Abstract
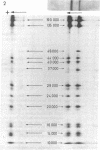
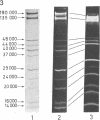
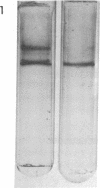
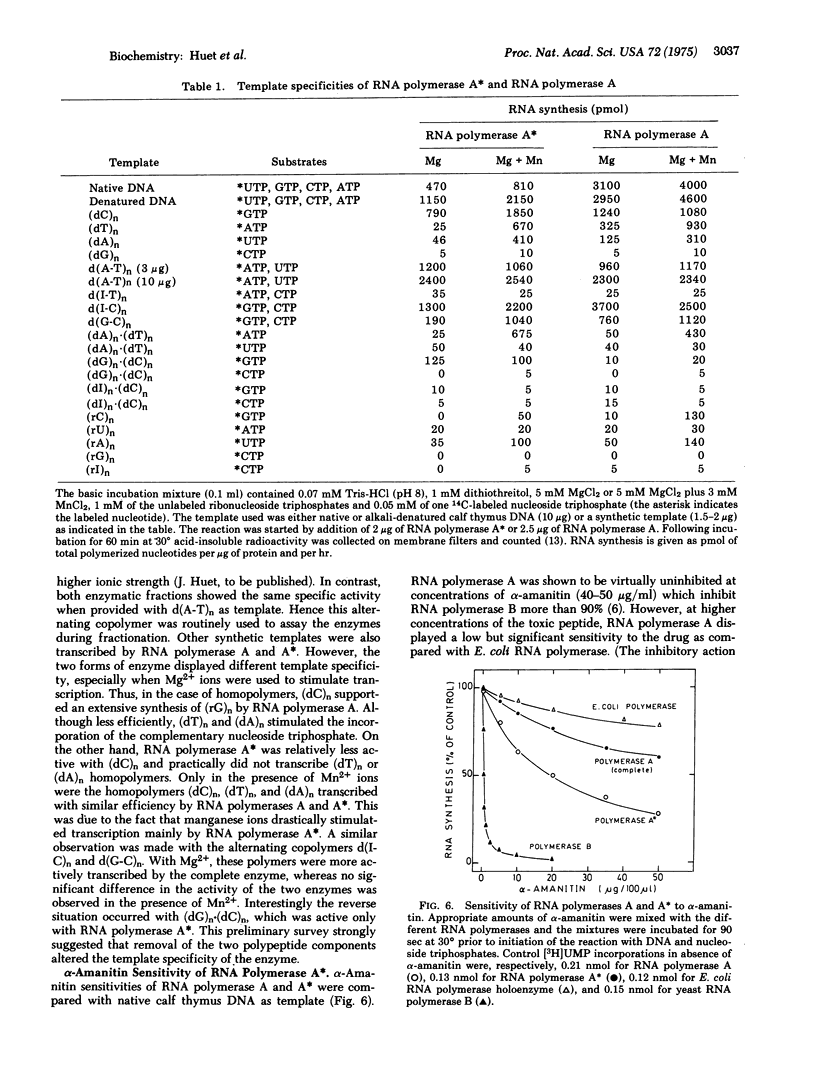
Yeast RNA polymerase A (RNA nucleotidyltransferase; nucleosidetriphosphate:RNA nucleotidyltransferase; EC 2.7.7.6) can be converted to a new form of enzyme, called RNA polymerase A*, which is lacking two polypeptide chains of 48,000 and 37,000 daltons. Apart from these two missing polypeptides the subunit structures of RNA polymerases A and A* are indistinguishable. RNA polymerase A* differs from the complete enzyme in its electrophoretic and chromatographic behavior, template requirements, and alpha-amanitin sensitivity. RNA polymerase A* transcribes the alternated copolymer d(A-T)n with the same efficiency as RNA polymerase A but its specific activity is greatly reduced with native calf thymus DNA as template. The transcription of a variety of synthetic templates is also altered by removal of the two polypeptide chains. RNA polymerase A* is inhibited by high concentrations of alpha-amanitin (500 mug/ml), whereas RNA polymerase A is comparatively less sensitive to the toxic peptide. The data are discussed in terms of possible roles of the two dissociable polypeptides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amalric F., Nicoloso M., Zalta J. -P. A comparative study of "soluble" RNA polymerase activity of Zajdela hepatoma ascites cells and calf thymus. FEBS Lett. 1972 Apr 15;22(1):67–72. doi: 10.1016/0014-5793(72)80221-7. [DOI] [PubMed] [Google Scholar]
- Buhler J. M., Sentenac A., Fromageot P. Isolation, structure, and general properties of yeast ribonucleic acid polymerase A (or I). J Biol Chem. 1974 Sep 25;249(18):5963–5970. [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Chang L. M. Low molecular weight deoxyribonucleic acid polymerase from calf thymus chromatin. I. Preparation of homogeneous enzyme. J Biol Chem. 1973 Jun 10;248(11):3789–3795. [PubMed] [Google Scholar]
- Dezelee S., Sentenac A., Fromageot P. Role of DNA-RNA hybrids in eukaryots 1. Purification of yeast RNA polymerase B. FEBS Lett. 1972 Mar;21(1):1–6. doi: 10.1016/0014-5793(72)80148-0. [DOI] [PubMed] [Google Scholar]
- Dezélée S., Sentenac A., Fromageot P. Role of deoxyribonucleic acid-ribonucleic acid hybrids in eukaryotes. Study of the template requirements of yeast ribonucleic acid polymerases and nature of the ribonucleic acid product. J Biol Chem. 1974 Sep 25;249(18):5971–5977. [PubMed] [Google Scholar]
- Dezélée S., Sentenac A., Fromageot P. Role of deoxyribonucleic acid-ribonucleic acid hybrids in eukaryotes. Synthetic ribo- and deoxyribopolynucleotides as template for yeast ribonucleic acid polymerase B (or II). J Biol Chem. 1974 Sep 25;249(18):5978–5983. [PubMed] [Google Scholar]
- Dezélée S., Sentenac A. Role of DNA-RNA hybrids in eukaryotes. Purification and properties of yeast RNA polymerase B. Eur J Biochem. 1973 Apr 2;34(1):41–52. doi: 10.1111/j.1432-1033.1973.tb02726.x. [DOI] [PubMed] [Google Scholar]
- Greenleaf A. L., Linn T. G., Losick R. Isolation of a new RNA polymerase-binding protein from sporulating Bacillus subtilis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):490–494. doi: 10.1073/pnas.70.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross K. J., Pogo A. O. Control mechanism of ribonucleic acid synthesis in eukaryotes. The effect of amino acid and glucose starvation and cycloheximide on yeast deoxyribonucleic acid-dependent ribonucleic acid polymerases. J Biol Chem. 1974 Jan 25;249(2):568–576. [PubMed] [Google Scholar]
- Kedinger C., Gissinger F., Chambon P. Animal DNA-dependent RNA polymerases. Molecular structures and immunological properties of calf-thymus enzyme AI and of calf-thymus and rat-liver enzymes B. Eur J Biochem. 1974 May 15;44(2):421–436. doi: 10.1111/j.1432-1033.1974.tb03500.x. [DOI] [PubMed] [Google Scholar]
- Kornberg A. Active center of DNA polymerase. Science. 1969 Mar 28;163(3874):1410–1418. doi: 10.1126/science.163.3874.1410. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lampert A., Feigelson P. A short lived polypeptide component of one of two discrete functional pools of hepatic nuclear alpha-amanitin resistant RNA polymerases. Biochem Biophys Res Commun. 1974 Jun 18;58(4):1030–1038. doi: 10.1016/s0006-291x(74)80247-0. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B., Roeder R. G. Purification and subunit structure of deoxyribonucleic acid-dependent ribonucleic acid polymerase I from the mouse myeloma, MOPC 315. J Biol Chem. 1974 Sep 25;249(18):5898–5906. [PubMed] [Google Scholar]
- Schwartz L. B., Sklar V. E., Jaehning J. A., Weinmann R., Roeder R. G. Isolation and partial characterization of the multiple forms of deoxyribonucleic acid-dependent ribonucleic acid polymerase in the mouse myeloma, MOPC 315. J Biol Chem. 1974 Sep 25;249(18):5889–5897. [PubMed] [Google Scholar]
- Seifart K. H., Benecke B. J., Juhasz P. P. Multiple RNA polymerase species from rat liver tissue: possible existence of a cytoplasmic enzyme. Arch Biochem Biophys. 1972 Aug;151(2):519–532. doi: 10.1016/0003-9861(72)90529-2. [DOI] [PubMed] [Google Scholar]
- Sklar V. E., Schwartz L. B., Roeder R. G. Distinct molecular structures of nuclear class I, II, and III DNA-dependent RNA polymerases. Proc Natl Acad Sci U S A. 1975 Jan;72(1):348–352. doi: 10.1073/pnas.72.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Blatti S. P., Rutter W. J. Molecular structures of DNA-dependent RNA polymerases (II) from calf thymus and rat liver. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2994–2999. doi: 10.1073/pnas.68.12.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F. L., Feigelson P. The rapid turnover of RNA polymerase of rat liver nucleolus, and of its messenger RNA. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2833–2837. doi: 10.1073/pnas.69.10.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]