Abstract
Background
Patients with peritoneal surface disease (PSD) often present with synchronous hepatic involvement (HI). The impact of addressing the hepatic component during CRS/HIPEC on operative and survival outcomes is not clearly defined.
Methods
A prospective database of 1,067 procedures was reviewed based on primary tumor, performance status, resection status, type of liver involvement (superficial or parenchymal) and hepatic resection, morbidity, mortality, and overall survival.
Results
There were 108 (10 %) CRS/HIPEC procedures performed with synchronous liver debulking in 99 patients with PSD from 27 (33 %) appendiceal and 32 (39 %) colorectal primary lesions. Ninety percent of patients underwent subsegmental hepatic resection, whereas 22 % had disease with hepatic parenchymal involvement. Median intensive care unit (ICU) and hospital stay were 3.5 and 13.6 days, respectively. Clavien grade III/IV morbidity was similar for patients with or without resected HI (18.9 vs. 22.5 %; p = 0.39). The 30-day mortality rate was 6.5 and 2.8 % (p = 0.07) for patients with and without resected HI, respectively. The median survival for all patients with low-grade appendiceal cancer was 42.1 months with resected HI and 95.5 months without HI (p = 0.03). Median survival for colorectal cancer patients after complete cytoreduction was 21.2 months with HI versus 33.6 months without HI (p = 0.03).
Conclusions
Synchronous resection of limited HI does not increase the morbidity or mortality of CRS/HIPEC procedures. The survival benefit, although still meaningful, was less for patients with HI. Resectable low volume HI in patients with PSD from colon and appendiceal primary lesions should not be considered a contraindication for CRS/HIPEC procedures.
The completeness of cytoreduction has been shown to predict survival for patients with peritoneal surface disease (PSD) from a variety of epithelial malignancies.1–3 However, patients with synchronous PSD and hepatic involvement (HI) have been traditionally considered suboptimal candidates for cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC). This is mainly due to concerns that obtaining locoregional control of PSD with CRS/HIPEC in tumors that also deploy biologic machinery necessary for parenchymal invasion has an increased theoretical risk of systemic failure. In addition, the cumulative morbidity and toxicity derived from the addition of a liver resection to CRS/HIPEC could be prohibitive.
The primary aim of this manuscript was to determine the operation specific morbidity and mortality of non-anatomic resection of limited hepatic disease during CRS/HIPEC. The secondary aims were to determine the biologic impact of superficial vs. parenchymal HI on survival and to determine the risk of systemic failure for patients with PSD from appendiceal and colorectal primary lesions.
METHODS
Patients who had a hepatectomy as a component of their cytoreduction were identified in a prospectively maintained database of 1,067 CRS/HIPEC procedures performed between 1991 and 2013, and were compared to those patients who had CRS/HIPEC without a hepatic resection. The variables included in our analysis were Eastern Cooperative Oncology Group (ECOG) performance status, 4 type of primary malignancy, and completeness of cytoreduction. Operative morbidity and mortality were also reviewed. Institutional review board approval was obtained.
The preoperative evaluation included a history and physical examination, relevant serum tumor markers, and a computed tomographic scan of the chest, abdomen, and pelvis. Eligibility criteria for CRS/HIPEC included histologic or cytologic diagnosis of peritoneal carcinomatosis, resectable or resected primary lesion, debulkable peritoneal disease, absence of extraabdominal disease, and complete recovery from previous radiation or chemotherapy treatments. 5 Presumably resectable HI, whether superficial or parenchymal, was not considered a contraindication.6 Morbidity and mortality were graded according to the Clavien and Dindo classification system.7
HI was defined as either “superficial” for cases in which HI was not invading Glisson’s capsule or “parenchymal” for cases with parenchymal invasion. Parenchymal invasion could have occurred via hematogenous spread identified on preoperative cross sectional imaging or via direct invasion from intraperitoneal dissemination. Because metastasis in the periphery of the liver could have arisen from hematogenous spread or direct invasion, we did not attempt to distinguish between the two. The extent of the liver resection was determined by review of the operative record, and the volume of the resected liver was calculated based on measurements from gross pathology.
Descriptive statistics included frequency and percentage for categorical variables and mean, and standard deviation for continuous variables. Fisher’s exact tests and Wilcoxon rank sum tests were used to compare categorical and continuous variables, respectively. OS was estimated using the Kaplan–Meier (product-limit) method from the date of CRS/HIPEC (or first CRS/HIPEC for cases in which a patient underwent more than one procedure) to the date of death or last follow-up. Disease-free survival (DFS) was only estimated for patients who underwent a complete cytoreduction. The log-rank test compared survival between groups. Statistical significance was defined as a p value < 0.05. All analyses were performed using SAS 9.3 (Cary, NC).
RESULTS
Overall, 108 of 1,067 (10.1 %) of CRS/HIPEC procedures performed at our institution during the study period included a liver resection. The total 108 liver resections were performed in 99 patients for 27 (33 %) appendiceal primary lesions and 32 (39 %) colorectal primary lesions. Patient characteristics were compared between those who underwent CRS/HIPEC with and without liver resection (Table 1). Resection of HI was more common in patients with colorectal cancer (p = 0.003). All patients with HI from colorectal adenocarcinoma and high-grade appendiceal adenocarcinoma received first-line systemic chemotherapy with FOLFOX or FOLFIRI with or without bevacizumab prior to CRS/HIPEC. In addition, 31 % of these patients received second-line chemotherapy and another 13 % received third-line chemotherapy prior to CRS/HIPEC. The mean number of visceral resections (excluding hepatic resection) served as a marker for both disease volume and extent of resection. For colorectal cancer patients, the mean number of visceral resections was 3.3 for those with HI and 3.1 for those without HI (p = 0.008), and for appendiceal cancer patients, it was 3.8 with HI and 3.2 without HI (p = 0.06). When looking at all patients, the mean number of visceral resections was higher in patients with HI (3.6 vs. 3.1; p = 0.04), yet rates of complete cytoreduction in patients proceeding to CRS/HIPEC were similar between those with and without HI (42.2 vs. 45.7 %; p = 0.57).
TABLE 1.
Patient characteristics
| Characteristics | No hepatic involvement (n = 875) | Hepatic involvement (n = 99) | p value |
|---|---|---|---|
| Female, n (%) | 461 (52.7) | 59 (59.6) | 0.20 |
| Age, mean (SD) years | 52.7 (12.4) | 53.8 (12.6) | 0.42 |
| Diabetes, n (%) | 85 (10.2) | 6 (6.3) | 0.28 |
| Heart disease, n (%) | 71 (8.5) | 8 (8.4) | 1.00 |
| Lung disease, n (%) | 30 (3.6) | 6 (6.3) | 0.25 |
| Any smoking history, n (%) | 260 (31.9) | 31 (33.0) | 0.82 |
| Body mass index, mean (SD) | 27.7 (6.0) | 27.5 (6.0) | 0.58 |
| Albumin, mean (SD) | 3.7 (0.59) | 3.7 (0.62) | 0.88 |
| ECOG performance status | 0.38 | ||
| 0/1 | 724 (83.9) | 86 (87.8) | |
| 2+ | 139 (16.1) | 12 (12.2) | |
| Type of primary, n (%) | 0.003 | ||
| Appendiceal | 425 (48.7) | 27 (32.9) | |
| Colorectal | 201 (23.0) | 32 (39.02) | |
| Mesothelioma | 65 (7.5) | 4 (4.9) | |
| Ovarian | 63 (7.2) | 4 (4.9) | |
| Gastric | 42 (4.8) | 2 (2.4) | |
| Other | 77 (8.8) | 13 (15.9) | |
| Complete resection, n (%) (n = 952) | 397 (45.7) | 35 (42.2) | 0.57 |
HI hepatic involvement, SD standard deviation, ECOG Eastern Cooperative Oncology Group
Hepatic resection was limited to a subsegmental resection in 89.8 % (n = 97) of cases. In 31 (28.7 %) cases, more than one liver resection was performed, and the mean volume of liver parenchyma resected was 87.2 cm3. Parenchymal involvement was noted in 22.2 % (n = 24). Patients with appendiceal primary lesions and HI had superficial disease confined to the liver capsule in 93.3 %. The remaining 6.7 % of appendiceal primary lesions had parenchymal invasion and were all high-grade lesions. Yet most of the high-grade appendiceal HI (75 %) was superficial. We did not identify parenchymal disease with low-grade appendiceal (LGA) lesions. Patients with colorectal primary lesions were more likely to have parenchymal disease than patients with appendiceal primary lesions (37.5 vs. 6.7 %; p < 0.001).
There was no statistically significant difference in overall minor (Clavien grade I and II) or major (Clavien grade III and IV) morbidity identified between patients with and without resection of HI during cytoreduction (Table 2). Mortality was not significantly different for patients with and without resection of HI (30-day mortality 6.5 vs. 2.8 %, respectively; p = 0.07). When evaluating outcomes based on the type of HI (superficial vs. parenchymal) and type of liver resection (anatomical vs. subsegmental), we identified no differences in minor morbidity, major morbidity, mortality, or 30-day readmission.
TABLE 2.
Morbidity and mortality after cases of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for patients with or without hepatic involvement and partial hepatectomy
| No hepatic involvement (n = 957) | Hepatic involvement (n = 108) | p value | |
|---|---|---|---|
| Minor morbidity, n (%) | 342 (35.7) | 30 (27.8) | 0.11 |
| Major morbidity, n (%) | 215 (22.5) | 20 (18.5) | 0.39 |
| 30-Day mortality, n (%) | 27 (2.8) | 7 (6.5) | 0.07 |
| 30-Day readmission, n (%) | 354 (37.0) | 30 (27.8) | 0.07 |
| Operation time, mean (SD) hours | 8.5 (3.1) | 8.8 (3.2) | 0.41 |
| Length of hospital stay, mean (SD) days | 14.2 (16.2) | 13.6 (16.4) | 0.71 |
| Intensive care unit stay, mean (SD) days | 3.3 (9.0) | 3.5 (7.6) | 0.92 |
SD standard deviation
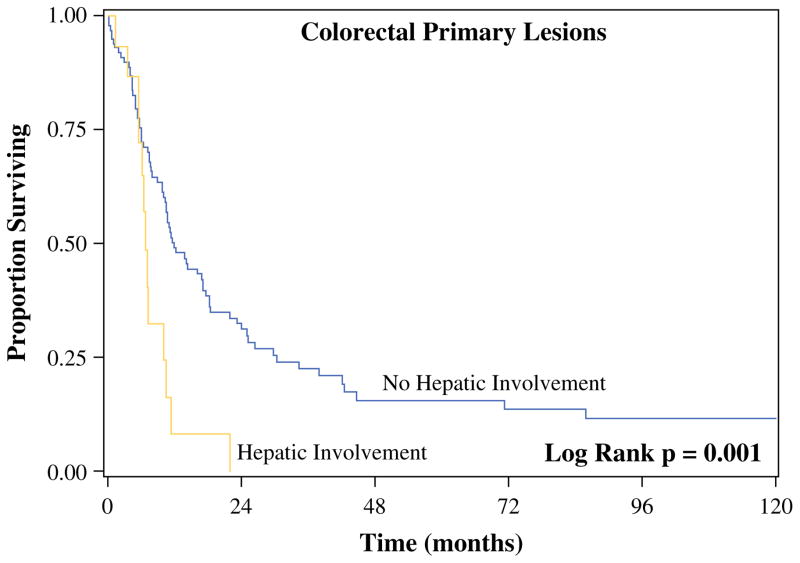
In the procedures in which a complete cytoreduction was obtained, recurrence rates were not significantly different for patients with colorectal primary lesions (64.7 vs. 53.3 %; p = 0.44) and LGA primary lesions (40.0 vs. 16.9 %; p = 0.22) based on the presence or absence of HI, respectively (Table 3). Of those patients with colon cancer and HI that recurred, only 12.5 % had high-grade lesions, whereas 71.9 % had lymph node involvement. Despite similar recurrence rates, recurrence occurred sooner in patients who had HI from colorectal primary lesions than in patients who did not have HI (DFS 6.8 vs. 12.0 months; p = 0.001) (Fig. 1). DFS after complete cytoreduction for patients with LGA primary lesions with and without HI was 118.9 versus 128.3 months, respectively (p = 0.23).
TABLE 3.
Disease recurrence after complete cytoreduction and hyperthermic intraperitoneal chemotherapy
| No hepatic involvement (n = 433) | Hepatic involvement (n = 37) | p value | |
|---|---|---|---|
| Recurrence, n/N (%) | |||
| Colorectal | 57/107 (53.3) | 11/17 (64.7) | 0.44 |
| Low-grade appendix | 20/118 (16.9) | 2/5 (40.0) | 0.22 |
| Median time to recurrence, months | |||
| Colorectal | 12.0 | 6.8 | 0.001 |
| Low-grade appendix | 128.3 | 118.9 | 0.23 |
| Site of recurrence, n/N (% of recurrences) | |||
| Liver | |||
| Colorectal | 22/57 (38.6) | 2/11 (18.2) | 0.30 |
| Low-grade appendix | 6/20 (30.0) | 1/2 (50.0) | 1.00 |
| Peritoneum | |||
| Colorectal | 21/57 (36.8) | 5/11 (45.5) | 0.74 |
| Low-grade appendix | 13/20 (65.0) | 1/2 (50.0) | 1.00 |
| Extra-abdominal | |||
| Colorectal | 14/57 (24.6) | 4/11 (36.4) | 0.46 |
| Low-grade appendix | 1/20 (5.0) | – | 1.00 |
FIG. 1.
Median disease-free survival for patients with colorectal cancer after cytoreductive surgery/hyperthermic intraperitoneal chemotherapy with complete cytoreduction
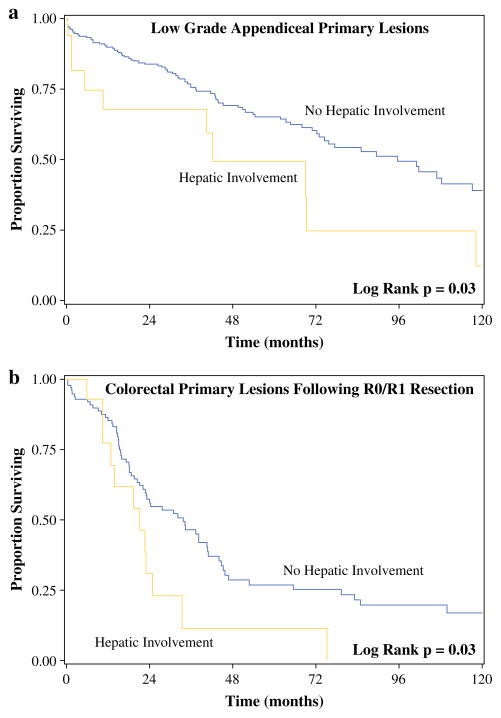
Median follow-up was 49.4 months for patients with resected HI and 49.9 months for those without HI. Median overall survival (OS) after CRS/HIPEC for patients with LGA primary lesions was 42.1 months for patients with HI and 95.5 months for patients without HI (p = 0.03) (Fig. 2a). Median OS was not reached for patients with LGA after complete cytoreduction, regardless of HI (p = 0.55). For patients with colorectal cancer, median OS after complete (R0/R1) cytoreduction and HIPEC was 21.2 months for those with HI and 33.6 months for those without HI (p = 0.03) (Fig. 2b). When evaluating all patients with colon cancer, regardless of resection status, those with parenchymal HI had similar survival when compared to those having HI limited to the surface of the liver (median OS 19.2 vs. 21.2 months; p = 0.97).
FIG. 2.
Median overall survival after cytoreductive surgery/hyperthermic intraperitoneal chemotherapy with and without hepatic resection for (a) patients with low-grade appendiceal cancer and (b) patients with colorectal cancer who underwent complete cytoreduction
DISCUSSION
The unexpected presence of PSD discovered upon attempted hepatectomy for colorectal cancer has traditionally served as a contraindication for continued resection. Recently, it has been demonstrated, however, that for colon primaries when the burden of PSD is low (<2 abdominal regions), hepatectomy should be undertaken as long as complete cytoreduction is possible.8 Recent meta-analysis, limited to the investigation of patients with colorectal primary lesions, suggested a trend toward increased survival among patients with HI and hepatic resection as a component of CRS/HIPEC when compared to patients treated with systemic chemotherapy.9 As partial hepatectomy is sometimes required to achieve complete cytoreduction in cases in which PSD involves the liver, it is important to determine the implications of hepatectomy as a component of CRS/HIPEC in terms of operative morbidity and mortality, and survival.
In this cohort, the addition of a nonanatomic hepatic resection to HIPEC was not associated with an increase in morbidity or ICU stay. Even though the higher mortality in patients with HI did not reach statistical significance, it serves to caution against hepatic resections in the presence of extensive peritoneal dissemination. Liver involvement, per se, did not preclude complete cytoreduction, yet this finding likely reflects careful preoperative patient selection, as those proceeding to surgery would have had disease preoperatively considered to be resectable.
This manuscript demonstrates that not all HI from PSD is equal. Patients with PSD from LGA primary lesions often present with an impressive volume of disease surrounding the liver capsule, yet this superficial disease very rarely invades the hepatic parenchyma and has no effect on the DFS or OS of patients after a complete macroscopic cytoreduction. In these patients, HI may function as a marker of greater volume of disease. The decreased survival observed for LGA with HI when incomplete cytoreductions were included in the analysis likely reflects the effect of residual PSD on survival and not the effect of the liver involvement itself. Therefore, in LGA, pericapsular liver disease alone should not be considered a sufficient reason to abort the operation.
On the contrary, HI by colorectal primary lesions is often parenchymal and denotes an aggressive biologic behavior with a statistically significant impact on both DFS and OS. This impact on survival is observed even when a complete macroscopic cytoreduction was achieved. In addition, 36 % of this cohort will develop extra-abdominal, systemic failure. Therefore, in patients with colorectal primary lesions, as well as high-grade appendiceal primary lesions, we reserve CRS/HIPEC only for patients who are treated with upfront systemic chemotherapy and are without progression of disease on repeat CT imaging after chemotherapy. We believe this practice is validated by the reported median OS of 21 months after CRS/HIPEC.
Although modern chemotherapy has resulted in survival outcomes of up to 23–26 months for patients with stage IV colon cancer, we measured survival from the date of surgery, which followed chemotherapy as opposed to the date of diagnosis and initiation of chemotherapy.10,11 The survival benefit provided by CRS/HIPEC is not in lieu of that provided by chemotherapy, but is additive to it. In this regard, CRS/HIPEC should not be compared to first-line chemotherapy in terms of survival benefits. A more accurate comparison could be made between CRS/HIPEC and second-line or third-line chemotherapy in which CRS/HIPEC has superior survival outcomes. More specifically, median survival for second-line chemotherapy is 10–14 months and for third-line (such as regorafenib), it is less than 3 months.12–14
In LGA, recurrence is not more commonly observed after CRS/HIPEC procedures that include resection of HI. When LGA does recur, it is limited to the peritoneal cavity or perihepatic area, but it does not involve liver parenchyma or extraabdominal sites. On the contrary, colorectal primary lesions have a 38 % potential for intrahepatic recurrence after CRS/HIPEC and a 25 % risk for the development of systemic disease outside the peritoneal cavity, even when HI is absent at CRS/HIPEC. Thus, colorectal cancer does not always behave as a peritoneal surface process. Until reliable methods of distinguishing patterns of dissemination are developed, these patients should be treated as having systemic disease with continuation of systemic chemotherapy after CRS/HIPEC.
The current study has several limitations. First, even though differences in the clinical significance of HI of two major histologies currently considered appropriate for CRS/HIPEC procedures are accurately depicted, this study represents a nonrandomized, single institution, retrospective analysis. Therefore, selection bias is inevitably incorporated in the reported outcomes. Although this study also represents the largest of its kind, small patient numbers were not avoided in the subgroup analyses.
In conclusion, synchronous resection of limited HI does not increase the morbidity or mortality of CRS/HIPEC procedures. HI in PSD from colorectal and appendiceal primary lesions is a negative prognostic indicator of survival. In patients with colorectal primary lesions, HI is associated with decreased OS and DFS, but CRS/HIPEC still offers a meaningful survival benefit when a complete cytoreduction can be achieved. In patients with LGA primary lesions, HI is generally superficial and should not be considered a contraindication for CRS/HIPEC procedures.
Acknowledgments
The study was supported by Wake Forest University Biostatistics shared resource NCI CCSG P30CA012197.
Footnotes
Presented as an oral presentation at the 9th Annual Academic Surgical Congress, San Diego, CA held February 4–6, 2014, and the 9th International Symposium on Regional Cancer Therapies, Steamboat Springs, CO held February 15–17, 2014.
DISCLOSURE None.
References
- 1.Levine EA, Stewart JH, Russell GB, Geisinger KR, Loggie BL, Shen P. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg. 2007;204:943–53. doi: 10.1016/j.jamcollsurg.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 2.Glockzin G, Schlitt HJ, Piso P. Peritoneal carcinomatosis: patients selection, perioperative complications and quality of life related to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol. 2009;7:5. doi: 10.1186/1477-7819-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7:69–76. doi: 10.1016/S1470-2045(05)70539-8. [DOI] [PubMed] [Google Scholar]
- 4.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 5.Stewart JH, Shen P, Levine EA. Intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: current status and future directions. Ann Surg Oncol. 2005;12:765–77. doi: 10.1245/ASO.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Shen P, Stewart JH, Levine EA. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancy: overview and rationale. Curr Probl Cancer. 2009;33:125–41. doi: 10.1016/j.currproblcancer.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allard MA, Adam R, Ruiz A, et al. Is unexpected peritoneal carcinomatosis still a contraindication for resection of colorectal liver metastases? Combined resection of colorectal liver metastases with peritoneal deposits discovered intra-operatively. Eur J Surg Oncol. 2013;39:981–7. doi: 10.1016/j.ejso.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 9.de Cuba EM, Kwakman R, Knol DL, Bonjer HJ, Meijer GA, Te Velde EA. Cytoreductive surgery and HIPEC for peritoneal metastases combined with curative treatment of colorectal liver metastases: Systematic review of all literature and meta-analysis of observational studies. Cancer Treat Rev. 2013;39:321–7. doi: 10.1016/j.ctrv.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 Treatment and RAS Mutations in Colorectal Cancer. N Engl J Med. 2013;369(11):1023–34. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs CS, Marshall J, Mitchell E, et al. Randomised, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol. 2007;25(30):4779–86. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 12.Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorn, and irinotecan improves survival in a phase III randomised trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499–506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 13.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebocontrolled, phase 3 trial. Lancet. 2013;381(9863):303–12. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 14.Haller DG, Rothenberg ML, Wong AO, et al. Oxaliplatin plus irinotecan compared with irinotecan alone as second-line treatment after single-agent fluoropyrimidine therapy for metastatic colorectal carcinoma. J Clin Oncol. 2008;26(28):4544–50. doi: 10.1200/JCO.2008.17.1249. Hepatic Involvement Treated with CRS/HIPEC. [DOI] [PubMed] [Google Scholar]