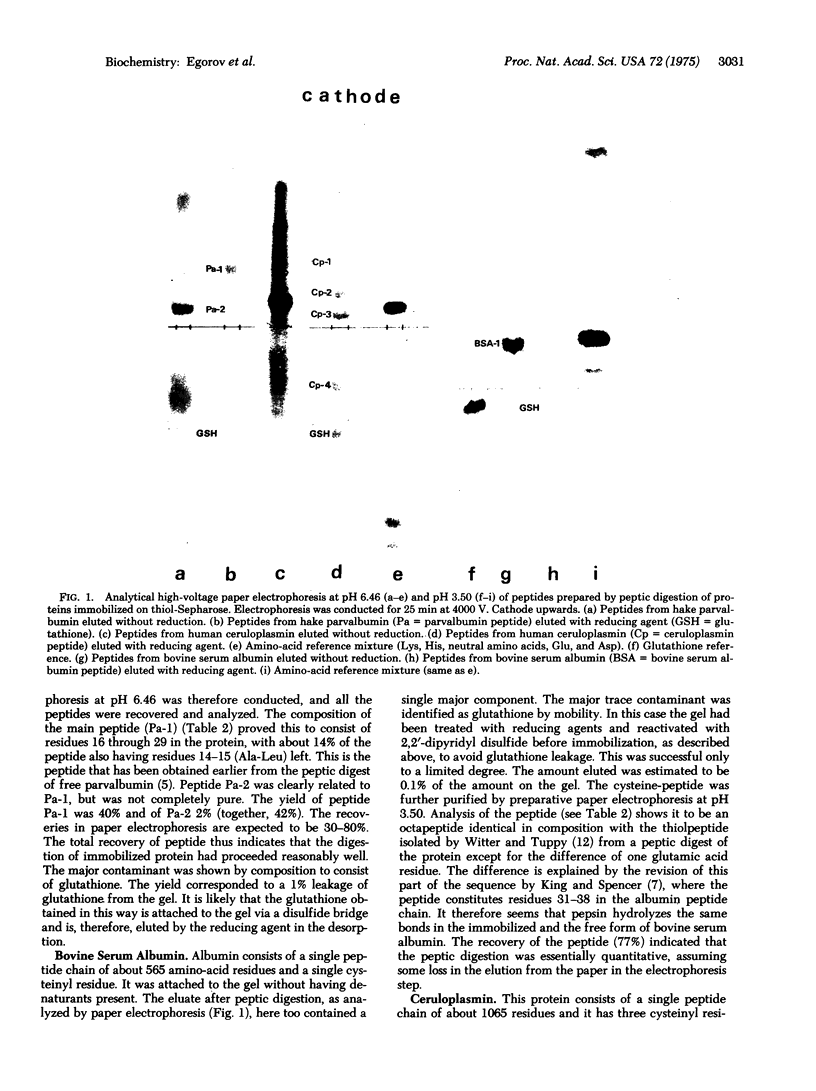
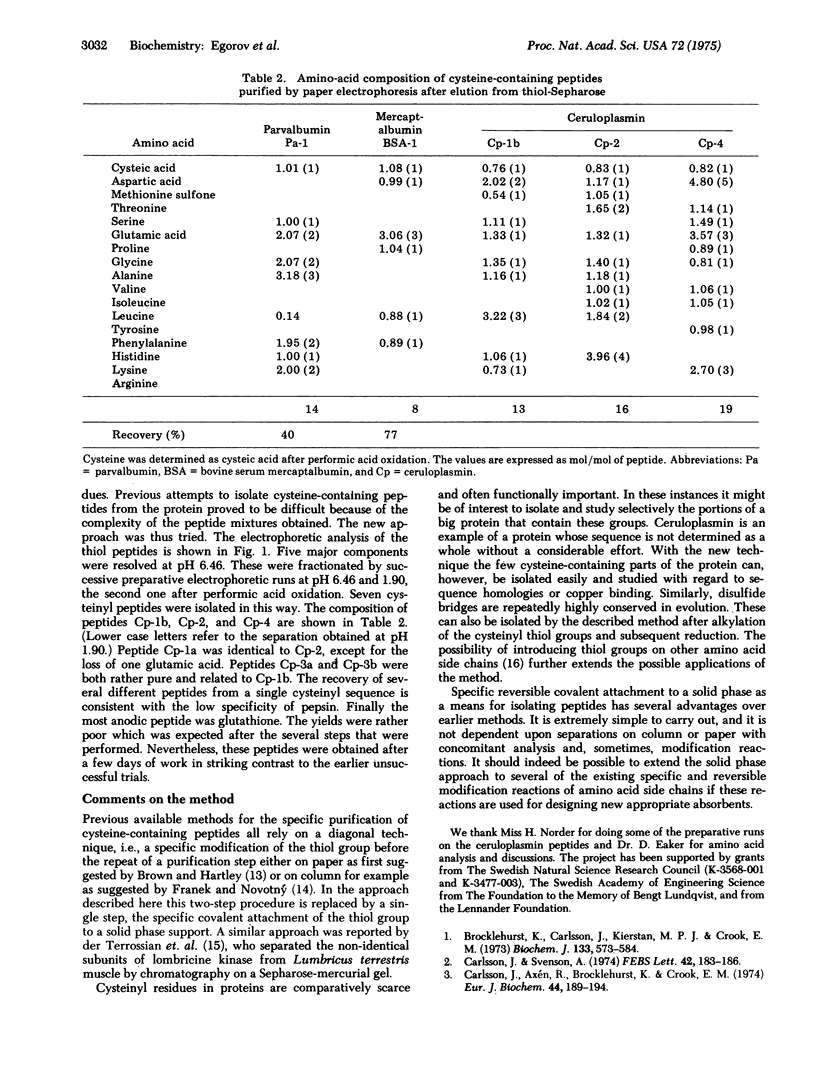
Abstract
Activated thiol-Sepharose [agarose-(glutathione-2-pyridyl disulfide) conjugate] has been used to immobilize proteins with a single or a few thiol groups via disulfide bridges. The immobilized proteins were subsequently proteolytically degraded. After washing, the thiol-containing peptides were eluted with a reducing agent. A single preparative paper electrophoresis, occasionally after a modification such as oxidation, was sufficient to obtain pure peptides in good yields. The method was applied to the major parvalbumin from hake muscle (a protein with 108 amino acid residues and one cysteine residue), to mercaptalbumin from bovine serum (565 residues and one cysteine), and to human serum ferroxidase [EC 1.16.3.1; iron (II):oxygen oxidoreductase] (ceruloplasmin) (1065 residues and three cysteines). The use of the technique, e.g., as a simple means of obtaining homologous peptides in related proteins, is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Carlsson J., Kierstan M. P., Crook E. M. Covalent chromatography. Preparation of fully active papain from dried papaya latex. Biochem J. 1973 Jul;133(3):573–584. doi: 10.1042/bj1330573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. R., Hartley B. S. Location of disulphide bridges by diagonal paper electrophoresis. The disulphide bridges of bovine chymotrypsinogen A. Biochem J. 1966 Oct;101(1):214–228. doi: 10.1042/bj1010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capony J. P., Rydèn L., Demaille J., Pechère J. F. The primary structure of the major parvalbumin from hake muscle. Overlapping peptides obtained with chemical and enzymatic methods. The complete amino-acid sequence. Eur J Biochem. 1973 Jan 3;32(1):97–108. doi: 10.1111/j.1432-1033.1973.tb02584.x. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Axén R., Brocklehurst K., Crook E. M. Immobilization of urease by thiol-disulphide interchange with concomitant purification. Eur J Biochem. 1974 May 2;44(1):189–194. doi: 10.1111/j.1432-1033.1974.tb03472.x. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Svenson A. Preparation of bovine mercaptalbumin by means of covalent chromatography. FEBS Lett. 1974 Jun 1;42(2):183–186. doi: 10.1016/0014-5793(74)80781-7. [DOI] [PubMed] [Google Scholar]
- Franek F., Novotný J. Selective isolation of large half-cystine-containing peptides. Amino acid sequence near some half-cystines in porcine immunoglobulin gamma-chains. FEBS Lett. 1973 Dec 1;37(2):162–166. doi: 10.1016/0014-5793(73)80449-1. [DOI] [PubMed] [Google Scholar]
- Grassetti D. R., Murray J. F., Jr Determination of sulfhydryl groups with 2,2'- or 4,4'-dithiodipyridine. Arch Biochem Biophys. 1967 Mar;119(1):41–49. doi: 10.1016/0003-9861(67)90426-2. [DOI] [PubMed] [Google Scholar]
- King T. P., Spencer E. M. Amino acid sequences of the amino and the carboxyl terminal cyanogen bromide peptides of bovine plasma albumin. Arch Biochem Biophys. 1972 Dec;153(2):627–640. doi: 10.1016/0003-9861(72)90382-7. [DOI] [PubMed] [Google Scholar]
- Pechère J. F., Capony J. P., Ryden L. The primary structure of the major parvalbumin from hake muscle. Isolation and general properties of the protein. Eur J Biochem. 1971 Dec 10;23(3):421–428. doi: 10.1111/j.1432-1033.1971.tb01636.x. [DOI] [PubMed] [Google Scholar]
- Perham R. N., Thomas J. O. Reaction of tobacco mosaic virus with a thiol-containing imidoester and a possible application to X-ray diffraction analysis. J Mol Biol. 1971 Dec 14;62(2):415–418. doi: 10.1016/0022-2836(71)90438-4. [DOI] [PubMed] [Google Scholar]
- Rydén L., Eaker D. Reactivities fo the cysteinyl residues of human ceruloplasmin (ferroxidase). FEBS Lett. 1975 May 15;53(3):279–281. doi: 10.1016/0014-5793(75)80036-6. [DOI] [PubMed] [Google Scholar]
- Rydén L., Eaker D. The amino-acid sequences of three tryptic glycopeptides from human ceruloplasmin. Eur J Biochem. 1974 May 2;44(1):171–180. doi: 10.1111/j.1432-1033.1974.tb03470.x. [DOI] [PubMed] [Google Scholar]
- Rydén L. Evidence for proteolytic fragments in commercial samples of human ceruloplasmin. FEBS Lett. 1971 Nov 1;18(2):321–325. doi: 10.1016/0014-5793(71)80477-5. [DOI] [PubMed] [Google Scholar]
- Terrossian E der, Pradel L. A., Kassab R., Desvages G. Separation of the two non-identical subunits of lombricine kinase from Lumbricus terrestris muscle by chromatography on sepharose-mercurial. Isolation of the tryptic peptide containing its essential thiol group. Eur J Biochem. 1974 Jun 1;45(1):243–251. doi: 10.1111/j.1432-1033.1974.tb03548.x. [DOI] [PubMed] [Google Scholar]
- WITTER A., TUPPY H. N-(4-Dimethylamino-3,5-dinitrophenyl)maleimide: a coloured sulfhydryl reagent. Isolation and investigation of cysteine-containing peptides from human and bovine serum albumin. Biochim Biophys Acta. 1960 Dec 18;45:429–442. doi: 10.1016/0006-3002(60)91480-3. [DOI] [PubMed] [Google Scholar]