.36(13) | 1.4±0.71(13) | 0.4685 | |
| 72h Rep | NIZ Endo RMBF (nl/min/g) | 0.72±0.42(13) | 0.71±0.43(13) | 0.9871 |
| NIZ Epi RMBF (nl/min/g) | 1.11±1.78(13) | 0.67±0.87(13) | 0.4307 | |
| NIZ Endo/Epi Ratio | 1.14±0.87(13) | 4.97±11.92(13) | 0.2697 | |
| RR Endo RMBF (nl/min/g) | 0.93±1.76(13) | 0.86±1.42(13) | 0.9110 | |
| RR Epi RMBF (nl/min/g) | 1.29±2.87(13) | 0.72±1.43(13) | 0.5278 | |
| RR Endo/Epi Ratio | 0.73±0.7(13) | 9.04±29.66(13) | 0.3324 | |
|
BDZ Endo RMBF
(nl/min/g) |
1.05±1.32(13) | 0.98±0.91(13) | 0.8785 | |
| BDZ Epi RMBF (nl/min/g) | 1.54±3.27(13) | 1.03±1.31(13) | 0.6135 | |
| BDZ Endo/Epi Ratio | 1.16±0.77(13) | 1.29±1.43(13) | 0.7750 | |
IPC – ischemic preconditioning; LV – left ventricle/ventricular; cTnI – cardiac troponin I; NIZ – nonischemic zone; BDZ – border zone; RR – region at risk; RMBF – regional myocardial blood flow; Endo – endocardial; Epi – epicardial.
Figure 5. Ischemic preconditioning reduces infarct size in pigs.
(A) Region at Risk (RR), Infarct (Inf), and Left Ventricular (LV) areas were determined with a dual-stain technique as described in the Methods. There was no difference in RR/LV between Control and Ischemic Preconditioning (IPC); however, IPC significantly reduced Inf/RR. (B) To determine cardiac damage with a different method, plasma levels of cardiac troponin I (cTnI) were determined with a pig-specific commercial ELISA kit. IPC significantly reduced cTnI levels early during reperfusion. (C) IPC also significantly reduced total cTnI release, as estimated by the area under the curve. Results are expressed as means ± SD *, p< 0.05.
Online Table III lists the summarized values for all of the assessed endpoints in pigs; the data are segregated by Center. Overall, these data indicate internal consistency within each Center (i.e. few statistically significant differences between the two groups, within a Center). Moreover, the experiences of the two Centers were highly similar, inasmuch as there were few differences between the Centers. These data indicate that the pig Centers can demonstrate the infarct-sparing effect of IPC and consistently achieve similar results.
DISCUSSION
The purpose of CAESAR is to verify, in a rigorous manner, the efficacy of promising therapies for which there is already some evidence of efficacy. This work is essential if we hope to break the abysmal chain of failures in translating preclinical “successes” into clinical practice5. CAESAR represents a major paradigm shift and an innovative effort to achieve such a goal. It is predicated on the premises that i) the translational failures that have plagued the field of cardioprotection for the past 40 years have been caused by the fact that most “positive” preclinical studies have not been reproducible and have not been conducted with sufficient rigor, and ii) therapies that prove reproducibly effective in rigorous preclinical models will be more likely to be effective in patients. In our view, the role of CAESAR is not only to identify promising (i.e. successful) infarct-sparing agents, but, equally importantly, to identify cardioprotective agents that are not likely to be efficacious in humans, thereby sparing millions of dollars and years of work involved in performing clinical trials of agents that do not withstand CAESAR’s test.
However, before CAESAR can be used to evaluate potentially cardioprotective therapies, it is necessary to demonstrate that it is able to detect infarct size limitation. This “positive control” is essential to validate any “negative” results that may be encountered in subsequent studies. IPC was selected as the initial test for CAESAR because it is the most reproducible infarct-sparing intervention discovered thus far. The first phase of CAESAR provided opportunities for protocol refinement, demonstrated the ability of the consortium to detect an effective intervention, and introduced the CAESAR investigators to the concept of multi-center integration of a single study - a common concept among clinician-investigators but a revolutionary concept for laboratory scientists.
These objectives were successfully achieved. The collective results of CAESAR’s six surgical Centers indicate that IPC significantly reduced infarct size in all three animal models used; furthermore, the reduction in infarct size by IPC, as well as the values of infarct size in the control and IPC groups, were consistent between the two Centers involved in each model. Thus, the infrastructure that we have developed i) can operate effectively in accordance with our goals of investigative rigor and reproducibility between Centers, and ii) can demonstrate cardioprotection. These findings are of paramount importance because they lay the necessary foundation for subsequent studies of novel infarct-sparing therapies by CAESAR.
Of even greater importance, however, is the fact that the present work provides information that could potentially transform basic research on cardioprotection: that is, a detailed description of state-of-the-art methodology that any laboratory, even outside of a network, can adopt to measure infarct size in a manner that is rigorous, accurate, and reproducible. In addition to the principles of randomization, investigator blinding, a priori sample size determination and exclusion criteria, and appropriate statistical analyses embodied by CAESAR, we provide herein detailed protocols (“CAESAR protocols”) for three major models of ischemia-reperfusion injury: the mouse, rabbit, and pig models. Each of these protocols is supported by a large body of previous work22, 31, 36-38 and is the result of extensive discussions among Centers and validation studies in the preliminary phase of CAESAR. Our results demonstrate that adherence to these protocols results in accurate and reproducible measurements of infarct size. Accordingly, these detailed protocols should be useful to other investigators who are engaged in studies of infarct size reduction.
The establishment of a multi-center, multi-species, preclinical consortium for the rigorous assessment of cardioprotective interventions described herein is truly a revolutionary approach, as this has never been done before in studies of infarct size limitation. Others have used various protocols at three sites in a single species to evaluate a single therapy39. CAESAR is unique also because it constitutes a public resource. The enormity of our goal required a relatively large operational structure (Figure 1), which we envisioned as operating in a manner analogous to a multicenter clinical trial. Investigators at four Universities (currently: UofL, LSUHSC-New Orleans [previously Emory University], Johns Hopkins University, and Virginia Commonwealth University) constitute the Consortium Operations Committee (COC), which functions as a Steering/Executive Committee for CAESAR. In addition, most of the COC members lead Surgical Centers and Cores and thus work on protocol implementation (Figure 1). Because the NIH U24 funding mechanism (which supports CAESAR) represents a bona fide partnership between the NHLBI and the CAESAR investigators, there are two non-voting members of the COC who represent the NHLBI (currently: Dr. Frank Evans and Ms. Lynn Rundhaugen; previously: Dr. Isabella Liang). These scientists provide important feedback from the NHLBI, make suggestions on protocol refinement, and participate in COC conference calls, which occur on a monthly basis or more frequently should specific issues arise.
Whereas a clinical trial has a Data Safety Monitoring Board, CAESAR has a Protocol Review and Monitoring Committee (PRMC), which consists of a non-voting Executive Secretary and four voting members (Figure 1). During CAESAR’s development, the PRMC provided critical feedback that improved the protocols at the Centers and Cores. Now that CAESAR has been validated and is actively testing candidate agents (see www.nihcaesar.org for current status), the PRMC’s primary functions are to approve the use of putative cardioprotective agents proposed by scientists and private companies outside of the COC and to evaluate the blinded data to determine whether ongoing studies should stop or continue. Throughout this process, the two members of the DCC (currently: Drs. Jones and Kong) are the only CAESAR investigators who have access to group assignments and information concerning summary data. All other CAESAR investigators will have seen, at most, only aggregate data (all groups combined into one group), which provide only general insight into overall protocol adherence (based on animal age, body weight, or other biometric parameters). Thus, the investigators generating the raw data (or data derived therefrom) are truly blinded throughout the entire process. Once reported to the PRMC, a study continues (if the report was an interim analysis) or is closed. When a study is closed and the minutes of the PRMC conference call are made official by the NHLBI, then, and only then, are the CAESAR investigators (including the overall PI, Dr. Bolli) notified of the results. The next step is for the CAESAR investigators to disseminate the findings via abstract presentations at conferences and via full-length, peer-reviewed manuscripts.
We found the development of CAESAR to be challenging. Converting several fiercely independent investigators into a cooperative and uniform team has rarely been achieved in basic research. In addition, achieving harmony among multiple Institutional Animal Care and Use Committees for the implementation of consistent protocols is difficult and time-intensive. Furthermore, such a multi-institutional and complex infrastructure requires a much higher administrative obligation, as illustrated in Figure 1. The challenge of developing CAESAR was not less than that inherent in launching a new multicenter clinical trial. Nevertheless, we believe that such an infrastructure is indispensable to move the field of cardioprotection forward beyond its current impasse.
Although in this study of IPC all Centers and Cores (with the exception of the DCC) were blinded to the results until the study was completed, IPC makes it virtually impossible to blind investigators to group allocation. In subsequent CAESAR studies40, 41, the most critical difference has been that neither the individuals performing the experiments nor those conducting subsequent analyses have had any knowledge of group assignment. Therefore, there are two differences between this IPC study and all subsequent CAESAR studies. First, although in the present study the pathologist was blinded to group assignment, the surgeons were not blinded to group assignment. Second, IPC is, by definition, an antecedent intervention; in subsequent studies, drugs tested in CAESAR have not been administered until late in ischemia. Such a design is motivated by our desire to bolster the translational potential of CAESAR’s efforts.
An important result of this study is that, in the absence of any intervention, results varied if the diet, age (size), or source of the animal was different. This finding has major implications for studies of infarct size because it reveals heretofore unappreciated confounding variables that may affect the outcome of many investigations published in the literature, thereby requiring rigorous standardization of these conditions in future studies. This finding emphasizes the importance of using a structure like CAESAR to achieve reproducible results and the importance of paying attention to even minute differences in operations among different laboratories.
Despite CAESAR’s unique strengths in terms of protocol rigor, statistical power, blinding, and randomization, certain limitations should be mentioned. Like in nearly all preclinical studies of infarct size reduction, CAESAR animals are young and healthy and, thus, devoid of risk factors. This aspect of CAESAR (and most preclinical studies of infarct size limitation to date) creates the possibility that some interventions may succeed in CAESAR, yet fail in humans because of the high prevalence of risk factors, comorbidities, and/or concurrent polypharmacy. Future iterations of CAESAR should include appropriate risk factors in the models13, 14. In addition, the ability of CAESAR to predict what will eventually succeed or fail in patients has no positive control. That is, because no drug is approved to limit infarct size in patients, there is nothing we can test in CAESAR to demonstrate that CAESAR can predict drugs that will be effective in patients (indeed, the very reason for CAESAR’s existence is that there is no intervention that has been accepted to limit infarct size in humans).
In summary, infarct size limitation remains a major unmet medical need. Despite enormous investments by NIH and private entities, and despite innumerable preclinical and clinical attempts, to date there is no drug that can be used in patients to limit infarct size. This colossal failure can be ascribed mainly to methodological limitations, i.e., to insufficient rigor in the studies conducted heretofore. CAESAR is a novel, revolutionary paradigm shift in cardioprotection, which provides a mechanism for assessing cardioprotective interventions with a level of rigor analogous to that used in multicenter randomized clinical trials. The present study demonstrates that CAESAR is operational, generates reproducible results, and can identify cardioprotection. Our experience also offers insights into how to build an effective and coordinated preclinical network. Most importantly, we provide here the “CAESAR protocols” – state-of-the-art, detailed protocols for measuring infarct size in mice, rabbits, and pigs in a manner that is rigorous, accurate, and reproducible; other investigators can adopt these protocols in their own laboratories. CAESAR is a public resource available to external investigators (www.nihcaesar.org). The CAESAR investigators look forward to continued engagement with the cardiovascular community in terms of proposal submissions and constructive feedback.
Novelty and Significance.
What Is Known?
After decades of effort and investment of hundreds of millions of dollars, there are still no drugs generally accepted to reduce infarct size in patients.
Many candidate drugs have arisen from preclinical studies of varying quality and reproducibility.
Because most laboratory studies of myocardial ischemia-reperfusion injury are not standardized and often poorly controlled, we need to develop a better approach to identify infarct-sparing drugs.
What New Knowledge Does This Article Contribute?
This study provides detailed experimental protocols for the rigorous assessment of myocardial infarct size in three commonly used laboratory species.
This article provides a roadmap for applying a clinical trial-like approach to preclinical studies of infarct-sparing drugs, which could easily be adopted in other areas of cardiovascular research.
Despite four decades of intense effort and substantial financial investment, the cardioprotection field has failed to deliver a single drug that is generally accepted to reduce myocardial infarct size in patients. The purpose of CAESAR is to verify, in a rigorous manner, the efficacy of promising therapies. This work is essential if we hope to break the abysmal chain of failures in translating preclinical “successes” into clinical practice. Our goal was to develop a multicenter randomized controlled trial (RCT)-like infrastructure to conduct rigorous and reproducible preclinical evaluation of cardioprotective therapies. CAESAR represents a major paradigm shift and an innovative effort to achieve such a goal. It is predicated on the premises that i) the translational failures that have plagued the field of cardioprotection for the past 40 years have been caused by the fact that most “positive” preclinical studies have not been reproducible and have not been conducted with sufficient rigor, and ii) therapies that prove reproducibly effective in rigorous preclinical models will be more likely to be effective in patients. CAESAR is operational, generates reproducible results, can detect cardioprotection, and provides a mechanism for assessing potential infarct-sparing therapies with a level of rigor analogous to multicenter RCTs.
ACKNOWLEDGEMENTS
The CAESAR investigators wish to acknowledge the dedication of various current/former NHLBI staff members, including Dr. Isabella Liang, Dr. Frank Evans, and Ms. Lynn Rundhaugen, and the current/former Protocol Review and Monitoring Committee members (Drs. Lisa Schwartz Longacre, Executive Secretary, John Canty, Chair, Karen Pzyklenk, James Sheets, Richard Vander Heide, and John Auchampach). We also acknowledge Ms. Sharon Nolan, Ms. Deanna Husted, and Ms. Mary Beth Oliver for administrative support with the multiple conference calls, website changes, and data site maintenance, Mr. Michael Book’s technical assistance and Ms. Angelica DeMartino’s preparation of the figures.
SOURCES OF FUNDING
This work was supported by a contract from the NIH (U24 HL094373), which was supplemented with divisional/departmental/center funds at the respective locations.
Nonstandard Abbreviations and Acronyms
- ANP
atrial natriuretic peptide
- BDZ
border zone
- CAESAR
Consoritum for preclinicAl assESsment of cARdioprotective therapies
- cTnI
cardiac troponin I
- COC
consortium operations committee
- DCC
data coordinating center
- Endo
endocardial
- Epi
epicardial
- HR
heart rate
- ICR
Institute of Cancer Research
- Inf
infarct
- IPC
ischemic preconditioning
- LAD
left anterior descending coronary artery
- LV
left ventricle or left ventricular
- NIZ
nonischemic zone
- PRMC
protocol review monitoring committee
- RCT
randomized controlled trial
- RMBF
regional myocardial blood flow
- RR
region at risk
- RV
right ventricle or right ventricular
- TTC
2,3,5-triphenyltetrazolium chloride
- UofL
University of Louisville
- VCU
Virginia Commonwealth University
- VF
ventricular fibrillation
Footnotes
DISCLOSURES
The authors have nothing to disclose.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart Disease and Stroke Statistics—2012 Update: A Report From the American Heart Association. Circulation. 2011 doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–72. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 3.St John Sutton M, Pfeffer MA, Moye L, Plappert T, Rouleau JL, Lamas G, Rouleau J, Parker JO, Arnold MO, Sussex B, Braunwald E. Cardiovascular death and left ventricular remodeling two years after myocardial infarction: baseline predictors and impact of long-term use of captopril: information from the Survival and Ventricular Enlargement (SAVE) trial. Circulation. 1997;96:3294–9. doi: 10.1161/01.cir.96.10.3294. [DOI] [PubMed] [Google Scholar]
- 4.Gibbons RJ, Valeti US, Araoz PA, Jaffe AS. The quantification of infarct size. J Am Coll Cardiol. 2004;44:1533–42. doi: 10.1016/j.jacc.2004.06.071. [DOI] [PubMed] [Google Scholar]
- 5.Bolli R, Becker L, Gross G, Mentzer R, Jr., Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy (NHLBI Working Group on the Translation of Therapies for Protecting the Heart from Ischemia) Circulation research. 2004;95:125–34. doi: 10.1161/01.RES.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- 6.Ibanez B, Macaya C, Sanchez-Brunete V, Pizarro G, Fernandez-Friera L, Mateos A, Fernandez-Ortiz A, Garcia-Ruiz JM, Garcia-Alvarez A, Iniguez A, Jimenez-Borreguero J, Lopez-Romero P, Fernandez-Jimenez R, Goicolea J, Ruiz-Mateos B, Bastante T, Arias M, Iglesias-Vazquez JA, Rodriguez MD, Escalera N, Acebal C, Cabrera JA, Valenciano J, Perez de Prado A, Fernandez-Campos MJ, Casado I, Garcia-Rubira JC, Garcia-Prieto J, Sanz-Rosa D, Cuellas C, Hernandez-Antolin R, Albarran A, Fernandez-Vazquez F, de la Torre-Hernandez JM, Pocock S, Sanz G, Fuster V. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: the Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation. 2013;128:1495–503. doi: 10.1161/CIRCULATIONAHA.113.003653. [DOI] [PubMed] [Google Scholar]
- 7.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. The New England journal of medicine. 2008;359:473–81. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 8.Mewton N, Croisille P, Gahide G, Rioufol G, Bonnefoy E, Sanchez I, Cung TT, Sportouch C, Angoulvant D, Finet G, Andre-Fouet X, Derumeaux G, Piot C, Vernhet H, Revel D, Ovize M. Effect of cyclosporine on left ventricular remodeling after reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:1200–5. doi: 10.1016/j.jacc.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 9.Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, Seguchi O, Myoishi M, Minamino T, Ohara T, Nagai Y, Nanto S, Watanabe K, Fukuzawa S, Hirayama A, Nakamura N, Kimura K, Fujii K, Ishihara M, Saito Y, Tomoike H, Kitamura S. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. The Lancet. 370:1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- 10.Woo JS, Kim W, Ha SJ, Kim JB, Kim S-J, Kim W-S, Seon HJ, Kim KS. Cardioprotective Effects of Exenatide in Patients With ST-Segment–Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: Results of Exenatide Myocardial Protection in Revascularization Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33:2252–2260. doi: 10.1161/ATVBAHA.113.301586. [DOI] [PubMed] [Google Scholar]
- 11.Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. The Lancet. 2013;381:166–175. doi: 10.1016/S0140-6736(12)60916-7. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz Longacre L, Kloner RA, Arai AE, Baines CP, Bolli R, Braunwald E, Downey J, Gibbons RJ, Gottlieb RA, Heusch G, Jennings RB, Lefer DJ, Mentzer RM, Murphy E, Ovize M, Ping P, Przyklenk K, Sack MN, Vander Heide RS, Vinten-Johansen J, Yellon DM, National Heart L. Blood Institute NIoH New horizons in cardioprotection: recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation. 2011;124:1172–9. doi: 10.1161/CIRCULATIONAHA.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacological reviews. 2014;66:1142–74. doi: 10.1124/pr.113.008300. [DOI] [PubMed] [Google Scholar]
- 14.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacological reviews. 2007;59:418–58. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 15.Sluijter JP, Condorelli G, Davidson SM, Engel FB, Ferdinandy P, Hausenloy DJ, Lecour S, Madonna R, Ovize M, Ruiz-Meana M, Schulz R, Van Laake LW, Nucleus of the European Society of Cardiology Working Group Cellular Biology of the H Novel therapeutic strategies for cardioprotection. Pharmacology & therapeutics. 2014;144:60–70. doi: 10.1016/j.pharmthera.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, Heusch G, Vinten-Johansen J, Yellon DM, Schulz R, Working Group of Cellular Biology of Heart of European Society of C Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovascular research. 2010;87:406–23. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- 17.Lecour S, Botker HE, Condorelli G, Davidson SM, Garcia-Dorado D, Engel FB, Ferdinandy P, Heusch G, Madonna R, Ovize M, Ruiz-Meana M, Schulz R, Sluijter JP, Van Laake LW, Yellon DM, Hausenloy DJ. ESC Working Group Cellular Biology of the Heart: Position Paper: improving the preclinical assessment of novel cardioprotective therapies. Cardiovascular research. 2014 doi: 10.1093/cvr/cvu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hausenloy DJ, Erik Botker H, Condorelli G, Ferdinandy P, Garcia-Dorado D, Heusch G, Lecour S, van Laake LW, Madonna R, Ruiz-Meana M, Schulz R, Sluijter JP, Yellon DM, Ovize M. Translating cardioprotection for patient benefit: position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovascular research. 2013;98:7–27. doi: 10.1093/cvr/cvt004. [DOI] [PubMed] [Google Scholar]
- 19.Lefer DJ, Bolli R. Development of an NIH consortium for preclinicAl AssESsment of CARdioprotective therapies (CAESAR): a paradigm shift in studies of infarct size limitation. Journal of cardiovascular pharmacology and therapeutics. 2011;16:332–9. doi: 10.1177/1074248411414155. [DOI] [PubMed] [Google Scholar]
- 20.Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014;505:612–3. doi: 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. The American journal of physiology. 1998;275:H1375–87. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong KU, Li QH, Guo Y, Patton NS, Moktar A, Bhatnagar A, Bolli R. A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic research in cardiology. 2013;108:346. doi: 10.1007/s00395-013-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Y, Tukaye DN, Wu WJ, Zhu X, Book M, Tan W, Jones SP, Rokosh G, Narumiya S, Li Q, Bolli R. The COX-2/PGI2 receptor axis plays an obligatory role in mediating the cardioprotection conferred by the late phase of ischemic preconditioning. PloS one. 2012;7:e41178. doi: 10.1371/journal.pone.0041178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Y, Sanganalmath SK, Wu W, Zhu X, Huang Y, Tan W, Ildstad ST, Li Q, Bolli R. Identification of inducible nitric oxide synthase in peripheral blood cells as a mediator of myocardial ischemia/reperfusion injury. Basic research in cardiology. 2012;107:253. doi: 10.1007/s00395-012-0253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Y, Flaherty MP, Wu WJ, Tan W, Zhu X, Li Q, Bolli R. Genetic background, gender, age, body temperature, and arterial blood pH have a major impact on myocardial infarct size in the mouse and need to be carefully measured and/or taken into account: results of a comprehensive analysis of determinants of infarct size in 1,074 mice. Basic research in cardiology. 2012;107:288. doi: 10.1007/s00395-012-0288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Guo Y, Tan W, Ou Q, Wu WJ, Sturza D, Dawn B, Hunt G, Cui C, Bolli R. Cardioprotection afforded by inducible nitric oxide synthase gene therapy is mediated by cyclooxygenase-2 via a nuclear factor-kappaB dependent pathway. Circulation. 2007;116:1577–84. doi: 10.1161/CIRCULATIONAHA.107.689810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q, Guo Y, Tan W, Stein AB, Dawn B, Wu WJ, Zhu X, Lu X, Xu X, Siddiqui T, Tiwari S, Bolli R. Gene therapy with iNOS provides long-term protection against myocardial infarction without adverse functional consequences. Am J Physiol Heart Circ Physiol. 2006;290:H584–9. doi: 10.1152/ajpheart.00855.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Y, Stein AB, Wu WJ, Zhu X, Tan W, Li Q, Bolli R. Late preconditioning induced by NO donors, adenosine A1 receptor agonists, and delta1-opioid receptor agonists is mediated by iNOS. Am J Physiol Heart Circ Physiol. 2005;289:H2251–7. doi: 10.1152/ajpheart.00341.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo Y, Stein AB, Wu WJ, Tan W, Zhu X, Li QH, Dawn B, Motterlini R, Bolli R. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H1649–53. doi: 10.1152/ajpheart.00971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Y, Bao W, Wu WJ, Shinmura K, Tang XL, Bolli R. Evidence for an essential role of cyclooxygenase-2 as a mediator of the late phase of ischemic preconditioning in mice. Basic Res Cardiol. 2000;95:479–84. doi: 10.1007/s003950070024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:11507–12. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reinhardt CP, Dalhberg S, Tries MA, Marcel R, Leppo JA. Stable labeled microspheres to measure perfusion: validation of a neutron activation assay technique. American journal of physiology Heart and circulatory physiology. 2001;280:H108–16. doi: 10.1152/ajpheart.2001.280.1.H108. [DOI] [PubMed] [Google Scholar]
- 34.Friedman J, Furberg C, DeMets D. Fundamentals of Clinical Trials. Springer; New York: 1998. [Google Scholar]
- 35.Little R, Stroup W, Freund R. SAS for Linear Models. SAS Institute, Inc.; Cary, NC: 2002. [Google Scholar]
- 36.Tang XL, Xuan YT, Zhu Y, Shirk G, Bolli R. Nicorandil induces late preconditioning against myocardial infarction in conscious rabbits. American journal of physiology Heart and circulatory physiology. 2004;286:H1273–80. doi: 10.1152/ajpheart.01055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun JZ, Tang XL, Knowlton AA, Park SW, Qiu Y, Bolli R. Late preconditioning against myocardial stunning. An endogenous protective mechanism that confers resistance to postischemic dysfunction 24 h after brief ischemia in conscious pigs. The Journal of clinical investigation. 1995;95:388–403. doi: 10.1172/JCI117667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang XL, Qiu Y, Turrens JF, Sun JZ, Bolli R. Late preconditioning against stunning is not mediated by increased antioxidant defenses in conscious pigs. The American journal of physiology. 1997;273:H1651–7. doi: 10.1152/ajpheart.1997.273.4.H1651. [DOI] [PubMed] [Google Scholar]
- 39.Baxter GF, Hale SL, Miki T, Kloner RA, Cohen MV, Downey JM, Yellon DM. Adenosine A1 agonist at reperfusion trial (AART): results of a three-center, blinded, randomized, controlled experimental infarct study. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 2000;14:607–14. doi: 10.1023/a:1007850527878. [DOI] [PubMed] [Google Scholar]
- 40.Lefer D, Jones S, Steenbergen C, Kukreja R, Guo Y, Tang X-L, Li Q, Ockaili R, Salloum F, Kong M, Polhemus D, Bhushan S, Goodchild T, Chang C, Book M,, Du J, Bolli R. Sodium Nitrite Fails to Limit Myocardial Infarct Size: Results from the CAESAR Cardioprotection Consortium (LB645) The FASEB Journal. 2014;28 [Google Scholar]
- 41.Kukreja R, Tang X-L, Lefer D, Steenbergen C, Jones S, Guo Y, Li Q, Kong M, Stowers H, Hunt G, Tokita Y, Wu W, Ockaili R, Salloum F, Book M, Du J, Bhushan S, Goodchild T, Chang C, Bolli R. Administration of Sildenafil at Reperfusion Fails to Reduce Infarct Size: Results from the CAESAR Cardioprotection Consortium (LB650) The FASEB Journal. 2014;28 [Google Scholar]






