Abstract
The purpose of early stage clinical trials is to determine the recommended dose and toxicity profile of an investigational agent or multi‐drug combination. Molecularly targeted agents (MTAs) and immunotherapies have distinct toxicities from chemotherapies that are often not dose dependent and can lead to chronic and sometimes unpredictable side effects. Therefore utilizing a dose escalation method that has toxicity based endpoints may not be as appropriate for determination of recommended dose, and alternative parameters such as pharmacokinetic or pharmacodynamic outcomes are potentially appealing options. Approaches to enhance safety and optimize dosing include improved preclinical models and assessment, innovative model based design and dose escalation strategies, patient selection, the use of expansion cohorts and extended toxicity assessments. Tailoring the design of phase I trials by adopting new strategies to address the different properties of MTAs is required to enhance the development of these agents. This review will focus on the limitations to safety and dose determination that have occurred in the development of MTAs and immunotherapies. In addition, strategies are proposed to overcome these challenges to develop phase I trials that can more accurately define the recommended dose and identify adverse events.
Keywords: Phase I trials, Recommended phase 2 dose, Toxicity
Highlights
Comprehensive review of optimal safety and dosing in early phase cancer trials.
Current strategies, limitations and optimization of phase 1 studies are discussed.
Various topics covered, including patient selection, start dose and study designs.
1. Introduction
Phase I trials of anticancer therapies classically involve cytotoxic agents that alter cell replication and metabolism. The need to more specifically target tumor cells and improve toxicity has led to the advent of molecularly targeted agents (MTAs) that include small molecule inhibitors and monoclonal antibodies, as well as immune based therapeutics. These new classes of drugs have different anticancer and toxicity profiles compared to cytotoxic chemotherapies and consequently challenge conventional early phase clinical testing (see Table 1). The primary objective of a phase I trial is to determine the recommended phase II dose (RP2D) of a drug or drug combination and to identify relevant treatment related toxicities. Given the different biological properties of MTAs and immunotherapies, trial designs developed during the era of cytotoxic treatments may be unsuitable to correctly define RP2D or adverse events.
Table 1.
Similarities and differences in phase I trials for different drug classes.
| Trial elements | Cytotoxics | MTAs and immunotherapies |
|---|---|---|
| Primary end point | RP2D | RP2D |
| Secondary end points | Toxicity (MTD, DLT), response rate | PK or PD (molecular) parameter, toxicity, response rate |
| Dose escalation decisions | Toxicity based | Escalate based on toxicity or to a desired on‐target effect |
| PK parameters | Cmax may correlate with toxicity | PK parameter (e.g. Cmax, Cmin, AUC) that correlates with desired target stimulation or suppression |
| t1/2 may predict recovery from toxicity | ||
| Reasons for selecting RP2D | Toxicity | Combination of toxicity and PD/PK parameters |
| RP2D must have tolerable toxicities and may demonstrate anti‐tumor activity | RP2D may demonstrate desired target effects with anti‐tumor activity and tolerable toxicity |
MTA, molecular targeted agent; RP2D, recommended phase II dose; MTD, maximum tolerated dose; DLT, dose limiting toxicity; PK, pharmacokinetic; PD, pharmacodynamic; AUC, area under the curve; SD, stable disease.
The fundamental elements of a phase I study are well described in several comprehensive reviews (Le Tourneau et al., 2009; LoRusso et al., 2010). Dose escalation designs influence the number of patients enrolled, the fraction of patients treated at sub‐therapeutic doses and the efficiency of the study. These designs are either rule‐ or model‐based. The former utilizes pre‐specified guidelines for observed toxicity based endpoints (e.g. dose limiting toxicity (DLT)) to determine subsequent dose levels, the maximum tolerated dose (MTD) or maximum administered dose (MAD) and RP2D (Figure 1). Model‐based designs estimate the dose toxicity relationship and assign dose levels by determining the statistical probability of observing a target event. Specific trial designs are listed in Table 2. The traditional 3 + 3 design is the most commonly applied rule‐based method (Storer, 1989), however it has been criticized for being slow, inefficient, inaccurate and treating a high proportion of patients at suboptimal doses (Reiner et al., 1999). Newer rule‐based methods have attempted to address these issues by optimizing efficiency without compromising safety, for example, by adopting an initial acceleration phase in which cohort sizes are reduced and dose increments are large (Simon et al., 1997).
Figure 1.
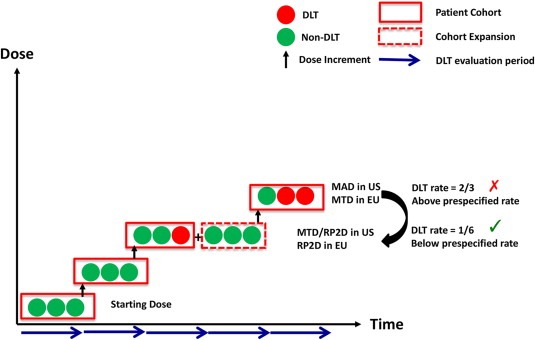
Elements of a dose escalation study.
Table 2.
Phase 1 trial designs.
| Trial design | Description | Comments |
|---|---|---|
| Rule‐Based Designs: Dose toxicity curve not assumedDecision to escalate based on pre‐defined rules and observed toxicities in the specified DLT period of current dose level | ||
| 3 + 3 | 3 patients treated per dose level. Depending on the number of DLTs, the dose is either escalated (0 DLTs) or de‐escalated (≥2 DLTs), or the cohort is expanded with 3 extra patients (1 DLT). Dose increments are pre‐determined eg. modified Fibonacci. RP2D is the highest dose with a pre‐specified DLT rate | • Commonly used design • Easy to implement • More patients may be treated at subtherapeutic doses • May not accurately define RP2D |
| Accelerated titration | Various designs proposed with fixed dose escalation increments EG:•3 + 3 design but with 40% dose increments•Single patient cohorts until a DLT or second intermediate toxicity occurs (trigger), then cohort expanded and reverted to design 1•Single patient cohorts with 80% dose increments. Revert to design 1 with the same trigger as design 2•As for design 3 but trigger to revert to design 1 is an anytime DLT or second anytime intermediate toxicity | • Acceleration and escalation in one design • Fewer patients may treated at subtherapeutic doses • Potentially faster • Delayed or cumulative toxicities masked if intrapatient dose‐escalation permitted |
| Model‐Based Design: Dose‐toxicity curve established a priori and then modified as toxicity data is collected on studyBiostatistical expertise required to construct and modify dose‐toxicity estimates | ||
| CRM | A target level of toxicity and the probabilities of observing a DLT at certain dose levels are pre‐defined at baseline for a fixed sample size. Initially doses for single patient cohorts are increased based on this model however with successive patients the model is re‐calculated according to Bayesian principles, which guides subsequent dose escalation. The RP2D is the dose associated with the target DLT rate consistent with the final dose‐toxicity model | • May overestimate RP2D • Limited data may exist to model initial dose‐toxicity curve • Intrapatient dose escalation may be permitted |
| Modified CRM | Similar to CRM except that a conservative starting dose is used with single dose level escalations per cohort. The next patient dose may not be escalated following a DLT. Cohorts may be larger than one and stopping rules are defined rather than using a fixed sample size | • Safety and efficiency improved compared to CRM |
| EWOC | Dose‐toxicity curve modeled to minimize the probability a patient will be treated at an unacceptably high dose | • Dose‐toxicity curve constantly remodeled requiring significant statistical support |
| TITE‐CRM | Data from all treated patients, including partial data, is incorporated into dose‐toxicity curve and subsequent dose calculationsPatients who have experienced DLT are fully weighted and those who have not experienced toxicity are weighted by the proportion of time observed on study | • Allows toxicity information of patients to contribute to RP2D determination • May account for chronic toxicities |
| Pharmacokinetically guided dose escalation (PGDE): Requires real time PK measurement and analysis for dose‐modificationAssumes DLT can be predicted by plasma drug concentration | ||
| PGDE | AUC measured for first cohort and dose escalation occurs according to distance to target AUC. This may occur initially by a factor equal to the square root of target AUC/initial AUC and then subsequently by a modified Fibonacci scheme. Another escalation strategy is to increase by a factor of 2 until 40% of target AUC is reached and then by a modified Fibonacci method | • Interpatient variability may limit dose escalation • May be suitable to estimate an ODB |
MTD, maximum tolerated dose; DLT, dose‐limiting toxicity; PK, pharmacokinetic; PD, pharmacodynamic; AUC, area under the curve; CRM, continuous reassessment method; EWOC, escalation with overdose control; TITE, time to event; RP2D, recommended phase II dose; MTAs, molecularly targeted agents; OBD, optimal biological dose.
Several reviews report that toxicity has been the most prevalent endpoint used to define RP2D in phase I trials (Le Tourneau et al., 2009; Parulekar and Eisenhauer, 2004). However given that MTAs and immunotherapies may not have dose dependent toxicities to identify an MTD, pharmacokinetic (PK) or pharmacodynamic (PD) parameters could be valuable tools to help determine RP2D. Establishing dosages based on the occurrence of a pre‐specified biomarker threshold, such as utilization of PK, PD or functional imaging parameters, is termed optimal biological dosing (OBD). OBD can be determined as a dose of a drug that reliably inhibits a key target in tumor or surrogate tissue, achieves a certain target plasma concentration, or reaches a pre‐specified immunologic parameter. Beyond escalation design and endpoints, patient selection is another important aspect of early phase clinical trials. Traditionally phase I trials enrolled all‐comers. However, increasingly contemporary phase I studies are restricting patients with specific pathology within a particular tumor type (e.g. esophageal cancers with squamous cell histology), or a specific molecular profile (e.g. solid tumors with PIK3CA mutations). High quality preclinical data and validated clinical assays are essential to this approach. The objective of this review is to highlight limitations in current phase I trial designs and discuss strategies to improve their accuracy and efficiency, with an emphasis on optimal dosing and safety.
2. Preclinical models
2.1. Current models
Numerous preclinical in vitro and in vivo models exist although no single system is considered the gold standard for evaluating toxicology and biological effects of a drug. Selecting the right system will depend on the mechanism of action and PK/PD properties of the studied agent, in addition to other practicalities such as cost, resource availability and animal model expertise. Two‐dimensional cell culture has typically been used to obtain mechanistic insight on new therapeutics. In recent years, various preclinical mouse model systems have become available, including autochthonous genetically engineered mouse models (GEMMs) and chemically induced tumor models, as well as ectopic models in which syngeneic or xenogeneic tumor or cells are implanted subcutaneously or orthotopically. Each of these systems has its own advantages and disadvantages with respect to biological validity, time investment and cost (Gutmann et al., 2006; Ocana et al., 2011). Toxicology studies, on the other hand, are often undertaken in non‐rodent species, such as dogs and monkeys that might be more predictive of human effects, however these models are seldom used to investigate molecular mechanisms.
2.2. Limitations and optimization
Human tumor cell line‐based xenografts are the most commonly used model system in preclinical research. Efficacy data from various xenografts were retrospectively reviewed and compared to patient data from early phase trials to assess their predictive value. Even though histology was matched in both data sets there was a low correlation in efficacy between the xenografts and patients when assessing cytotoxic compounds (Johnson et al., 2001). Xenografts and cultured cell lines have limited utility in predicting antitumor effects for new therapies, in part because they typically do not reproduce complex tumor biology and tumor–stromal interactions. Important components of the tumor microenvironment that are altered in xenograft tumors include cancer‐associated fibroblasts, the vasculature, lymphatics, and immune cells (Frese and Tuveson, 2007; Sikder et al., 2003). Due to interspecies differences in epitope antigenicity and immune responses, preclinical models for immunotherapies may have limited applicability to humans. Despite these caveats the majority of new therapies continue to be investigated in these systems due to their relatively low cost and quick turnaround compared to more suitable models.
GEMMs and patient derived xenografts (PDXs), have been developed to closely replicate the genetics and biology of specific cancer types and thus provide a more representative physiological system to evaluate PK and PD effects of study drugs (Tentler et al., 2012). Despite this progress these models are not widely implemented because significant resources are required to develop and maintain them and there is lack of a standardized translational approach to the clinic (Tentler et al., 2012). However recently GEMMs have been used to identify the importance of mTOR and EGFR inhibitors in neuroendocrine cancers, leading to the successful translation of mTOR inhibitors into clinical practice in this tumor type (Chiu et al., 2010; Yao et al., 2011). PDXs are also increasingly used to guide personalised therapy (Hidalgo et al., 2011; Morelli et al., 2012). The relatively new paradigm of co‐clinical studies, in which mouse trials are performed concurrently with human trials, is presently under investigation (Clohessy and de Stanchina, 2013). A co‐clinical trial utilizing a KRAS‐driven GEMM of non‐small cell lung cancer demonstrated that TP53 and LKB1 mutations were predictive of response to the MEK inhibitor selumitinib given in combination with docetaxel (Chen et al., 2012). Variability and reproducibility of results from co‐clinical trials may be addressed by implementing standardized guidelines, assays and methodology (Clohessy and de Stanchina, 2013). Uniform reporting of experiments with PDXs and GEMMs would facilitate comparisons between studies. New therapeutics should be tested in relevant preclinical models with pre‐defined biological and efficacy endpoints. Additionally, it would be ideal if more than one non‐rodent model be used to assess toxicities. Close collaboration between the pharmaceutical industry and academia is required to ensure this information is available prior to embarking in an early phase trial, as many toxicology and early biological studies are performed in industry.
Reproducing pre‐clinical data across different laboratories and groups presents another challenge. A recent report revealed less than 25% of pre‐clinical results from landmark publications could be validated (Begley and Ellis, 2012). These discrepancies have been attributed to poor experimental design, a paucity of robust supportive data, inappropriate controls, the failure to report negative results, and reliance on one or few model systems. Even studies carried out by respected institutions that utilize hundreds of cell lines may not accurately control for variation. Comparison of two recent large‐scale pharmacogenomic studies revealed discordant results that were presumably due, at least in part, to a lack of standardized methodology (Haibe‐Kains et al., 2013). Ideally pre‐clinical studies should be blinded, utilize only validated reagents and standardized drug‐response measurements, incorporate appropriate positive and negative controls, and be replicated by independent investigators. Improving the quality, reliability, and reproducibility of this preclinical information will provide greater knowledge of toxicities, integration of PK and PD endpoints and a more transparent discovery process for the successful translation to phase I trials.
3. Starting dose determination
3.1. Current models
In 2010 the US Food and Drug Administration (FDA) released guidelines for the nonclinical development of novel oncology drugs which recommended that the starting dose of an investigational agent should have a pharmacologic effect and be reasonably safe to use (ICH S9) (Food and Drug Administration, 2010). Both rodent and non‐rodent models are used for preclinical safety assessments, but it has been demonstrated that non‐rodent models may be better at predicting MTD in humans (Tomaszewski, 2004). Traditionally one tenth of the lethal dose for mice (LD10), or one sixth (1/6th) the highest non‐severely toxic dose (HNSTD) in a more sensitive species (e.g. monkey), is considered an appropriate starting dose. For drugs defined as having a high risk of adverse events in humans, the European Medicines Agency (EMA) has recommended using the minimal anticipated biological effect level (MABEL), which incorporates all in vivo and in vitro data to calculate the anticipated dose that will have a biological effect in humans (Agoram, 2009; Committee for Medicinal Products for Human use (CHMP), 2007).
Selecting a safe starting dose must be balanced against the proportion of patients treated at sub‐therapeutic doses. This is especially important for agents that demonstrate minimal toxicity in preclinical testing or drugs that are unlikely to ever reach MTD. A report examining choice of starting dose for MTAs in 81 first in human phase I trials revealed the starting dose to MTD or MAD ratio was widely variable, as were the toxicological parameters used to select the starting dose (Le Tourneau et al., 2010). Despite this variability the choice of starting dose was safe in the majority of studies examined (96.3%), and a hypothetical doubling of the starting dose was found to be non‐toxic in most of the trials.
3.2. Limitations and optimization
The FDA's guidelines for starting dose determination refer mainly to small molecules and cytotoxic chemotherapeutics. The applicability of these guidelines to larger molecules such as monoclonal antibodies, antibody drug conjugates or cytokines is unknown. Additional work is needed to prospectively demonstrate the suitability of 1/6 HNSTD for start dose determination for large biological agents and immunotherapies. Several FDA approved agents, such as bevacizumab, imatinib and vismodegib, did not have a MTD established in the phase I setting. Instead, endpoints of PK and/or PD were used to determine the RP2D. The application of biological parameters to assist determination of starting doses of MTAs has not been validated. If a range of biologically active doses could be predicted from preclinical models, using PK or PD endpoints, this information could be applied alongside preclinical toxicology data to inform starting dose decisions. This binomial approach has the potential to reduce the number of dose escalations while preventing patients from being treated at overly toxic doses that lack incremental biological activity. This approach may be particularly valuable for drugs that do not have MTD defined in animals and are unlikely to reach MTD in humans, and could represent an alternative to 1/6 HNSTD or LD10 in determining the human starting dose.
4. Patient selection
4.1. Current models
Traditionally, phase I clinical trials enrol patients with all disease types who have exhausted available anti‐cancer therapy. The advent of rapid, inexpensive genomic testing presents an opportunity to design biomarker‐driven trials. Since some MTAs are only active in patients with corresponding molecular abnormalities, accurate selection of the study population will spare those who are unlikely to respond from the toxicities of investigational MTAs or immunotherapies. The strategy of selecting patients for phase I trials based on genomic aberrations is mostly unproven, although trials employing vemurafenib in BRAF V600E mutant melanomas (Flaherty et al., 2010) and imatinib in BCR‐ABL‐positive CML (Druker et al., 2001) have successfully utilized an enrichment approach. Various molecular profiling programs have been integrated into the clinic with the promise of enriching trials with patients of certain molecular subtypes (Bedard et al., 2013). Furthermore, some molecular profiling programs that utilized genotype‐matched therapies have demonstrated improved response rates and clinical outcomes (Tsimberidou et al., 2012; Von Hoff et al., 2010). However a recent publication demonstrated no significant clinical benefit in molecularly profiled chemorefractory colorectal cancer patients matched to treatments (Dienstmann et al., 2012).
4.2. Limitations and optimization
Large‐scale population sequencing initiatives have identified multiple genomic aberrations that drive oncogenesis and may act as treatment targets. Apart from a small number of recurrently mutated genes, such as BRAF in melanoma or PIK3CA in breast cancer, the majority of these abnormalities have been reported with low frequency (Agrawal et al., 2011; Jones et al., 2008; Parsons et al., 2008). Therefore, the identification of a sufficient number of patients with a specific molecular aberration can significantly slow clinical trial accrual. This may also hinder the optimal detection and evaluation of treatment related adverse effects, if each investigator site only treats a small number of patients. In these cases multi‐institutional studies with frequent communications between investigator sites should ameliorate these limitations. Additionally, technological advances may permit genomic profiling on smaller or scant tumor samples with lower quality DNA and thus increase the number of patients who would be eligible for testing.
Typically tumor from an archived diagnostic biopsy or surgical specimen is used for molecular characterization. However these assessments may be limited by geographic or temporal intra‐tumor heterogeneity. Geographic heterogeneity due to spatial variations in molecular aberrations has been demonstrated within a single tumor, or between different lesions (e.g. between primary tumor and metastases, or between different metastases). Temporal heterogeneity and clonal evolution represent molecular changes that emerge over time (Campbell et al., 2010; Gerlinger et al., 2012; Shah et al., 2012; Walter et al., 2012). Consequently using only a biopsy or archived surgical specimen may fail to detect treatment resistant or low frequency subclones and may not provide the complete and current molecular landscape of a patient's tumor. There is no standardized approach for evaluating intra‐tumor heterogeneity. Multiple tumor biopsies, ultra deep sequencing and non‐invasive tumor imaging could potentially overcome the limitations of geographic heterogeneity. To address the problem of clonal evolution, patient enrollment should be restricted to those who have had a recent tumor biopsy. In addition, there are emerging techniques to detect and molecularly characterize circulating tumor cells or free tumor DNA, with the hope of dynamically capturing relevant genomic aberrations in a non‐invasive manner. While it may not be appropriate to attempt these types of assessment in a phase I study, it does pose a barrier which must be addressed when developing biomarker studies.
5. Dose escalation
5.1. Current models
The traditional 3 + 3 study design remains widely employed in phase I oncology trials. However, model‐based escalation designs have been promoted as more accurate and efficient in defining RP2D. The continual reassessment method (CRM) was the original model‐based design and it has been modified into several subsequent schemes such as modified CRM, escalation with overdose control (EWOC) and time to event CRM (TITE‐CRM) to improve patient safety and prevent overestimation of the RP2D (Babb et al., 1998; Cheung and Chappell, 2000; Goodman et al., 1995; O'Quigley et al., 1990). Although model‐based escalation studies are thought to be labor and cost intensive, it can be highly efficient and safer in the appropriate setting. Although there is no head to head comparison of rule‐ versus model‐based designs in terms of efficiency, a recent review demonstrated the mean number of patients exposed to doses exceeding the MTD was at least twice as high in trials using a standard “3 + 3” compared to model‐based designs, indicating the efficiency of model‐based design (Le Tourneau et al., 2012).
5.2. Limitations and optimization
Despite their potential advantages model‐based designs have not been routinely incorporated into phase I trials. A review of 1235 phase I clinical trials revealed only 20 trials (1.6%) had utilized model‐based strategies (Rogatko et al., 2007). Beyond accuracy of RP2D determination and safety, chronic or delayed toxicities present a particular challenge to dose escalation. Modifications to model‐based methods may account for cumulative adverse events resulting in a more accurate determination of the RP2D. Several designs have been proposed such as TITE‐CRM, mixed effect proportional odds model (POM) and fractional dose finding (see Table 2) (Doussau et al., 2013) (Yin et al., 2013). TITE‐CRM has been reported to more accurately define the MTD without exposing patients to excessive toxicity when compared with the standard 3 + 3 method, provided that preclinical data do not unacceptably underestimate the side effects (Normolle and Lawrence, 2006). Currently only TITE‐CRM has been used in early phase clinical trials with mixed results (Desai et al., 2007; Muler et al., 2004; Tevaarwerk et al., 2012). The limited application of these other designs may be due to the need for considerable biostatistical expertise, resources and software. This infrastructure may not be available or readily accessible at many institutions, and thus precludes the wider implementation of novel model‐based methods. Furthermore, in most model‐based designs substantial preclinical evidence is required to estimate the potential dose toxicity curve prior to study commencement and this may not always be available. Where scant preclinical evidence exists to estimate the dose toxicity relationship, or the expected level of drug toxicity is high, a conventional 3 + 3 rule‐based design may be more appropriate. However for agents that are expected to have minimal toxicities or that demonstrate few adverse events in animal testing, a rapid dose escalation with either a model‐ or accelerated rule‐based method may be safely employed as an alternative.
In the absence of toxicity endpoints, determining the optimal biologically active dose (OBD) is an attractive alternative but remains challenging. This would require incorporation of endpoints such as PK, PD or functional imaging as part of dose escalation, necessitating serial collections of blood and tumor tissue or imaging such as positron emission tomography (PET). Pharmacokinetically guided dose escalation (PGDE) utilizes real‐time PK measurements to determine dose increments and thus would be an appealing approach for molecules where MTD cannot be determined. However PGDE is resource intensive, preventing widespread application into phase I trials. Assessing PD markers as primary endpoints in dose escalation phases of early stage trials requires a strong scientific rationale, and a reliable, reproducible assay. Developing non‐invasive assays or functional imaging such as PET scans may permit PD markers to be tracked easily with minimal harm to the patient. Currently no single dose escalation scheme has been shown superior for assessing OBD.
6. Dose expansion
6.1. Current models
Expansion cohorts in early phase clinical trials have been used to improve the volume and quality of data by enrolling additional subjects at the RP2D. A recent publication revealed a significant increase in expansion cohorts in single agent phase I trials, from 12% in 2006 to 38% in 2011, particularly in multi‐institutional trials and with MTAs (Manji et al., 2013). The most common reasons for undertaking a dose expansion cohort were safety (80%), efficacy (45%), PK assessment (28%), PD assessment (23%) and patient enrichment (14%). The majority of expansion cohorts provided meaningful new safety data, even leading to a modification of RP2D in some of the studies (Manji et al., 2013). In light of the benefits of expansion cohorts for adverse event identification and dose determination their use in early phase clinical trials will likely increase.
6.2. Limitations and optimization
Larger sample sizes increase the chance of identifying relevant toxicities of an investigational agent, thus permitting improved modification of the dose‐defining process. The probability of observing an adverse event is strengthened with an increased sample size, for example sample sizes of 57–82 patients have a 90–99% probability of identifying an adverse event that occurs in 5% of patients (DeMichele et al., 2013). Therefore the addition of expansion cohorts could improve the safety of a trial. However, with a requirement for larger numbers of patients the study is more likely to be performed across multiple institutions, with the requisite limitations that can occur with multi‐institutional trials. As well designed dose expansion cohorts could be used to guide go‐no go decision, these “tail” additions to phase I studies should also have clearly stated objectives, justification for number of patients accrued and a strong scientific rationale for any pre‐determined biological endpoints or biomarkers.
7. Endpoints in early phase clinical trials
7.1. Current models
Scant clinical data exist on the accuracy at which phase I clinical trials identify the RP2D and capture toxicities. The RP2D has typically been determined using MTD, a toxicity‐based endpoint. Increasingly biological endpoints are also taken into consideration (Table 3). Dosing to MTD with cytotoxic agents is based on preclinical data demonstrating that higher drug exposure increases tumor cell death, which in turn correlates with improved clinical efficacy (Sleijfer and Wiemer, 2008). MTD was used to define the RP2D in 77% of phase I trials of cytotoxic treatments compared to only 58% of MTA phase I trials (Fontes Jardim et al., 2014). In the event that an MTD is identified, non‐toxicity measures, such as PK or PD parameters, can verify that this dose is below, equivalent to, or exceeds the OBD. Non‐toxicity endpoints are typically incorporated as secondary or exploratory objectives and can be used to define the most biologically active dose when MTD is not reached. If MTD or OBD cannot be determined concerns should be raised about the drug's mechanism of action, and further developmental plans of the drug should be revisited.
Table 3.
Basis for RP2D and important toxicities of FDA approved MTAs in solid tumors.
| Drug | Basis for RP2D | Select adverse events |
|---|---|---|
| Imatinib | PK/PD | Rash, edema, decreased LVEF, myelosuppression, myalgias and arthralgias |
| Trastuzumab | PK | Cardiomyopathy, asthenia, fever, chills |
| Pertuzumab | PK | Diarrhea, fatigue, nausea, anemia |
| Lapatinib | Toxicity + efficacy | Decreased LVEF, rash, hand‐foot syndrome, diarrhea, elevated LFTs. |
| Erlotinib | Toxicity | Acneiform rash, diarrhea, interstitial lung disease |
| Gefitinib | PK + efficacy | Acneiform rash, diarrhea, interstitial lung disease |
| Cetuximab | PK | Acneiform rash, nail changes, diarrhea, hypomagnesemia, interstitial lung disease |
| Panitumumab | PK/PD | Acneiform rash, diarrhea, hypomagnesemia, hypocalcaemia, interstitial lung disease |
| Temsirolimus | Efficacy | Emesis, myelosuppression, dyslipidemia, diarrhea, rash and nephrotoxicity |
| Everolimus | PK/PD | Mucositis, rash, electrolyte abnormalities, dyslipidemia, diarrhea, pneumonitis, peripheral edema |
| Vemurafenib | Toxicity | Arthralgias, rash, squamous cell ca, keratocanthomas |
| Crizotinib | Toxicity | Nausea, vomiting, diarrhea, hepatotoxicity |
| Aflibercept | Toxicity + PK | Neutropenia, diarrhea, hypertension, eye irritation or visual disturbance |
| Bevacizumab | PK | Hypertension, thromboembolism, gastrointestinal perforation, poor wound healing |
| Sorafenib | Toxicity | Hypertension, rash, hand‐foot syndrome, diarrhea, emesis, myelosuppression, delayed wound healing, hypophosphatemia |
| Sunitinib | Toxicity | Hypertension, emesis, myelosuppression, hypothyroidism, adrenal dysfunction, decreased LVEF, yellow skin discolouration and mucositis |
| Pazopanib | PK/PD + efficacy | Fatigue, increased LFTs, diarrhea, hypothyroidism |
| Regorafenib | Toxicity + PK/PD | Hypertension, hand foot syndrome and diarrhea |
| Cabazantinib | Toxicity + efficacy | Hand foot syndrome and mucositis |
| Vandetanib | Toxicity and PK | QTc prolongation, diarrhea, asthenia and fatigue |
| Ipilimumab | Efficacy | Autoimmune colitis, dermatitis and hepatitis. Various endocrinopathies. |
| Enzalutamide | Toxicity | Fatigue, diarrhea, flushing, edema |
| Axitinib | Toxicity | Diarrhea, hypertension, weight decrease, anorexia |
| Vismodegib | PK | Muscle spasms, alopecia, dysgeusia, weight loss, fatigue |
LFTs, liver function tests; LVEF, left ventricular ejection fraction; PK, pharmacokinetic; PD, pharmacodynamic.
7.2. Toxicity as an endpoint: limitations and optimization
Conventionally DLTs occurring in the first cycle of treatment guide dose escalation decisions. The occurrence of delayed toxicities is particularly relevant in the context of continuous dosing for MTAs or maintenance schedules of immunotherapies. In a review from two European Union drug development programs of 445 patients on 36 phase I MTA trials, half the patients experienced their most severe toxicity after the DLT assessment period and 50% of grade ≥3 adverse events occurred after the first cycle (Postel‐Vinay et al., 2011; Sophie Postel‐Vinay, 2013). Beyond these severe events, chronic low grade toxicities are equally important and can affect the overall tolerability of a drug at a particular dose or schedule. The DLT‐TARGETT initiative, led by the European Organisation for Research and Treatment and Cancer, evaluated over 2000 patients on 54 single agent phase I MTA trials and reported that a significant proportion of patients had a dose reduction for grade ≤2 toxicities as early as cycle 1 (Sophie Postel‐Vinay, 2013). To enable a more accurate assessment of the RP2D, all available information from phase I trials should be incorporated upon their completion, including high grade toxicities and DLTs observed beyond the first cycle, as well as lower grade toxicities leading to significant reductions of relative dose intensity. This integration of information occurs at the end of phase I trials. In contrast, dose escalation or de‐escalation decisions between dose levels should be based on toxicities observed in the first cycle to maintain trial efficiency.
The side effect profile of MTAs can be markedly different from traditional chemotherapies (Table 3). Investigators must be vigilant when examining for more unusual toxicities, such as ocular changes with MEK inhibitors (Renouf et al., 2012) and dermatological events with BRAF inhibitors (Belum et al., 2013). Immunotherapy related toxicities are sometimes subtle and go unrecognized, such as hypophysitis. They can also lead to potentially life threatening situations such as adrenalitis or severe colitis (Arnheiter et al., 1996; Corsello et al., 2013). Management may be challenging, often requiring prolonged corticosteroids, hormone replacement therapy and additional immunosuppressive therapy. Frequent thorough clinical examinations by experienced investigators and pertinent laboratory tests are necessary to evaluate these toxicities. Photography, skin biopsies and urgent radiological studies may be required. Regular interactions between investigators from all participating sites should enable early recognition of complex patterns of toxicity. Prompt involvement of other medical specialities, such as ophthalmology and endocrinology, is essential for optimal long term management of these conditions.
7.3. PK and PD endpoints: limitations and optimization
In contrast to cytotoxic agents, MTAs do not necessarily have a monotonic dose toxicity relationship. A recent study examined 24 phase I MTA trials demonstrating antitumor activity at levels ≤25% of the MTD (Jain et al., 2010). Therefore further investigation of targeted agents and inclusion of biological endpoints may aid in the identification of lower, well tolerated yet active dose levels. However several barriers prohibit the implementation of PK and PD assays as endpoints, including intensive serial blood draws, fresh tumor procurement, stringent companion diagnostics, and lack of definitive correlation between target inhibition in PK or PD biomarkers and clinical efficacy. PK and PD assays are typically not tested rigorously in relevant preclinical tumor models, and the relationships between PK, PD and tumor growth modulation in such models are often not well characterized. With these limitations, the role of these assays in phase I trials frequently remains exploratory, as they lack the validity to define the RP2D or OBD. Importantly, the strategy of using a nontoxicity endpoint is only feasible for drugs that have a suitable PK threshold or PD marker identified. Strong scientific rationale for analyzing biological endpoints is required both to balance the risk of harm to patients undergoing biopsy or other invasive procedures, and to prevent unnecessary exclusion of patients who have inaccessible tumors.
PK studies examining different formulations or dosing schedules of drugs should be encouraged as they may reveal a more optimal route or administration frequency that yields biologically relevant exposures. Studies investigating drug–drug interactions and effects on food consumption evaluate pharmacologic properties of new drugs in the presence of other substances. Furthermore, the conduct of phase I studies in ethnically unique populations and those with organ dysfunction are important to verify PK variations in these groups. The inclusion of PK and PD endpoints in early phase trials is informative, however in situations whereby these biological endpoints are attained without reaching DLT, dose escalation should continue, unless there is compelling evidence that these assays are highly predictive of antitumor activity. For agents which do not reach toxicity based MTD, the utility of target specific biological endpoints becomes essential.
8. Study logistics
8.1. Current model
Early phase clinical trials are becoming increasingly complex, due to larger patient numbers, multi‐institutional involvement, extensive correlative components, and intricate study protocols, schedules and data collection. Considerable manpower and resources are required for the successful performance of a study, including study coordinators, nurses, doctors, pharmacists, radiologists and translational scientists. Concern for patient safety in clinical trials has resulted in strict regulatory requirements. To manage this, the trial sponsor, national and local regulatory bodies oversee the conduct of clinical trials to ensure the safety of their participants. The costs to undertake these trials have escalated in the context of reduced health care budgets, therefore participating institutions are under pressure to manage their resources even more efficiently (James et al., 2011; Roche et al., 2002).
8.2. Limitations and optimization
Increasing trial complexity, particularly with multi‐institutional trials, may lead to problems with fewer patients being treated at one center, resulting in individual investigators having less familiarity with toxicities with certain therapies. Isolated symptoms may only be recognized as being related to therapy when awareness is raised for the same toxicity or pattern in subsequent patients (Dowlati, 2009). Low grade toxicities are generally not reported in real time, and thus may not be appreciated to the same degree at each institution. Therefore when a new drug is tested in dose escalation, a limited number of investigators and centres may be preferable. In this way, investigators would develop a broader understanding of acute and possibly chronic toxicities and their collective experience and expertise could be used to inform those participating in the expansion phase of the study.
Extensive detailed data collection is required for early stage trials however this can increase the costs of the trial and lead to reduced trial efficiency. To improve recording and reporting of AEs computerized platforms are now available which allow the study site to enter data directly into electronic data capture systems, allowing the monitor to remotely view the data as soon as they are submitted. The growing use of electronic medical records could facilitate the sharing of source documents, accessed electronically in a secure manner over the internet, which may permit remote access for source data verification (Uren et al., 2013). Close collaboration between industry and academia is essential for successful completion of early stage trials. Comprehensive preclinical data and detailed information about proposed clinical biological endpoints and potential toxicology effects should be freely available from the sponsor of the trial. Frequent sharing of experiences between industry and site investigators supports timely communication and distribution of information, whether this be in person or via a teleconference. Regular meetings within a study centre are also crucial to ensure information about trial participants is dispersed in a timely and detailed manner.
9. Conclusions
Conventional methods of drug development are not sustainable with only 5% of agents demonstrating anticancer activity in preclinical testing eventually being approved for clinical use (Hutchinson and Kirk, 2011). Capitalizing on advances in preclinical testing with GEMMs, patient derived xenografts and co‐clinical oncology models will allow for a better assessment of drug target engagement, potential identification of predictive biomarkers and greater knowledge of expected toxicities. Improved starting dose selection and incorporation of PK and PD parameters may safely reduce the number of escalations required to reach MTD or MAD. Biomarker driven studies or enrichment strategies will enable preclinical hypothesis to be tested to facilitate go/no‐go decisions about a drug's development. Model‐based escalation designs have the potential to accurately define the dose toxicity curve, safely estimate dose increments and consequently determine the RP2D.
For agents where an MTD is not reached, collecting PK and PD measures will be critical to determine the OBD. Even when a therapy has an MTD, biological parameters should be examined. Often the OBD cannot be determined until later phase clinical trials, however the PK and PD information already collected can contribute to a thorough understanding of the drug's mechanism of action. Accounting for delayed and cumulative toxicity may refine the current method of dose determination, which relies solely on DLT frequency in an initial phase of treatment. Greater collaborations between academia and industry, to share information and make publicly available clinically annotated databases could enhance the development of experimental agents. Harnessing innovations in technology and trial design will permit rationale development of MTAs to ensure efficacious drugs reach the clinic and prevent valuable resources from being consumed by ineffective therapies.
Cook Natalie, Hansen Aaron R., Siu Lillian L., Abdul Razak Albiruni R., (2015), Early phase clinical trials to identify optimal dosing and safety, Molecular Oncology, 9, doi: 10.1016/j.molonc.2014.07.025.
References
- Agoram, B.M. , 2009. Use of pharmacokinetic/pharmacodynamic modelling for starting dose selection in first-in-human trials of high-risk biologics. Br. J. Clin. Pharmacol. 67, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal, N. , Frederick, M.J. , Pickering, C.R. , Bettegowda, C. , Chang, K. , Li, R.J. , Fakhry, C. , Xie, T.X. , Zhang, J. , Wang, J. , Zhang, N. , El-Naggar, A.K. , Jasser, S.A. , Weinstein, J.N. , Trevino, L. , Drummond, J.A. , Muzny, D.M. , Wu, Y. , Wood, L.D. , Hruban, R.H. , Westra, W.H. , Koch, W.M. , Califano, J.A. , Gibbs, R.A. , Sidransky, D. , Vogelstein, B. , Velculescu, V.E. , Papadopoulos, N. , Wheeler, D.A. , Kinzler, K.W. , Myers, J.N. , 2011. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 333, 1154–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnheiter, H. , Frese, M. , Kambadur, R. , Meier, E. , Haller, O. , 1996. Mx transgenic mice–animal models of health. Curr. Top. Microbiol. Immunol. 206, 119–147. [DOI] [PubMed] [Google Scholar]
- Babb, J. , Rogatko, A. , Zacks, S. , 1998. Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat. Med. 17, 1103–1120. [DOI] [PubMed] [Google Scholar]
- Bedard, P.L. , Hansen, A.R. , Ratain, M.J. , Siu, L.L. , 2013. Tumour heterogeneity in the clinic. Nature. 501, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley, C.G. , Ellis, L.M. , 2012. Drug development: raise standards for preclinical cancer research. Nature. 483, 531–533. [DOI] [PubMed] [Google Scholar]
- Belum, V.R. , Fischer, A. , Choi, J.N. , Lacouture, M.E. , 2013. Dermatological adverse events from BRAF inhibitors: a growing problem. Curr. Oncol. Rep. 15, 249–259. [DOI] [PubMed] [Google Scholar]
- Campbell, P.J. , Yachida, S. , Mudie, L.J. , Stephens, P.J. , Pleasance, E.D. , Stebbings, L.A. , Morsberger, L.A. , Latimer, C. , McLaren, S. , Lin, M.L. , McBride, D.J. , Varela, I. , Nik-Zainal, S.A. , Leroy, C. , Jia, M. , Menzies, A. , Butler, A.P. , Teague, J.W. , Griffin, C.A. , Burton, J. , Swerdlow, H. , Quail, M.A. , Stratton, M.R. , Iacobuzio-Donahue, C. , Futreal, P.A. , 2010. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 467, 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Cheng, K. , Walton, Z. , Wang, Y. , Ebi, H. , Shimamura, T. , Liu, Y. , Tupper, T. , Ouyang, J. , Li, J. , Gao, P. , Woo, M.S. , Xu, C. , Yanagita, M. , Altabef, A. , Wang, S. , Lee, C. , Nakada, Y. , Pena, C.G. , Sun, Y. , Franchetti, Y. , Yao, C. , Saur, A. , Cameron, M.D. , Nishino, M. , Hayes, D.N. , Wilkerson, M.D. , Roberts, P.J. , Lee, C.B. , Bardeesy, N. , Butaney, M. , Chirieac, L.R. , Costa, D.B. , Jackman, D. , Sharpless, N.E. , Castrillon, D.H. , Demetri, G.D. , Janne, P.A. , Pandolfi, P.P. , Cantley, L.C. , Kung, A.L. , Engelman, J.A. , Wong, K.K. , 2012. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature. 483, 613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, Y.K. , Chappell, R. , 2000. Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics. 56, 1177–1182. [DOI] [PubMed] [Google Scholar]
- Chiu, C.W. , Nozawa, H. , Hanahan, D. , 2010. Survival benefit with proapoptotic molecular and pathologic responses from dual targeting of mammalian target of rapamycin and epidermal growth factor receptor in a preclinical model of pancreatic neuroendocrine carcinogenesis. J. Clin. Oncol.: Official J. Am. Soc. Clin. Oncol. 28, 4425–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clohessy, J.G. , de Stanchina, E. , 2013. Infrastructure needs for translational integration of mouse and human trials. Cold Spring Harbor Protoc. 2013, [DOI] [PubMed] [Google Scholar]
- Committee for Medicinal Products for Human use (CHMP), 2007. Guideline on Strategies to Identify and Mitigate Risks for First-in-human Clinical Trials with Investigational Medicinal Products. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsello, S.M. , Barnabei, A. , Marchetti, P. , De Vecchis, L. , Salvatori, R. , Torino, F. , 2013. Endocrine side effects induced by immune checkpoint inhibitors. J. Clin. Endocrinol. Metab. 98, 1361–1375. [DOI] [PubMed] [Google Scholar]
- DeMichele, A. , Berry, D.A. , Zujewski, J. , Hunsberger, S. , Rubinstein, L. , Tomaszewski, J.E. , Kelloff, G. , Perlmutter, J. , Buxton, M. , Lyandres, J. , Albain, K.S. , Benz, C. , Jo Chien, A. , Haluska, P. , Leyland-Jones, B. , Liu, M.C. , Munster, P. , Olopade, O. , Park, J.W. , Parker, B.A. , Pusztai, L. , Tripathy, D. , Rugo, H. , Yee, D. , Esserman, L. , 2013. Developing safety criteria for introducing new agents into neoadjuvant trials. Clin. Cancer Res. 19, 2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai, S.P. , Ben-Josef, E. , Normolle, D.P. , Francis, I.R. , Greenson, J.K. , Simeone, D.M. , Chang, A.E. , Colletti, L.M. , Lawrence, T.S. , Zalupski, M.M. , 2007. Phase I study of oxaliplatin, full-dose gemcitabine, and concurrent radiation therapy in pancreatic cancer. J. Clin. Oncol. 25, 4587–4592. [DOI] [PubMed] [Google Scholar]
- Dienstmann, R. , Serpico, D. , Rodon, J. , Saura, C. , Macarulla, T. , Elez, E. , Alsina, M. , Capdevila, J. , Perez-Garcia, J. , Sanchez-Olle, G. , Aura, C. , Prudkin, L. , Landolfi, S. , Hernandez-Losa, J. , Vivancos, A. , Tabernero, J. , 2012. Molecular profiling of patients with colorectal cancer and matched targeted therapy in phase I clinical trials. Mol. Cancer Ther. 11, 2062–2071. [DOI] [PubMed] [Google Scholar]
- Doussau, A. , Thiébaut, R. , Paoletti, X. , 2013. Dose-finding design using mixed-effect proportional odds model for longitudinal graded toxicity data in phase I oncology clinical trials. Stat. Med. 32, 5430–5447. [DOI] [PubMed] [Google Scholar]
- Dowlati, A. , 2009. Controversies in multi-institutional phase I clinical trials. Clin. Adv. Hematol. Oncol.: H&O. 7, 518–520. [PubMed] [Google Scholar]
- Druker, B.J. , Talpaz, M. , Resta, D.J. , Peng, B. , Buchdunger, E. , Ford, J.M. , Lydon, N.B. , Kantarjian, H. , Capdeville, R. , Ohno-Jones, S. , Sawyers, C.L. , 2001. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 344, 1031–1037. [DOI] [PubMed] [Google Scholar]
- Flaherty, K.T. , Puzanov, I. , Kim, K.B. , Ribas, A. , McArthur, G.A. , Sosman, J.A. , O'Dwyer, P.J. , Lee, R.J. , Grippo, J.F. , Nolop, K. , Chapman, P.B. , 2010. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363, 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes Jardim, D.L. , Hess, K.R. , LoRusso, P.M. , Kurzrock, R. , Hong, D.S. , 2014. Predictive value of phase I trials for safety in later trials and final approved dose: analysis of 61 approved cancer drugs. Clin. Cancer Res. 20, (2) 281–288. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration, H.H.S, 2010. International conference on harmonisation; guidance on S9 nonclincal evaluation for anticancer pharmaceuticals; availability. Notice. Federal Register. 75, 10487–10488. [PubMed] [Google Scholar]
- Frese, K.K. , Tuveson, D.A. , 2007. Maximizing mouse cancer models. Nat. Rev. Cancer. 7, 645–658. [DOI] [PubMed] [Google Scholar]
- Gerlinger, M. , Rowan, A.J. , Horswell, S. , Larkin, J. , Endesfelder, D. , Gronroos, E. , Martinez, P. , Matthews, N. , Stewart, A. , Tarpey, P. , Varela, I. , Phillimore, B. , Begum, S. , McDonald, N.Q. , Butler, A. , Jones, D. , Raine, K. , Latimer, C. , Santos, C.R. , Nohadani, M. , Eklund, A.C. , Spencer-Dene, B. , Clark, G. , Pickering, L. , Stamp, G. , Gore, M. , Szallasi, Z. , Downward, J. , Futreal, P.A. , Swanton, C. , 2012. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, S.N. , Zahurak, M.L. , Piantadosi, S. , 1995. Some practical improvements in the continual reassessment method for phase I studies. Stat. Med. 14, 1149–1161. [DOI] [PubMed] [Google Scholar]
- Gutmann, D.H. , Hunter-Schaedle, K. , Shannon, K.M. , 2006. Harnessing preclinical mouse models to inform human clinical cancer trials. J. Clin. Invest. 116, 847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haibe-Kains, B. , El-Hachem, N. , Birkbak, N.J. , Jin, A.C. , Beck, A.H. , Aerts, H.J. , Quackenbush, J. , 2013. Inconsistency in large pharmacogenomic studies. Nature. 504, 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo, M. , Bruckheimer, E. , Rajeshkumar, N.V. , Garrido-Laguna, I. , De Oliveira, E. , Rubio-Viqueira, B. , Strawn, S. , Wick, M.J. , Martell, J. , Sidransky, D. , 2011. A pilot clinical study of treatment guided by personalized tumorgrafts in patients with advanced cancer. Mol. Cancer Ther. 10, 1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson, L. , Kirk, R. , 2011. High drug attrition rates–where are we going wrong? Nature reviews. Clin. Oncol. 8, 189–190. [DOI] [PubMed] [Google Scholar]
- Jain, R.K. , Lee, J.J. , Hong, D. , Markman, M. , Gong, J. , Naing, A. , Wheler, J. , Kurzrock, R. , 2010. Phase I oncology studies: evidence that in the era of targeted therapies patients on lower doses do not fare worse. Clin. Cancer Res. 16, 1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, P. , Bebee, P. , Beekman, L. , Browning, D. , Innes, M. , Kain, J. , Royce-Westcott, T. , Waldinger, M. , 2011. Effort tracking metrics provide data for optimal budgeting and workload management in therapeutic cancer clinical trials. J. Natl. Compr. Cancer Netw.: JNCCN. 9, 1343–1352. [DOI] [PubMed] [Google Scholar]
- Johnson, J.I. , Decker, S. , Zaharevitz, D. , Rubinstein, L.V. , Venditti, J.M. , Schepartz, S. , Kalyandrug, S. , Christian, M. , Arbuck, S. , Hollingshead, M. , Sausville, E.A. , 2001. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br. J. Cancer. 84, 1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, S. , Zhang, X. , Parsons, D.W. , Lin, J.C. , Leary, R.J. , Angenendt, P. , Mankoo, P. , Carter, H. , Kamiyama, H. , Jimeno, A. , Hong, S.M. , Fu, B. , Lin, M.T. , Calhoun, E.S. , Kamiyama, M. , Walter, K. , Nikolskaya, T. , Nikolsky, Y. , Hartigan, J. , Smith, D.R. , Hidalgo, M. , Leach, S.D. , Klein, A.P. , Jaffee, E.M. , Goggins, M. , Maitra, A. , Iacobuzio-Donahue, C. , Eshleman, J.R. , Kern, S.E. , Hruban, R.H. , Karchin, R. , Papadopoulos, N. , Parmigiani, G. , Vogelstein, B. , Velculescu, V.E. , Kinzler, K.W. , 2008. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 321, 1801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Tourneau, C. , Gan, H.K. , Razak, A.R. , Paoletti, X. , 2012. Efficiency of new dose escalation designs in dose-finding phase I trials of molecularly targeted agents. PLoS One. 7, e51039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Tourneau, C. , Lee, J.J. , Siu, L.L. , 2009. Dose escalation methods in phase I cancer clinical trials. J. Natl. Cancer Inst. 101, 708–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Tourneau, C. , Stathis, A. , Vidal, L. , Moore, M.J. , Siu, L.L. , 2010. Choice of starting dose for molecularly targeted agents evaluated in first-in-human phase I cancer clinical trials. J. Clin. Oncol. 28, 1401–1407. [DOI] [PubMed] [Google Scholar]
- LoRusso, P.M. , Boerner, S.A. , Seymour, L. , 2010. An overview of the optimal planning, design, and conduct of phase I studies of new therapeutics. Clin. Cancer Res. 16, 1710–1718. [DOI] [PubMed] [Google Scholar]
- Manji, A. , Brana, I. , Amir, E. , Tomlinson, G. , Tannock, I.F. , Bedard, P.L. , Oza, A. , Siu, L.L. , Abdul Razak, A.R. , 2013. Evolution of clinical trial design in early drug development: systematic review of expansion cohort use in single-agent phase I cancer trials. J. Clin. Oncol. 31, (33) 4260–4267. [DOI] [PubMed] [Google Scholar]
- Morelli, M.P. , Calvo, E. , Ordonez, E. , Wick, M.J. , Viqueira, B.R. , Lopez-Casas, P.P. , Bruckheimer, E. , Calles-Blanco, A. , Sidransky, D. , Hidalgo, M. , 2012. Prioritizing phase I treatment options through preclinical testing on personalized tumorgraft. J. Clin. Oncol.: Official J. Am. Soc. Clin. Oncol. 30, e45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muler, J.H. , McGinn, C.J. , Normolle, D. , Lawrence, T. , Brown, D. , Hejna, G. , Zalupski, M.M. , 2004. Phase I trial using a time-to-event continual reassessment strategy for dose escalation of cisplatin combined with gemcitabine and radiation therapy in pancreatic cancer. J. Clin. Oncol. 22, 238–243. [DOI] [PubMed] [Google Scholar]
- Normolle, D. , Lawrence, T. , 2006. Designing dose-escalation trials with late-onset toxicities using the time-to-event continual reassessment method. J. Clin. Oncol. 24, 4426–4433. [DOI] [PubMed] [Google Scholar]
- O'Quigley, J. , Pepe, M. , Fisher, L. , 1990. Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics. 33–48. [PubMed] [Google Scholar]
- Ocana, A. , Pandiella, A. , Siu, L.L. , Tannock, I.F. , 2011. Preclinical development of molecular-targeted agents for cancer. Nature reviews. Clin. Oncol. 8, 200–209. [DOI] [PubMed] [Google Scholar]
- Parsons, D.W. , Jones, S. , Zhang, X. , Lin, J.C. , Leary, R.J. , Angenendt, P. , Mankoo, P. , Carter, H. , Siu, I.M. , Gallia, G.L. , Olivi, A. , McLendon, R. , Rasheed, B.A. , Keir, S. , Nikolskaya, T. , Nikolsky, Y. , Busam, D.A. , Tekleab, H. , Diaz, L.A. , Hartigan, J. , Smith, D.R. , Strausberg, R.L. , Marie, S.K. , Shinjo, S.M. , Yan, H. , Riggins, G.J. , Bigner, D.D. , Karchin, R. , Papadopoulos, N. , Parmigiani, G. , Vogelstein, B. , Velculescu, V.E. , Kinzler, K.W. , 2008. An integrated genomic analysis of human glioblastoma multiforme. Science. 321, 1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parulekar, W.R. , Eisenhauer, E.A. , 2004. Phase I trial design for solid tumor studies of targeted, non-cytotoxic agents: theory and practice. J. Natl. Cancer Inst. 96, 990–997. [DOI] [PubMed] [Google Scholar]
- Postel-Vinay, S. , Gomez-Roca, C. , Molife, L.R. , Anghan, B. , Levy, A. , Judson, I. , De Bono, J. , Soria, J.-C. , Kaye, S. , Paoletti, X. , 2011. Phase I trials of molecularly targeted agents: should we pay more attention to late toxicities?. J. Clin. Oncol. 29, 1728–1735. [DOI] [PubMed] [Google Scholar]
- Reiner, E. , Paoletti, X. , O'Quigley, J. , 1999. Operating characteristics of the standard phase I clinical trial design. Comput. Stat. Data Anal. 30, 303–315. [Google Scholar]
- Renouf, D.J. , Velazquez-Martin, J.P. , Simpson, R. , Siu, L.L. , Bedard, P.L. , 2012. Ocular toxicity of targeted therapies. J. Clin. Oncol.: Official J. Am. Soc. Clin. Oncol. 30, 3277–3286. [DOI] [PubMed] [Google Scholar]
- Roche, K. , Paul, N. , Smuck, B. , Whitehead, M. , Zee, B. , Pater, J. , Hiatt, M.A. , Walker, H. , 2002. Factors affecting workload of cancer clinical trials: results of a multicenter study of the national cancer institute of Canada clinical trials group. J. Clin. Oncol.: Official J. Am. Soc. Clin. Oncol. 20, 545–556. [DOI] [PubMed] [Google Scholar]
- Rogatko, A. , Schoeneck, D. , Jonas, W. , Tighiouart, M. , Khuri, F.R. , Porter, A. , 2007. Translation of innovative designs into phase I trials. J. Clin. Oncol. 25, 4982–4986. [DOI] [PubMed] [Google Scholar]
- Shah, S.P. , Roth, A. , Goya, R. , Oloumi, A. , Ha, G. , Zhao, Y. , Turashvili, G. , Ding, J. , Tse, K. , Haffari, G. , Bashashati, A. , Prentice, L.M. , Khattra, J. , Burleigh, A. , Yap, D. , Bernard, V. , McPherson, A. , Shumansky, K. , Crisan, A. , Giuliany, R. , Heravi-Moussavi, A. , Rosner, J. , Lai, D. , Birol, I. , Varhol, R. , Tam, A. , Dhalla, N. , Zeng, T. , Ma, K. , Chan, S.K. , Griffith, M. , Moradian, A. , Cheng, S.W. , Morin, G.B. , Watson, P. , Gelmon, K. , Chia, S. , Chin, S.F. , Curtis, C. , Rueda, O.M. , Pharoah, P.D. , Damaraju, S. , Mackey, J. , Hoon, K. , Harkins, T. , Tadigotla, V. , Sigaroudinia, M. , Gascard, P. , Tlsty, T. , Costello, J.F. , Meyer, I.M. , Eaves, C.J. , Wasserman, W.W. , Jones, S. , Huntsman, D. , Hirst, M. , Caldas, C. , Marra, M.A. , Aparicio, S. , 2012. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 486, 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikder, H. , Huso, D.L. , Zhang, H. , Wang, B. , Ryu, B. , Hwang, S.T. , Powell, J.D. , Alani, R.M. , 2003. Disruption of Id1 reveals major differences in angiogenesis between transplanted and autochthonous tumors. Cancer Cell. 4, 291–299. [DOI] [PubMed] [Google Scholar]
- Simon, R. , Rubinstein, L. , Arbuck, S.G. , Christian, M.C. , Freidlin, B. , Collins, J. , 1997. Accelerated titration designs for phase I clinical trials in oncology. J. Natl. Cancer Inst. 89, 1138–1147. [DOI] [PubMed] [Google Scholar]
- Sleijfer, S. , Wiemer, E. , 2008. Dose selection in phase I studies: why we should always go for the top. J. Clin. Oncol.: Official J. Am. Soc. Clin. Oncol. 26, 1576–1578. [DOI] [PubMed] [Google Scholar]
- Sophie Postel-Vinay, E.R. , Le Tourneau, Christophe , Olmos, David , Massard, Christophe , Ivy, Percy , Seymour, Lesley , Siu, Lillian L. , Lacombe, Denis , Paoletti, Xavier , Kaye, Stan B. , Verweij, Jaap , Collette, Laurence , Soria, Jean-Charles , 2013. Towards New Methods for the Determination of Dose Limiting Toxicities and Recommended Dose of Molecularly Targeted Agents European Cancer Congress (ECCO-ESMO-ESTRO) Amsterdam: [Google Scholar]
- Storer, B.E. , 1989. Design and analysis of phase I clinical trials. Biometrics. 925–937. [PubMed] [Google Scholar]
- Tentler, J.J. , Tan, A.C. , Weekes, C.D. , Jimeno, A. , Leong, S. , Pitts, T.M. , Arcaroli, J.J. , Messersmith, W.A. , Eckhardt, S.G. , 2012. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 9, 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevaarwerk, A. , Wilding, G. , Eickhoff, J. , Chappell, R. , Sidor, C. , Arnott, J. , Bailey, H. , Schelman, W. , Liu, G. , 2012. Phase I study of continuous MKC-1 in patients with advanced or metastatic solid malignancies using the modified time-to-event continual reassessment method (TITE-CRM) dose escalation design. Invest. New Drugs. 30, 1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewski, J.E. , 2004. Multi-species toxicology approaches for oncology drugs: the US perspective. Eur. J. Cancer. 40, 907–913. [DOI] [PubMed] [Google Scholar]
- Tsimberidou, A.M. , Iskander, N.G. , Hong, D.S. , Wheler, J.J. , Falchook, G.S. , Fu, S. , Piha-Paul, S. , Naing, A. , Janku, F. , Luthra, R. , Ye, Y. , Wen, S. , Berry, D. , Kurzrock, R. , 2012. Personalized medicine in a phase I clinical trials program: the MD Anderson cancer center initiative. Clin. Cancer Res.: J. Am. Assoc. Cancer Res. 18, 6373–6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uren, S.C. , Kirkman, M.B. , Dalton, B.S. , Zalcberg, J.R. , 2013. Reducing clinical trial monitoring resource allocation and costs through remote access to electronic medical records. J. Oncol. Prac./Am. Soc. Clin. Oncol. 9, e13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hoff, D.D. , Stephenson, J.J. , Rosen, P. , Loesch, D.M. , Borad, M.J. , Anthony, S. , Jameson, G. , Brown, S. , Cantafio, N. , Richards, D.A. , Fitch, T.R. , Wasserman, E. , Fernandez, C. , Green, S. , Sutherland, W. , Bittner, M. , Alarcon, A. , Mallery, D. , Penny, R. , 2010. Pilot study using molecular profiling of patients' tumors to find potential targets and select treatments for their refractory cancers. J. Clin. Oncol.: Official J. Am. Soc. Clin. Oncol. 28, 4877–4883. [DOI] [PubMed] [Google Scholar]
- Walter, M.J. , Shen, D. , Ding, L. , Shao, J. , Koboldt, D.C. , Chen, K. , Larson, D.E. , McLellan, M.D. , Dooling, D. , Abbott, R. , Fulton, R. , Magrini, V. , Schmidt, H. , Kalicki-Veizer, J. , O'Laughlin, M. , Fan, X. , Grillot, M. , Witowski, S. , Heath, S. , Frater, J.L. , Eades, W. , Tomasson, M. , Westervelt, P. , DiPersio, J.F. , Link, D.C. , Mardis, E.R. , Ley, T.J. , Wilson, R.K. , Graubert, T.A. , 2012. Clonal architecture of secondary acute myeloid leukemia. N. Engl. J. Med. 366, 1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, J.C. , Shah, M.H. , Ito, T. , Bohas, C.L. , Wolin, E.M. , Van Cutsem, E. , Hobday, T.J. , Okusaka, T. , Capdevila, J. , de Vries, E.G. , Tomassetti, P. , Pavel, M.E. , Hoosen, S. , Haas, T. , Lincy, J. , Lebwohl, D. , Oberg, K. , Rad001 in Advanced Neuroendocrine Tumors, T.T.S.G2011. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 364, 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, G. , Zheng, S. , Xu, J. , 2013. Fractional dose-finding methods with late-onset toxicity in phase I clinical trials. J. Biopharm. Stat. 23, 856–870. [DOI] [PubMed] [Google Scholar]


