Abstract
Objective
To investigate if repopulating the degenerating intervertebral disc (IVD) with articular chondrocytes (ACs) will decrease inflammation and restore disc structure. In this study, we aimed to determine if well-differentiated AC alone or transduced with adenovirus overexpressing BMP-7 gene may survive and inhibit inflammation or repair disc structure in the degenerating rabbit IVD.
Design
This was a biological study in a rabbit IVD-injury model in vivo. Dual cell tracking methods (IR dye-labeling and adenovirus transduction) were used to demonstrate the viability of allogeneic AC injected into degenerating rabbit IVDs. Interleukin (IL)-8 gene expression was determined via real-time PCR. Infiltrating inflammatory cells (macrophages, T-cells or neutrophils) were examined with immunohistochemistry. The IVDs were also examined by routine histology.
Results
ACs labeled with infrared (IR) dye were detected in the degenerating IVDs at both 2 and 8 weeks after injection. At the 2-week time point, IL-8 gene expression was comparable in IVDs injected with chondrocytes and in intact discs as control (P=0.647), while its expression in IVDs injected with saline increased 50fold (p=0.028). Transgene expression of red fluorescent protein, β-galactosidase, and BMP-7 diminished at 8 weeks post injection. IVDs injected with chondrocytes overexpressing hBMP-7 did not show lower IL-8 gene expression or improved histology. Macrophages were consistently detected by immunohistochemistry in the cartilage formation around the needle insertion sites in both the saline and chondrocyte groups, while neither T cells nor neutrophils were detected.
Conclusions
Allogeneic rabbit AC survived in the degenerating rabbit IVDs for at least 8 weeks. Cell treatment resulted in reduced IVD inflammation, but did not significantly improve IVD structure.
Keywords: cell therapy, inflammation, intervertebral disc, articular chondrocytes
INTRODUCTION
Annual direct medical costs for spine-related conditions totaled over $100 billion in the US.1, 2 Back pain is the most common reason for a physician visit in the US.2 In many affected patients, intervertebral disc (IVD) degeneration is a common finding and appears to play a role in the etiology of pain. Biological approaches that promote matrix repair and reduce inflammation may serve as alternatives to surgery for millions of people with back pain.
Because endogenous progenitor cells in the IVD decline with aging and degeneration,3 cells to repopulate the aging IVD are being rigorously sought after.4, 5 Mesenchymal stem cells (MSC) from bone marrow,6-9 adipose tissue,10, 11 umbilical cord blood12 or umbilical cord tissue13, 14 have been considered as possible candidates for repairing a degenerating IVD. Transplanted autologous IVD cells survived for at least one year in a canine model15 and in sand rats.16 Autologous human IVD cells derived from a therapeutic discectomy have also been tested in a pilot clinical study to repair the IVD.17-19 However, it is difficult to obtain nucleus pulposus (NP) cells uncontaminated by other cell types (e.g., fibroblasts, leukocytes).
Articular chondrocytes (ACs) serve as another attractive potential source for disc repair because these cells are already chondrocytes, and are available via allogeneic (deceased donor) or autologous sources (non-weight bearing regions of the patient’s joints). Acosta et al have recently compared MSCs and juvenile ACs in a porcine model of IVD degeneration. These authors found that IVDs injected with ACs contained almost exclusively type II collagen, while scar tissues rich in type I and II collagen formed in IVDs injected with MSCs.20
The use of ACs to repair the degenerating IVD has a distinct advantage over other cell types: they already have the chondrocytic phenotype at the time of implantation. When these cells are cultured in alginate beads, they immediately begin to reform an abundant extracellular matrix that retains cartilage-like properties for months.21 Allogeneic ACs can be isolated from unrelated deceased donors in greater numbers than IVD cells. Allogeneic chondrocytes are not used to repair cartilage defects because of the potential for rejection.22 Unlike articular tissue in a synovial joint, the disc tissue may be relatively immunoprivileged.23 Because allogeneic ACs could be obtained from unrelated deceased donors, these cells may prove useful in clinical applications. Autologous ACs have long been used to repair articular cartilage defects.24, 25 Autologous cells would be a reasonable cell source, but this would require a separate invasive procedure for cartilage procurement from the patient.
Bone morphogenetic proteins (BMPs) have been shown to stimulate IVD cell matrix production.4 We have compared 12 different BMPs and found that BMP-7 is the most effective in stimulating nucleus pulposus (NP) proteoglycan accumulation,26, 27 likely by stimulating both proteoglycan synthesis28 and slow down degradation.29 Viral vectors could be utilized to deliver growth factors or inhibitors of proteolytic enzymes.30, 31 These studies support the use of gene transfer to repair IVDs. A novel feature of the present study is the use of ACs transduced ex vivo to deliver genes to repair IVDs, a relatively safe approach because the host is not directly exposed to the virus.
Our hypothesis is that well-differentiated chondrocytes isolated from articular cartilage may survive in the degenerating IVD and reduce disc inflammation, thereby improving disc structure. Transplantation of cells into the degenerating discs must overcome several hurdles before achieving success. The first hurdle for transplanted cells is to survive in the harsh IVD environment, which is low in nutrients and in oxygen, and experiences fluctuations between high and low pressure. Another hurdle is the threat of rejection by the host. Although the intervertebral disc is a relatively immunoprivileged organ, a degenerative disc may be more accessible to inflammatory cells than a healthy one, thus increasing the possibility of rejection of transplanted cells. In this pilot study, we aim to present evidence that allogeneic ACs i) can survive; ii) can distribute to both NP and annulus fibrosus (AF) tissues in the injured IVD; and iii) reduce host inflammatory responses to injuries. Further, infiltrating inflammatory cells (macrophages, T lymphocytes, and neutrophils) were examined by immunostaining. The present findings will lay the groundwork for a cell therapy approach using allogeneic ACs to treat degenerative disc disease.
MATERIALS AND METHODS
Injury-induced intervertebral disc (IVD) degeneration
After approval from the Institutional Animal Care and Use Committee was obtained (IACUC # 08-039), young adult NZW rabbits weighing about 3.2 kg (approximately 3 months old) were used for the disc-injury model. Twenty four rabbits were used in this study. To induce IVD degeneration, 4 IVDs (L2/3, L3/4, L4/5, and L5/6) in each rabbit were exposed, and injury was induced with an 18-gauge (G) needle via a left retroperitoneal approach, as previously described.32 The rabbits were randomly assigned to the following groups: i) ACs transduced with AdhBMP-7 (n = 4); ii) ACs transduced with AdRFP-β-gal (n = 5); iii) unmodified ACs (as control, n = 4); or iv) saline (as control). During the initial phase of experiments (14 animals), each rabbit received only one type of treatment. During the second phase of the experiments (8 rabbits), as we perfected our surgical techniques, L2/3 and L3/4 received one type of treatment, while L4/5 and L5/6 received a different treatment; the treatments were randomly assigned. Four weeks post injury, 8 μl of cells suspended in medium were injected into the degenerating rabbit IVDs on the opposite side, via a right posterior-lateral approach. Two rabbits received sham surgeries and served as intact controls.
Rabbit AC culture, gene transfer and infrared (IR) dye-labeling
Younger rabbits (10 weeks old) were selected as chondrocyte donors because their cells have a better potential for ex vivo expansion, and may produce more proteoglycan-rich extracellular matrix compared with cells from old rabbits. Rabbit articular cartilage was harvested from the tibial plateau and femoral condyles of 10-week-old NZW rabbits (weighing about 2.5 kg; n = 3). Immediately after sacrifice of these animals, cartilage fragments were shaved from their knees, and ACs were released by serial enzyme digestion with pronase and collagenase as previously described.27 Primary cells were culture in monolayer at a density of 8×104 cells/cm2 for 3-5 days, until reaching 80% confluency. Cells were trypsinized, replated (these are considered passage-1 cells), and cultured for an additional 2-3 days before transplantation.
Adenovirus expressing red fluorescent protein (RFP) and β-galactosidase (β-gal) (AdRFP-β-gal) and adenovirus expressing green fluorescent protein (GFP) and human bone morphogenetic protein-7 (AdGFP-hBMP-7) were constructed using the AdEasy system.33 Briefly, the coding regions of the genes of interest (i.e., RFP and β-gal, or GFP and BMP-7) driven by cytomegalovirus (CMV) promoter were cloned into a shuttle vector to generate replication-deficient adenoviruses. The virus was subsequently amplified in 293 cells (E1-transformed human embryonic kidney cells). For the cells undergoing gene transfer, cells were transduced with AdRFP-β-gal or AdhBMP-7, and cultured for 2-4 days to achieve about 90% transduction efficiency as confirmed by visualizing RFP or GFP by fluorescence microscopy. On the day of injection, chondrocytes were detached with trypsin and labeled with the infrared (IR) dye (CellVue® NIR815 Fluorescent dye, LI-COR, Lincoln, NE) for 4 minutes, according to the manufacturer’s instructions. Chondrocytes were washed 3 times to eliminate unincoporated dye, then resuspended at 1×107 cells per ml of serum-free medium. Eight μl of the IR dye labeled cells were injected into each disc (8×104 cells/disc).
Detection of transplanted cells labeled with IR dye
For cell tracking, the IR dye was incorporated stably into the cell membranes of intact chondrocytes, which were then injected into the degenerating rabbit IVDs. Two or 8 weeks after cell transplantation, rabbits were euthanized with an overdose of pentobarbital and the spines removed en bloc. The spine segments and individual discs were scanned with an IR imager (LI-COR Biosciences, Lincoln, NE). IR fluorescence intensity (counts per mm3 of individual discs) was determined using the imaging software and exported to Microsoft Excel. The IR fluorescence intensities at the two time points were compared using a non-parametric t-test. At the time of sacrifice, only IVDs with detectable IR signals were used for data analysis; IVDs without good IR signals, or for which signals were outside the IVD because of variable surgical technique,32 were not used for data analysis.
Detection of RFP or β-gal expression in the rabbit IVDs
Nucleus pulposus cells containing RFP were examined by the Fluoview Laser Confocal system equipped with an Olympus microscope (Olympus 1X70, excitation at 588 nm). To examine the distribution of the injected cells (transduced with AdRFP-β-gal) within the IVD, β-gal activity was examined. Rabbit discs were isolated 2 or 8 weeks after cell transplantation. Each disc was transected to expose both NP and AF tissues and lightly fixed with 0.05 % glutaraldehyde in phosphate buffered saline (PBS) for 1 hour. The discs were washed with PBS and then incubated in X-gal staining solution {1 mg/ml of X-gal, 100 mM Na3PO4, 3 mM K3Fe(CN)6, 3mM K4Fe(CN)6, 1.3 mM MgCl2 in water} for 3 hours at 37°C. Digital images were taken with a digital camera (Sony).
Real-time PCR for BMP-7 or IL-8 gene expression in the rabbit IVDs
Chondrocytes transduced with Ad-hBMP-7 were injected into the degenerating rabbit discs, and hBMP-7 gene expression was examined by real-time PCR at 2 or 8 weeks after injection. Human BMP-7 (hBMP-7) mRNA levels were measured with real-time polymerase chain reaction (real-time PCR). Specifically, 2 or 8 weeks after transplantation, spine segments and individual discs were scanned with the IR imager to determine the presence of transplanted cells. Total cellular RNA was isolated from the IVDs (including both the nucleus pulposus and annulus fibrosus) using Trizol reagent (Invitrogen) and tissue homogenization using a rotor-stator device (Omni International). Fifty nanograms of total RNA from each sample was reverse-transcribed to generate first-strand cDNA, using the High Capacity RNA-to-cDNA Kit (Invitrogen). To determine the expression levels of hBMP-7 mRNA, a real-time PCR method was used with the hBMP-7-specific Taqman Gene Expression Assay [Applied Biosystems (ABI)]. Amplification was performed with a spectrofluorometric thermal cycler (7300 Real-time PCR System; Applied Biosystems). To standardize mRNA levels, the eukaryotic 18S ribosomal RNA (18S rRNA) was amplified as an internal control. IL-8 gene expression was quantified using rabbit IL-8-specific Taqman Gene Expression Assay (ABI). Four to 11 rabbits were used in each group, 1-2 discs were examined from each rabbit, and the average gene expression in each group of animals was determined. IL-8 gene expression among the groups was compared, with analysis of variance (ANOVA), followed by non-parametric Student’s t-test.
Immunostaining for examination of inflammatory cell infiltrates in the IVD
Inflammatory cell infiltrates at 2 weeks post chondrocyte or saline treatment were examined. The IVD tissue near the needle entry sites was isolated, separating the disc/cartilaginous outgrowth from the bony vertebral body. The tissues were cryosectioned to 10μM thickness. The sections were fixed briefly in 4% paraformaldehyde, washed with PBS, and incubated with mouse IgG antibody recognizing a cytoplasmic antigen in rabbit macrophages (clone RAM11, Dako, Denmark). Neutrophils were examined with a mouse monoclonal antibody to rabbit neutrophil defensin-5 (NP-5, Hycult Biotech, Plymouth Meeting, PA). T cells were examined with a mouse monoclonal antibody raised against pan T-cell markers in rabbit thymocytes (KEN-5, Santa Cruz Biotechnology, Santa Cruz, CA). Specifically, primary antibodies were used at a dilution of 1:100, and incubated at 4°C overnight. Secondary horse biotinylated anti-mouse IgG was then applied (Vector Laboratories Inc., CA, USA). Horseradish peroxidase activity was detected by incubation with 3,3′-diaminobenzidine (DAB) and development of a light brown color, and the sections were lightly counterstained with hematoxylin as described previously.12
RESULTS
Infrared dye-labeled cells persisted in the degenerating rabbit intervertebral disc (IVD)
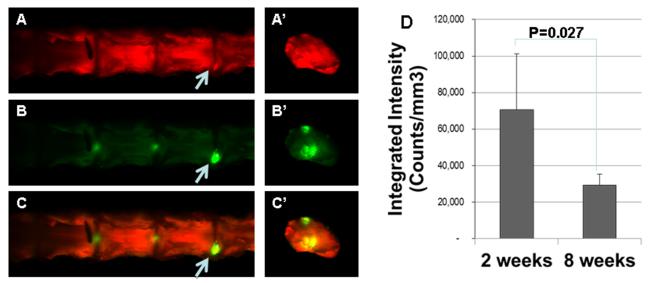
The left panels of Figure 1 show that at 2 weeks post cell transplantation, rabbit spine segments were scanned with an IR imager, and coronal images were obtained (panels A to C). Subsequently the individual IVDs from these spine segments were further separated at the growth plates, to isolate intact IVDs with adjacent endplates, and a transverse view of the disc was obtained (panels A’ to C’). To show the contour of the lumbar spine, tissues were detected in the 700 nm channel and represented in red (panels A and A’). IR dye-labeled cells were detected in the 800 nm channel and represented in green (panels B and B’). When the IR dye-labeled cells and the tissue contour images are superimposed, the signals overlap and are represented in yellow (panels C and C’). Arrows point to areas of intense IR dye-labeling, which may represent the needle entry point.
Figure1. Allogeneic rabbit articular chondrocytes survived in the degenerating rabbit disc.
Chondrocytes labeled with infrared dye were injected into the degenerating disc. Left Panels: the rabbit spines and discs were imaged at 2 weeks post injection. A & A’: coronal view of the spine and transverse view of an individual disc showing tissue contour; B & B’: infrared dye labeled cells; C & C’: overlay of the above panels. Right Panel: intensity of the infrared dye at 2 and 8 weeks post injection.
The right panel of Figure 1 shows the integrated intensities of the rabbit IVDs that were injected with AC transduced with Ad-hBMP-7, and labeled with IR dye before injection. At 2 or 8 weeks post injection, as analyzed with the IR imaging software. The average integrated intensity of IVDs at 2 weeks post injection is 70,654 (n=4), and the average intensity at 8 weeks post injection is 29,407 (n=7). The IR dye intensity level decreased by 58.5% at the 8-week time point compared with that at the 2-week time point (p = 0.027). It is worth noting that only rabbits that received the same batch of IR dye-labeled chondrocytes were compared here in order to decrease variability introduced during IR-dye labeling.
Cell tracking using allogeneic rabbit ACs transduced with Ad-RFP-β-gal to confirm the presence and location of the injected cells in the rabbit IVD
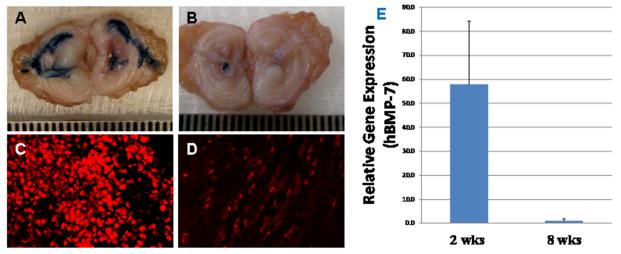
Figure 2, panels A and B show disc stained for β-gal activity at 2 weeks (n=2) or 8 weeks (n=3) post transplantation. The injected cells were stained blue, demonstrating production of the reporter protein. Figure 2A shows that transplanted cells, which were injected into the NP, spread to the annulus likely via annular fissures. In panel 2A (2 weeks post injection), the blue areas are larger and more intense compared with panel 2B (8 weeks post injection).
Figure2. Allogeneic rabbit articular chondrocytes are viable in the degenerating rabbit disc, although transgene expression diminished over time.
Chondrocytes transduced with adenovirus expressing red fluorescent protein (RFP) and β-galactosidase (Ad-RFP-β-gal) continued to express these transgenes. A&B: β-gal (blue) at 2 and 8 weeks, respectively, post injection; C&D: RFP (red) at 2 and 8 weeks, respectively, post injection. E: Expression of hBMP-7 in IVDs at 2 and 8 weeks, respectively, post injection of AC transduced with AdGFP-hBMP-7.
Figure 2, panels C and D show transplanted cells (identifiable by RFP fluorescence) in the NP tissues at 2 weeks (n=3) and 8 weeks (n=3), respectively, post chondrocyte transplantation. Similar to the β-gal activity, RFP fluorescence is more intense at 2 weeks compared with 8 weeks post transplantation.
Articular chondrocytes transduced with Ad-hBMP-7 expressed the human BMP-7 (hBMP-7) gene in the degenerating IVDs
Panel E shows hBMP-7 gene expression in IVDs injected with AC transduced with Ad-hBMP-7. Real-time PCR was performed with primers for hBMP-7 and 18S rRNA (as internal control). HBMP-7 was detected in the IVDs injected with chondrocytes transduced with Ad-hBMP-7, but not in IVDs injected with chondrocytes without Ad-hBMP-7. Figure 2, panel E shows that the average hBMP-7 gene expression at the 8-week time point (n=2) was only 1.7% of that at the 2-week time point (n=2).
Articular chondrocyte treatment resulted in decreased IL-8 expression
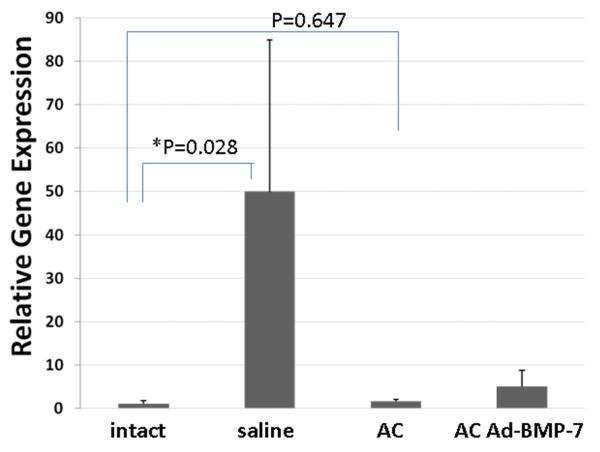
IL-8 gene expression, as a marker of acute inflammation, was assessed in IVDs injected with ACs and compared with those injected with saline (as control) at 2 or 8 weeks post injection. Figure 3 shows that at 2 weeks post-injection, IL-8 gene expression in IVDs injected with chondrocytes (n=5) did not differ significantly from that in intact discs (as control, n=11, P=0.647), whereas IL-8 gene expression in IVDs injected with saline increased 50-fold (n=4, p=0.028) compared with intact IVDs (n=11).
Figure 3. Interleukin (IL)-8 gene expression in rabbit intervertebral discs (IVDs).
Two weeks after chondrocyte injection, IL-8 gene expression does not differ in IVDs injected with chondrocytes compared with that in intact discs (as control, P=0.647), while its expression in IVDs injected with saline increased 50-fold (p=0.028).
At 8 weeks post-injection, IL-8 gene expression in IVDs injected with saline is only 30.0% of that at 2 weeks post-injection (n=2 and 5, respectively). In the IVD injected with AC, at the 8-week time-point, IL-8 gene expression is 69.5% of those at 2-week time point. (n=2 and 4, respectively). This finding suggests that inflammation subsides at 8 weeks after both treatments. At 8 weeks, IVDs injected with saline (n=2) was still 4.4 fold of that in the discs injected with ACs (n=2).
Cell therapy did not result in significant improvement in IVD histology or biochemical content
No significant changes were detected by examining histological sections at either of the 2- or 8-week post-injection time points (n=3). In addition, we evaluated total proteoglycan content and cell number (by DNA assays) to evaluate the biochemical content (proteoglycan, collagen, and DNA) of the IVDs treated with AC or saline. No significant changes were found (n=8 in each group, p>0.05, data not shown).
Inflammatory cell infiltration in the rabbit IVD
Macrophage infiltration was detected in AC- and saline-injected, as well as injured with sham injection groups, especially in the anterior annulus where injury and injection were performed. Figure 4 shows representative images of IVDs immunostained with antibody recognizing a rabbit macrophage cytoplasmic antigen (panels A-C) or with no primary antibody (panel D). Note that the anterior annulus in IVDs injected with AC (panel A) contains cells resembling chondrocytes, while cells in the IVDs in saline or sham injection groups retain the fibrocyte-like phenotype.
Figure 4. Macrophage infiltration into the injured intervertebral discs (IVDs).
A-C are stained immunohistochemically to show macrophages. A: injured IVD injected with articular chondrocytes; B: injured IVD injected with saline; C: injured IVD, sham injection; D: injured IVD, no primary antibody (as negative control).
No T-lymphocyte infiltration was detected in either the chondrocyte- or saline-injected IVDs (data not shown). Data on neutrophil staining are inconclusive because IVD cells stained positive with the anti-neutrophil antibody, suggesting that they may express surface antigens recognized by this antibody. We did not detect any cell stained with anti-neutrophil antibody and displaying neutrophil morphology (data not shown).
DISCUSSION
In conclusion, we have established the technical feasibility of a cell-based gene therapy approach to repairing the degenerating IVD in the rabbit. We have demonstrated that allogeneic rabbit articular chondrocytes transplanted into the IVDs in vivo survived for at least 8 weeks. The articular chondrocytes transduced with Ad-hBMP-7 also expressed the hBMP-7 gene. No immune cell infiltration was observed in the IVD injected with allogeneic chondrocytes. The cell survival and absence of immune cell infiltration at the 8-week time point suggest that the IVD tissue is relatively immunoprivileged.
The β-gal gene was expressed 2 and 8 weeks after injection, although β-gal activity was reduced at the 8-week time point when compared to the 2-week time point. RFP was also decreased in the NP at the 8-week time point compared with 2 weeks post injection. The articular chondrocytes transduced with Ad-hBMP-7 expressed the human BMP-7 gene at the 2-week time point, though with diminished intensity at the 8-week time point. The IR dye intensity decreased from the 2-week to the 8-week time point, likely due to loss of transplanted cells. The decrease in RFP and in β-gal expression is more dramatic than the decrease in IR-dye intensity. This is most likely due to a decrease in transgene expression, in addition to a reduction in number of cells expressing the RFP or β-gal gene. This is not surprising: adenovirus-mediated gene expression is usually transient because the viral genes are not incorporated into the host genome. It is worth noting that sources of measured IR dye might include cell membrane fragments retained in the IVD after cell death. Thus, further cell staining with a dye that is sensitive to mitochondrial membrane potential is needed to conclusively demonstrate cell viability.
We have recently established a novel method to quantify injected cells labeled with an infrared dye, by measuring the dye intensity (shown in Figure 1).12 This method greatly improves our ability to quantify injected cells, compared with the visual inspection of GFP described previously.34 We suggest that this cell tracking method could be routinely used in future studies, to verify that cells are delivered to a particular disc space.
In this study, we injected 8×104 cells/disc. The decision on the number of cells to inject is based on previous experiments in cultured IVD explants and calculations according to Stairmand et al.35 We estimated normal rabbit IVD tissue cell number by comparing a whole disc DNA content with the DNA content of a known number of cultured cells: in a single rabbit IVD, the NP contains an average of 3.6×106 cells, while the AF contains 16.2 ×106 cells (n = 17). The 8×104 cells that we injected were merely 2% of the normal NP cell content. Because of limited space and nutrition in the IVD, cell apoptosis may occur if too many cells are injected.36 In the future, we plan to examine a wider range of cells for transplantation. In this study, we used allogeneic articular chondrocytes from 10-week old rabbits as donors, and 4-month old rabbits (young adults) as recipients. Endplate permeability may decline in older animals, and the number of surviving cells may be smaller than in younger animals.
Although the donor rabbits are from the same colony as the recipient NZW rabbits, these rabbits did not undergo a rigorous inbreeding process. Consequently, the grafts in this study are technically “allografts”, rather than “isografts”. NZW rabbits are in the Leporidae (hares, rabbits) family. In the future, in order to study cell survival, we plan to use donor allogeneic cells from other animals (e.g. the Ochotonidae family), and to examine immune responses to the transplanted cells. Consistent with our previous data supporting survival of xenogeneic human stem cells in the rabbit (unpublished data), the rabbit AC derived from a different animal have survived after injection into the degenerative IVDs in this study.
The injected cells, targeted to the center of the NP, also dispersed through the needle track and annular fissures. We did not detect significant cell leakage beyond the cartilagenous outgrowth around the needle insertion point in 4-month old rabbits by the infrared imager. In more severely degenerative IVDs of older animals or humans, cell leakage through the annular fissures might occur. An alternative approach is to use carriers for chondrocytes, and glues to seal off the needle track. Various pertinent materials are available: we have some experience using a chondroitin sulfate based hydrogel as a carrier [12]. Temperature-sensitive gels may be advantageous if they solidify quickly.
Future studies to examine the effects of cell therapy on IVD biochemical composition and biomechanical properties, as well as on the inflammatory status of the IVDs, will help to determine the efficacy of the treatment. Studies in a large animal model may also be indicated before clinical trials.
ACKNOWLEDGEMENTS
The authors gratefully thank Eugene Thonar, PhD for valuable insights and discussions towards experiment design, and Carla Scanzello, MD, PhD, for helpful discussions on inflammatory cell infiltrates. We thank Nancy Ruel, PhD, for help in quantifying IR dye intensity, Motomi Enomoto-Iwamoto, DDS, PhD for technical advice on β-gal assays, and Thomas Cha, MD, Ting-Hsien Kao, MD, and QingYi He, MD for performing surgery. The authors thank Martin Heyworth, MD, for critically editing the manuscript. Yejia Zhang, MD, PhD is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, 1K08 HD049598). This work is supported, in part, by a research grant from the Cervical Spine Research Society (CSRS).
Footnotes
AUTHORS’ CONTRIBUTIONS IM performed surgery; SP performed surgery, rabbit dissections, and histological examinations; AC cultured, transduced and labeled chondrocytes, scanned the rabbit spines and discs using the infrared imager, and performed the β-gal assays and RT-PCR analysis; EC performed confocal microscopy; NR quantified the infrared fluorescence intensity; TCH provided Ad-RFP-β-gal and Ad-hBMP-7; YZ and HSA are responsible for the conception and design of experiments, data interpretation, and for obtaining funding. RW performed immunostaining for inflammatory cells.
REFERENCES
- 1.United States bone and joint decade: The burden of musculoskeletal diseases in the United States. The American Academy of Orthopaedic Surgeons; Rosemont, IL: 2008. [Google Scholar]
- 2.Katz JN. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 3.Sakai D, Nakamura Y, Nakai T, et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Chee A, Thonar EJ, An HS. Intervertebral disk repair by protein, gene, or cell injection: A framework for rehabilitation-focused biologics in the spine. PM R. 2011;3(6 Suppl 1):S88–94. doi: 10.1016/j.pmrj.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Kregar Velikonja N, Urban J, Frohlich M, et al. Cell sources for nucleus pulposus regeneration. Eur Spine J. 2013 doi: 10.1007/s00586-013-3106-9. [DOI] [PubMed] [Google Scholar]
- 6.Sakai D. Stem cell regeneration of the intervertebral disk. Orthop Clin North Am. 2011;42(4):555–62. viii–ix. doi: 10.1016/j.ocl.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Risbud MV, Shapiro IM, Vaccaro AR, Albert TJ. Stem cell regeneration of the nucleus pulposus. Spine J. 2004;4(6 Suppl):348S–353S. doi: 10.1016/j.spinee.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert HT, Hoyland JA, Richardson SM. Stem cell regeneration of degenerated intervertebral discs: Current status (update) Curr Pain Headache Rep. 2013;17(12):377-013–0377-0. doi: 10.1007/s11916-013-0377-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Drapeau S, Howard SA, Thonar EJ, Anderson DG. Transplantation of goat bone marrow stromal cells to the degenerating intervertebral disc in a goat disc injury model. Spine (Phila Pa 1976) 2011;36(5):372–377. doi: 10.1097/BRS.0b013e3181d10401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganey T, Hutton WC, Moseley T, Hedrick M, Meisel HJ. Intervertebral disc repair using adipose tissue-derived stem and regenerative cells: Experiments in a canine model. Spine (Phila Pa 1976) 2009;34(21):2297–2304. doi: 10.1097/BRS.0b013e3181a54157. [DOI] [PubMed] [Google Scholar]
- 11.Tapp H, Deepe R, Ingram JA, Kuremsky M, Hanley EN, Jr, Gruber HE. Adipose-derived mesenchymal stem cells from the sand rat: Transforming growth factor beta and 3D co-culture with human disc cells stimulate proteoglycan and collagen type I rich extracellular matrix. Arthritis Res Ther. 2008;10(4):R89. doi: 10.1186/ar2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson DG, Markova D, An HS, et al. Human umbilical cord blood-derived mesenchymal stem cells in the cultured rabbit intervertebral disc: A novel cell source for disc repair. Am J Phys Med Rehabil. 2013;92(5):420–429. doi: 10.1097/PHM.0b013e31825f148a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruan D, Zhang Y, Wang D, et al. Differentiation of human Wharton’s jelly cells toward nucleus pulposus-like cells after coculture with nucleus pulposus cells in vitro. Tissue Eng Part A. 2012;18(1-2):167–175. doi: 10.1089/ten.TEA.2011.0186. [DOI] [PubMed] [Google Scholar]
- 14.Leckie SK, Sowa GA, Bechara BP, et al. Injection of human umbilical tissue-derived cells into the nucleus pulposus alters the course of intervertebral disc degeneration in vivo. Spine J. 2013;13(3):263–272. doi: 10.1016/j.spinee.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganey T, Libera J, Moos V, et al. Disc chondrocyte transplantation in a canine model: A treatment for degenerated or damaged intervertebral disc. Spine (Phila Pa 1976) 2003;28(23):2609–2620. doi: 10.1097/01.BRS.0000097891.63063.78. [DOI] [PubMed] [Google Scholar]
- 16.Gruber HE, Johnson TL, Leslie K, et al. Autologous intervertebral disc cell implantation: A model using Psammomys obesus , the sand rat. Spine (Phila Pa 1976) 2002;27(15):1626–1633. doi: 10.1097/00007632-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 17.Hohaus C, Ganey TM, Minkus Y, Meisel HJ. Cell transplantation in lumbar spine disc degeneration disease. Eur Spine J. 2008;17(Suppl 4):492–503. doi: 10.1007/s00586-008-0750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meisel HJ, Siodla V, Ganey T, Minkus Y, Hutton WC, Alasevic OJ. Clinical experience in cell-based therapeutics: Disc chondrocyte transplantation. A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24(1):5–21. doi: 10.1016/j.bioeng.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Meisel HJ, Ganey T, Hutton WC, Libera J, Minkus Y, Alasevic O. Clinical experience in cell-based therapeutics: Intervention and outcome. Eur Spine J. 2006;15(Suppl 3):S397–405. doi: 10.1007/s00586-006-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acosta FL, Jr, Metz L, Adkisson HD, et al. Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells. Tissue Eng Part A. 2011;17(23-24):3045–3055. doi: 10.1089/ten.tea.2011.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauselmann HJ, Fernandes RJ, Mok SS, et al. Phenotypic stability of bovine articular chondrocytes after long-term culture in alginate beads. J Cell Sci. 1994;107(Pt 1):17–27. doi: 10.1242/jcs.107.1.17. [DOI] [PubMed] [Google Scholar]
- 22.Romaniuk A, Malejczyk J, Kubicka U, Hyc A, Olszewski WL, Moskalewski S. Rejection of cartilage formed by transplanted allogeneic chondrocytes: Evaluation with monoclonal antibodies. Transpl Immunol. 1995;3(3):251–257. doi: 10.1016/0966-3274(95)80032-8. [DOI] [PubMed] [Google Scholar]
- 23.Wei A, Tao H, Chung SA, Brisby H, Ma DD, Diwan AD. The fate of transplanted xenogeneic bone marrow-derived stem cells in rat intervertebral discs. J Orthop Res. 2009;27(3):374–379. doi: 10.1002/jor.20567. [DOI] [PubMed] [Google Scholar]
- 24.de Windt TS, Welsch GH, Brittberg M, et al. Is magnetic resonance imaging reliable in predicting clinical outcome after articular cartilage repair of the knee? A systematic review and meta-analysis. Am J Sports Med. 2013;41(7):1695–1702. doi: 10.1177/0363546512473258. [DOI] [PubMed] [Google Scholar]
- 25.Ebert JR, Smith A, Wood DJ, Ackland TR. A comparison of the responsiveness of 4 commonly used patient-reported outcome instruments at 5 years after matrix-induced autologous chondrocyte implantation. Am J Sports Med. 2013;41(12):2791–2799. doi: 10.1177/0363546513502314. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Anderson DG, Phillips FM, et al. Comparative effects of bone morphogenetic proteins and Sox9 overexpression on matrix accumulation by bovine anulus fibrosus cells: Implications for anular repair. Spine (Phila Pa 1976) 2007;32(23):2515–2520. doi: 10.1097/BRS.0b013e318158cc09. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Li Z, Thonar EJ, et al. Transduced bovine articular chondrocytes affect the metabolism of cocultured nucleus pulposus cells in vitro: Implications for chondrocyte transplantation into the intervertebral disc. Spine (Phila Pa 1976) 2005;30(23):2601–2607. doi: 10.1097/01.brs.0000187880.39298.f0. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, An HS, Song S, et al. Growth factor osteogenic protein-1: Differing effects on cells from three distinct zones in the bovine intervertebral disc. Am J Phys Med Rehabil. 2004;83(7):515–521. doi: 10.1097/01.phm.0000130031.64343.59. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Hutton WC, Yoon ST. Bone morphogenetic protein-7 antagonizes tumor necrosis factor-alpha-induced activation of nuclear factor kappaB and up-regulation of the ADAMTS, leading to decreased degradation of disc matrix macromolecules aggrecan and collagen II. Spine J. 2013 doi: 10.1016/j.spinee.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Leckie SK, Bechara BP, Hartman RA, et al. Injection of AAV2-BMP2 and AAV2-TIMP1 into the nucleus pulposus slows the course of intervertebral disc degeneration in an in vivo rabbit model. Spine J. 2012;12(1):7–20. doi: 10.1016/j.spinee.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woods BI, Vo N, Sowa G, Kang JD. Gene therapy for intervertebral disk degeneration. Orthop Clin North Am. 2011;42(4):563–74. ix. doi: 10.1016/j.ocl.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Moss IL, Zhang Y, Shi P, Chee A, Piel MJ, An HS. Retroperitoneal approach to the intervertebral disc for the annular puncture model of intervertebral disc degeneration in the rabbit. Spine J. 2013;13(3):229–234. doi: 10.1016/j.spinee.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 33.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95(5):2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Phillips FM, Thonar EJ, et al. Cell therapy using articular chondrocytes overexpressing BMP-7 or BMP-10 in a rabbit disc organ culture model. Spine (Phila Pa 1976) 2008;33(8):831–838. doi: 10.1097/BRS.0b013e31816b1f38. [DOI] [PubMed] [Google Scholar]
- 35.Stairmand JW, Holm S, Urban JP. Factors influencing oxygen concentration gradients in the intervertebral disc. A theoretical analysis. Spine (Phila Pa 1976) 1991;16(4):444–449. doi: 10.1097/00007632-199104000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Serigano K, Sakai D, Hiyama A, Tamura F, Tanaka M, Mochida J. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J Orthop Res. 2010;28(10):1267–1275. doi: 10.1002/jor.21147. [DOI] [PubMed] [Google Scholar]