Abstract
Introduction: Trichophyton rubrum is one of the most common species of dermatophytes which affects superficial keratinous tissue. It is not especially virulent but it can be responsible for considerable morbidity. Although there are different therapeutic modalities to treat fungal infections, clinicians are searching for alternative treatment because of the various side effects of the present therapeutic methods. As a new procedure, Laser therapy has brought on many advantages in clinical management of dermatophytes. Possible inhibitory potential of laser irradiation on fungal colonies was investigated invitro in this study.
Methods: A total of 240 fungal plates of standard size of trichophyton rubrum colonies that had been cultured from the lesions of different patients at the mycology laboratory, were selected. Each fungal plate was assigned as control or experimental group. Experimental plates were irradiated by a laser system (low power laser or different wavelength of high power laser). The effects of different laser wavelengths and energies on isolated colonies were assessed. After laser irradiation, final size of colonies was measured on the first, the 7th and the 14th day after laser irradiation.
Results: Although low power laser irradiation did not have any inhibitory effect on fungal growth, the Q-Switched Neodymium-Doped Yttrium Aluminium Garnet (Nd:YAG) laser 532nm at 8j/cm2, Q-Switched Nd:YAG laser 1064nm at 4j/cm2 to 8j/cm2 and Pulsed dye laser 595nm at 8j/cm2 to 14j/cm2 significantly inhibited growth of trichophyton rubrum in vitro.
Conclusion: Q-Switched Nd:YAG 532nm at 8j/cm2, Q-Switched Nd:YAG laser 1064nm at 4j/cm2 to 8j/cm2 and pulsed dye laser (PDL) 595nm at 8j/cm2 to 14j/cm2 can be effective to suppress trichophyton rubrum growth.
Keywords: laser, dermatophyte, Q-Switched, Nd:YAG lasers, PDL
Introduction
Dermatophyte infection is a common superficial skin disease which represents the dominant type of fungal infection of keratinous tissues such as skin, hair and nails1. After colonizing keratinous tissues, inflammation reaction occurs because of the host response to the organism2. It is believed that widespread invasion of the infection is due to metabolizing enzyme produced by dermatophytes3. Although dermatophytes generally infect healthy individuals, immunocompromised patients are especially more susceptible4. According to morphological and pathological features, dermatophytes are classified as Microsporum, Epidermophyton and Trichophyton.
Trichophyton Rubrum is one of the most common causes of dermatophyte infections all over the world. This type of fungal infection has a wide range of clinical presentations. Prevalence and severity of this infection considerably varies from one place to another and is associated with several factors 5-7. Current management of Trichophyton infection includes systemic and topical synthetic antifungal therapies. Due to several reasons such as various side effects of synthetic pharmacological antifungal drug in systemic use, insufficient concentration of the medication in target area, inconvenient use of topical agents and long term application of management in many situations8, more effective, available, and safer methods of management have been sought9. A probable useful technique for this purpose is laser therapy, as a novel application in medical management10. Laser in medical science has become indispensable due to rapidly expanding use of laser technology in various field of medicine11. Photodynamic therapy, considered as a therapeutic method in skin diseases, has been proposed to be effective in treating dermatophyte infections 12-14.
This study aimed to assess the inhibitory effect of several systems of low power and high power lasers with different frequencies and energies on the growth of Trichophyton rubrum in vitro and compare the result of different systems with each other and control group.
Methods
Assessing the inhibitory effect of different laser systems on the growth of Trichophyton rubrum was an experimental controlled study conducted in Mycology laboratory, Laser and skin research center of Shahid Beheshti University of Medical Sciences through the year 2010 to 2013. The study was conducted after achieving approval of the Ethics Committee of Medical Researches.
Isolated colonies of Trichophyton rubrum, were cultured in standard plates containing Sabour and dextrose agar and mycobiotic in Mycology laboratory. These samples were serially passed on standard conditions. When pure Trichophyton rubrum colonies were obtained, a total of 240 fungal plates were used for this experiment. 120 fungal plates were assigned as control group and 120 fungal plates were used for laser irradiation. According to the results of spectrophotometry, wavelengths in which the fungi absorbed the highest rate of energy were applied for the study. In consideration of the outcome of spectrophotometry experiments, various low power and high power laser systems were considered for this study (Table 1).
Table 1 . Laser systems, category, wavelength and power applied throughout the first phase of the assay .
| Laser system | Category | Wavelength | Power |
| Blue LED | low power | 450nm | 350mw |
| Red LED | low power | 630nm | 225mw |
| LD PUMPED green laser | low power | 532nm | 100mw |
| Infrared laser diode | low power | 808nm, 980nm | 130mw,100mw |
| Q – Switched Nd: YAG laser | high power | 532nm | 2 J/cm 2 , 4 J/cm 2 , 8 J/cm 2 |
| Q – Switched Nd: YAG laser | high power | 1064nm | 2 J/cm 2 , 4 J/cm 2 , 8 J/cm 2 |
| Intense pulsed light (IPL) | high power | 535nm, 872nm | 20 J/cm 2 , 80 J/cm 2 |
| Infrared laser | high power | 808nm | 5 J/cm 2 , 20 J/cm 2 |
| Erbium YAG laser | high power | 2940nm | 20 J/cm 2 |
| Pulsed dye laser (PDL) | high power | 595nm | 8 J/cm 2 , 14 J/cm 2 |
The variation in power and wavelength of the laser was based on previous studies and clinical usage of laser in fungal skin diseases13-15. During the initial phase of the experiment, results of laser irradiations to the colonies showed that 532nm and 1064nm Q-Switched Nd: YAG laser and Pulsed dye laser 595nm decreased the size of fungal colonies. Therefore, these three laser systems were used for the second study phase. In this phase 532nm and 1064nm Q– Switched Nd: YAG laser ( Nd: YAG short pulse, lutronic, korea) were used at the intensity of either 2 J/cm2, 4 J/cm2 and up to 8 J/cm2 with 2Hz pulse frequency, 10 ns pulse duration, 2 mm diameter laser beam spot size. Pulsed dye laser (Vbeam candela, america) 595nm was used at 8 j/cm2 to 14 j/cm2, 1.5 ms pulse duration, 7 mm diameter laser beam spot size. The number of laser pulses used for each colony depends on the area of the colony surface. Laser beams were applied to cover the whole surface of the colony by moving the laser beam in a circular pattern centrifugally16. Each colony received only one kind of laser system irradiation throughout the study.
In order to recognize every change in size and morphology of the colonies and compare colonies with each other, isolated colonies in each plate were photographed by manual focus digital camera (Canon), immediately after preparation, on the first, 7th and 14th day after laser irradiation. Digital photos were used to assess the change in growth of the colonies (Figure 1).
Figure 1 .
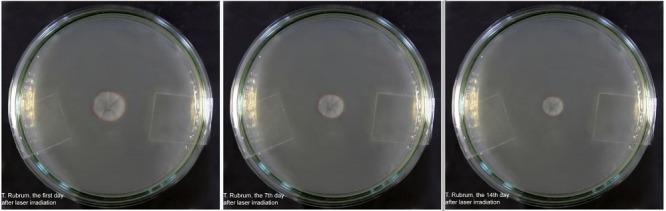
Trichophyton Rubrum on the first, 7th and 14th day after laser irradiation by Q – Switched Nd: YAG laser 1064nm at 4j/cm2
The surface area of the colonies was calculated precisely by standard pixel ruler software. The normal distribution of the initial size of colonies was investigated at the beginning of the experiment. The significance of the results of this study was approved by Paired-Sample T Test and the changes in colonies of experimental group versus control group were analyzed. The software used in this study to process and analyze the data was SPSS (PASW Statistics version 20). In all tests, P-value less than 0.05 was considered statistically significant.
Results
Low power laser systems modified colonies but did not have any inhibition effects on the fungal colonies. In the high power laser category, Q–Switched Nd: YAG laser 532nm at 8j/cm2 and Q–Switched Nd: YAG laser 1064nm at 4j/cm2 to 8j/cm2 with 2Hz pulse frequency, 10ns pulse duration, 2mm laser beam spot size and also Pulsed dye laser (PDL) 595nm at 8j/cm2 to 14j/cm2 with 1.5 ms pulse duration, 7 mm diameter laser beam spot size had significantly inhibitory effects on the growth of Trichophyton rubrum. The results of laser irradiations on the colonies are showed in figures 2-5. In this study, the distribution pattern of fungal colonies size was initially evaluated by One-Sample Kolmogorov- Smirnov test. Asymp. Sig. (2- tailed) = 0/384 > 0/05.
Figure 2 .
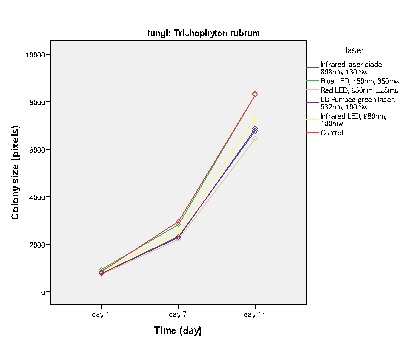
The effect of low power laser on the growth of T. rubrum
Figure 5 .
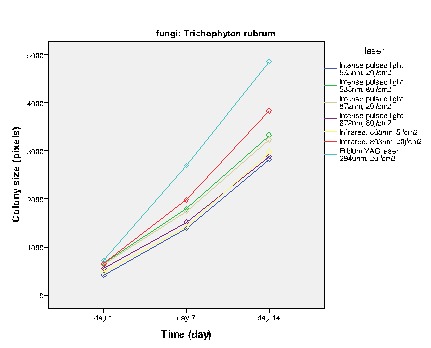
The effect of high power laser except Q-Switched lasers and PDL on the growth of T.rubrum
Figure 3 .
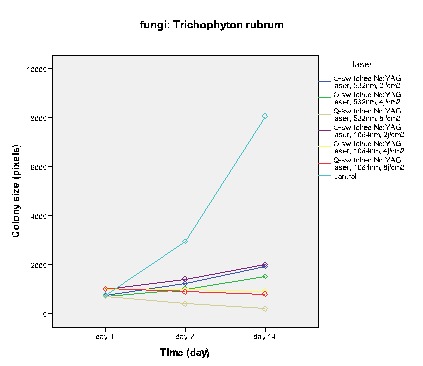
The effect of Q -Switched Nd: YAG laser on the growth of T. rubrum
Figure 4 .
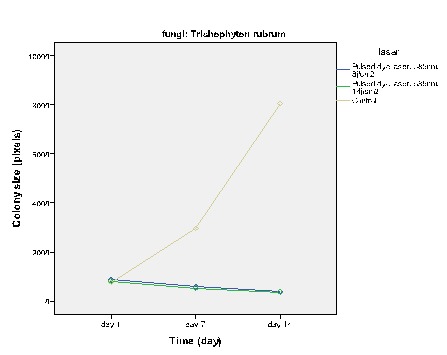
The effect of Pulsed Dye Laser on the growth of T.rubrum
P-value greater than 0/05 conveyed that the pattern distribution of the size of colonies was normal.
Figures 2-5 show the average colony size of T. rubrum, on the first, 7th and 14th day after laser irradiation separately, based on laser systems. These diagrams demonstrate that laser systems affected fungal colony in variable ways, they had either macroscopic or microscopic effects on the structure of colonies. Assessment proved that there is a correlation between power of Q – Switched Nd: YAG 1064nm and the final size of T. rubrum colony. It means that by increasing this laser power, the inhibitory effects rise alongside.
It is noteworthy, in this study, p-value< 0/05 was considered statistically significant. Results of General Linear Model – Repeated Measures test showed that each of the laser systems significantly affected T. rubrum colonies differently. In this study, Paired – Sample T test was used to approve the significance of the growth inhibition by effective laser systems. Q-Switched Neodymium-Doped Yttrium Aluminium Garnet (Nd:YAG) 532nm at 8j/cm2, Q-Switched Nd:YAG laser 1064nm at 4j/cm2 to 8j/cm2 and PDL 595nm at 8j/cm2 to 14j/cm2 used in this experiment, had an inhibitory effect on the growth of fungal colonies, Sig. (2-tailed) < 0/05 , therefore effects of these lasers on Trichophyton rubrum are statistically significant.
Discussion
In this study, we investigated the possible inhibitory effects of different laser systems on the growth of Trichophyton rubrum. This paper revealed that Q–Switched Nd:YAG laser 532nm at 8j/cm2 and Q–Switched Nd: YAG laser 1064nm at 4j/cm2 to 8j/cm2 (with 2Hz pulse frequency, 10ns pulse duration, 2 mm diameter laser beam spot size) and Pulsed dye laser (PDL) 595nm at 8j/cm2 to 14j/cm2 (1.5 ms pulse duration, 7 mm diameter laser beam spot size) have statistically significant inhibitory effects on the growth of T. rubrum.
Dermatophyte infections affect many people all over the world; therefore, therapeutic methods are considered by clinicians17. There are high rates of drug resistance, recurrence and persistence of dermatophyte infections. Pharmacological therapies may cause allergic reaction, drug interactions and other side effects8. There are many researches on the role of photodynamic therapy as a novel management of these infections18. They assess advantages and limitations of this therapeutic method. Previous studies showed that high power laser is supposed to be efficacious in treating patients, infected by dermatophytes. Although there are many papers on high power laser therapy in this field, they are not supported by sufficient experiment to assay low power laser therapy in dermatophyte infections19. The present study attempts to discover the effects of low and high power laser irradiations on the growth of fungal colonies. It is known that low power laser systems through optical excitation of light sensitive cells can cause photochemical reactions in the tissues without any thermal impairment20. Otherwise, high power lasers generate heat and increase the kinetic energy of the target cells and induce their denouement through evaporation, coagulation or necrosis reactions21,22. It is obvious if low power laser systems have inhibitory effects on fungal growth, its convenience and availability is more than high power lasers. In this study, low power lasers were examined and the results showed that there is no acceptable reason for their inhibitory effects in vitro. The practical clinical laser therapy depends on many components to be mentioned such as the laser output power, wavelength, spot size, pulse frequency, duration of tissue exposure to the laser and number of the sessions. In this study different laser systems were selected accurately according to basic spectrophotometric experiments. The spectrophotometric experiments occurred before setting out the laser investigation, therefore the laser systems were chosen precisely. The species of Trichophyton rubrum contains xanthomegnin as a dominant diffusing red pigment which is susceptible to laser irradiations at the wavelength of 532nm and 598nm13. In this study a control group was considered, so that there was a chance of comparison between experimental and control group. While the survey was in progress, conditions of the colonies were evaluated and captured serially, thus there was no concern of missing data. Finally the collected data of colony measurement was analyzed through digital pixel counter and precise result was achieved. The following features are some of the most prominent aspects of this study.
The susceptibility of T. rubrum to the laser irradiation due to pigmented structure of this fungi justified the effects of Q –Switched Nd:YAG laser on the growth of this dermatophyte. In this survey 120 fungal colonies were examined by laser systems so the reliability of study was justified. Nowadays, due to increasing use of laser therapy in various clinical situations, research in this field is growing rapidly. The results of this study are compatible with other studies in this field.
This study examined the effect of laser irradiations on dermatophyte in vitro. Although the results of this study revealed that Q–Switched Nd:YAG laser 532nm and 1064nm and also Pulsed dye laser 595nm have an inhibitory effect on the growth of T. rubrum, further in vitro and in vivo studies are necessary to approve laser therapy as a clinical modality for treating T. rubrum infections. More research in this field may compare effects of number of laser therapy sessions with unique laser system to find how many sessions is needed to eradicate trichophyton rubrum. Additional Surveys on the effect of laser irradiation on dermatophyte infections in vivo will be useful. These investigations guide clinicians and researchers to develop new concepts for treatment of dermatophyte infections.
Please cite this article as follows:
Ghavam SA, Aref S , Mohajerani E, Shidfar MR, Moravvej H. The Effect of Laser Irradiation on the Growth of Trichophyton Rubrum: An in Vitro Study. J Lasers Med Sci 2015;6(1):10-6
References
- 1.Aly R. Ecology and epidemiology of dermatophyte infections. J Am Acad Dermatol. 1994;31:S21–5. doi: 10.1016/s0190-9622(08)81262-5. [DOI] [PubMed] [Google Scholar]
- 2.Haroon S, Samdani AJ. Epidemiology of dermatophyte infection Comparison of clinical and mycological findings. Saudi Med J. 2005;26:680–1. [PubMed] [Google Scholar]
- 3.Maleszka R, Adamski Z. Clinical and diagnostic aspects of dermatophyte onychomycosis. Mycoses. 1998;41:67–72. doi: 10.1111/j.1439-0507.1998.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 4.Peres NT, Maranhao FC, Rossi A, Martinez-Rossi NM. Dermatophytes: host-pathogen interaction and antifungal resistance. Ana Bras Dermatol. 2010;85:657–67. doi: 10.1590/s0365-05962010000500009. [DOI] [PubMed] [Google Scholar]
- 5.Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335–52. doi: 10.1007/s11046-008-9100-9. [DOI] [PubMed] [Google Scholar]
- 6.Bassiri-Jahromi S, Khaksari AA. Epidemiological survey of dermatophytosis in Tehran, Iran, from 2000 to 2005. Indian J Dermatology, Venereol Leprol. 2009;75:142–7. doi: 10.4103/0378-6323.48658. [DOI] [PubMed] [Google Scholar]
- 7.Rassai S, Feily A, Sina N, Derakhshanmehr F. Some epidemiological aspects of dermatophyte infections in Southwest Iran. Acta Dermatovenerol Croat : ADC. 2011;19:13–5. [PubMed] [Google Scholar]
- 8.Thappa DM. Current treatment of onychomycosis. Indian J Dermatol Venereol Leprol. 2007;73:373–6. doi: 10.4103/0378-6323.37052. [DOI] [PubMed] [Google Scholar]
- 9.Finch JJ, Warshaw EM. Toenail onychomycosis: current and future treatment options. Dermatol Ther. 2007;20:31–46. doi: 10.1111/j.1529-8019.2007.00109.x. [DOI] [PubMed] [Google Scholar]
- 10.Smijs TG, Pavel S. The susceptibility of dermatophytes to photodynamic treatment with special focus on Trichophyton rubrum. Photochem Photobiol. 2011;87:2–13. doi: 10.1111/j.1751-1097.2010.00848.x. [DOI] [PubMed] [Google Scholar]
- 11.Prindeze NJ, Moffatt LT, Shupp JW. Mechanisms of action for light therapy: a review of molecular interactions. Exp Biol Med. 2012;237:1241–8. doi: 10.1258/ebm.2012.012180. [DOI] [PubMed] [Google Scholar]
- 12.Calzavara-Pinton PG, Venturini M, Sala R. A comprehensive overview of photodynamic therapy in the treatment of superficial fungal infections of the skin. J Photochem Photobiol B. 2005;78(1):1–6. doi: 10.1016/j.jphotobiol.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Vural E, Winfield HL, Shingleton AW, Horn TD, Shafirstein G. The effects of laser irradiation on Trichophyton rubrum growth. Lasers Med Sci. 2008;23:349–53. doi: 10.1007/s10103-007-0492-4. [DOI] [PubMed] [Google Scholar]
- 14.Manevitch Z, Lev D, Hochberg M, Palhan M, Lewis A, Enk CD. Direct antifungal effect of femtosecond laser on Trichophyton rubrum onychomycosis. Photochem Photobiol. 2010;86(2):476–9. doi: 10.1111/j.1751-1097.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- 15.Xu ZL, Xu J, Zhuo FL, Wang L, Xu W, Xu Y. et al. Effects of laser irradiation on Trichophyton rubrum growth and ultrastructure. Chin Med J. 2012;125:3697–700. [PubMed] [Google Scholar]
- 16.Hochman LG. Laser treatment of onychomycosis using a novel 065-millisecond pulsed Nd:YAG 1064-nm laser. J Cosmet Laser Ther. 2011;13:2–5. doi: 10.3109/14764172.2011.552616. [DOI] [PubMed] [Google Scholar]
- 17.Hainer BL. Dermatophyte infections. Am Fam Physician. 2003;67:101–8. [PubMed] [Google Scholar]
- 18.Laser treatment of onychomycosis. Med Lett Drugs Ther. 2013;55:15. [PubMed] [Google Scholar]
- 19.Amorim JC, Soares BM, Alves OA, Ferreira MV, Sousa GR, Silveira Lde B. et al. Phototoxic action of light emitting diode in the in vitro viability of Trichophyton rubrum. Ana Bras Dermatol. 2012;87:250–5. doi: 10.1590/s0365-05962012000200009. [DOI] [PubMed] [Google Scholar]
- 20.Smijs TG, Mulder AA, Pavel S, Onderwater JJ, Koerten HK, Bouwstra JA. Morphological changes of the dermatophyte Trichophyton rubrum after photodynamic treatment: a scanning electron microscopy study. Med Mycol. 2008;46:315–25. doi: 10.1080/13693780701836977. [DOI] [PubMed] [Google Scholar]
- 21.Noguchi H, Miyata K, Sugita T, Hiruma M, Hiruma M. Treatment of onychomycosis using a 1064nm Nd:YAG laser. Med Mycol J. 2013;54:333–9. doi: 10.3314/mmj.54.333. [DOI] [PubMed] [Google Scholar]
- 22.Ledon JA, Savas J, Franca K, Chacon A, Nouri K. Laser and light therapy for onychomycosis: a systematic review. Lasers Med Sci. 2014;29:823–9. doi: 10.1007/s10103-012-1232-y. [DOI] [PubMed] [Google Scholar]


