Abstract
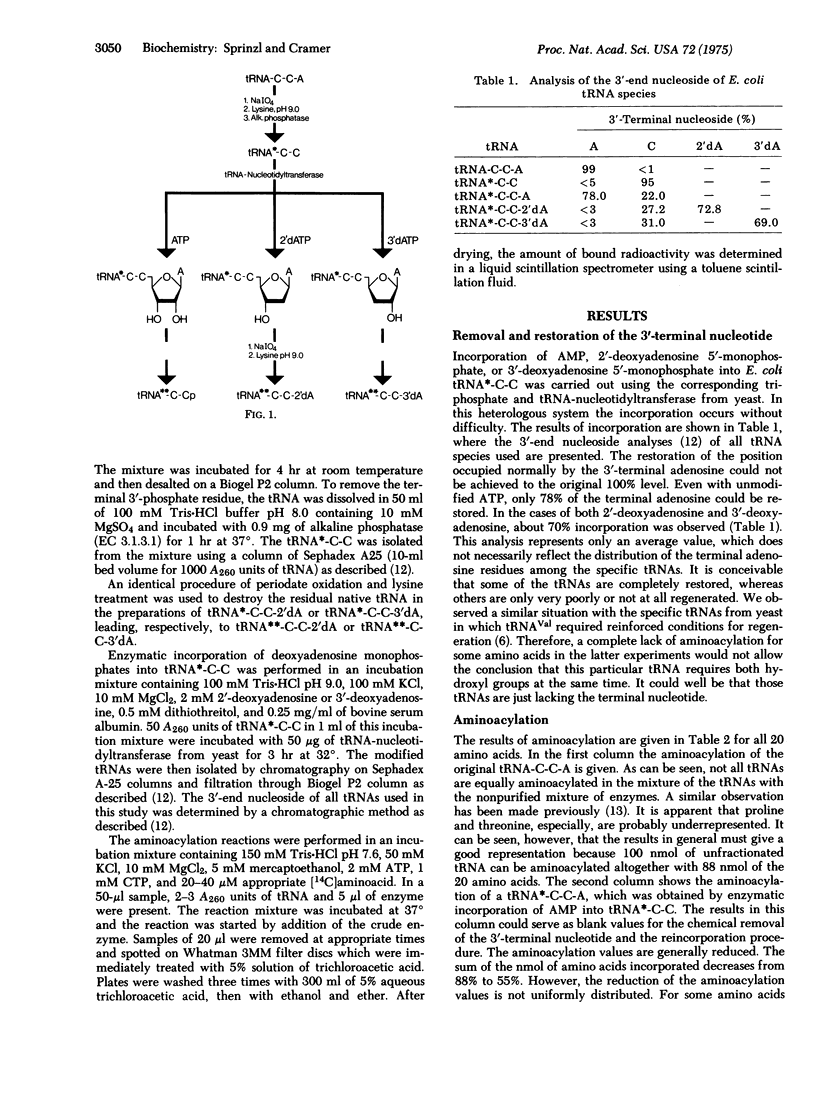
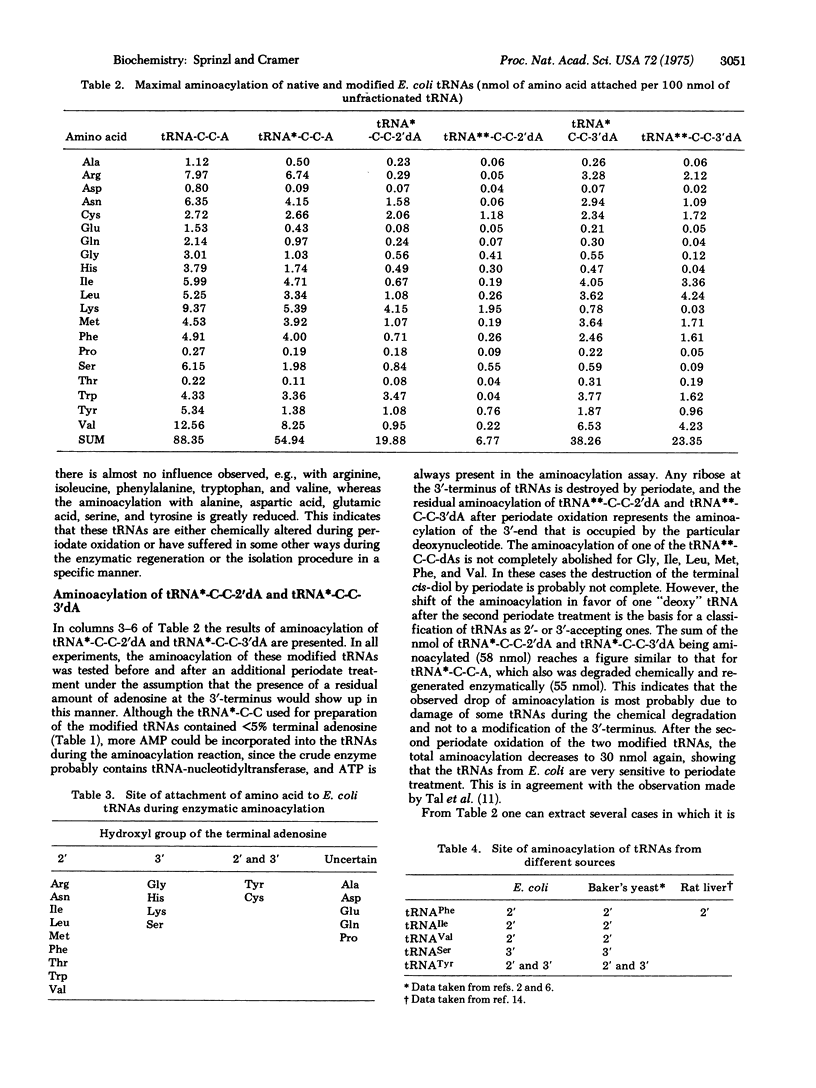
A method is presented by which the site of primary attachment of the amino acids with respect to the 2'- or 3'-hydroxyl group of the terminal adenosine of E. coli tRNAs can be determined. It is found that the aminoacyl-tRNA synthetases (EC 6.1.1.-) with specificity for Arg, Asn, Ile, Leu, Met, Phe, Thr, Trp, and Val attach the amino acid to the 2'-position; those with specificity for Gly, His, Lys, and Ser attach the amino acid to the 3'-position; and that Tyr and Cys can be enzymatically attached to both the 2'- and 3'-positions. Together with previous experiments on yeast aminoacyl-tRNA synthetases, it is now shown that the specificity for one particular hydroxyl group is preserved during the evolution from prokaryotic to eukaryotic systems.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beikirch H., von der Haar F., Cramer F. Tyrosyl-tRNA synthetase from baker's yeast. Isolation and some properties. Eur J Biochem. 1972 Mar 27;26(2):182–190. doi: 10.1111/j.1432-1033.1972.tb01755.x. [DOI] [PubMed] [Google Scholar]
- Chinali G., Sprinzl M., Parmeggiani A., Cramer F. Participation in protein biosynthesis of transfer ribonucleic acids bearing altered 3'-terminal ribosyl residues. Biochemistry. 1974 Jul 16;13(15):3001–3010. doi: 10.1021/bi00712a001. [DOI] [PubMed] [Google Scholar]
- Chousterman S., Chapeville F. Tyrosyl-tRNA synthetase of Escherichia coli B. Binding of various ligands. Eur J Biochem. 1973 May;35(1):51–56. doi: 10.1111/j.1432-1033.1973.tb02808.x. [DOI] [PubMed] [Google Scholar]
- Cramer F., Erdmann V. A., von der Haar F., Schlimme E. Structure and reactivity of tRNA. J Cell Physiol. 1969 Oct;74(2 Suppl):163+–163+. doi: 10.1002/jcp.1040740416. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Seno T., Söll G. Is there a discriminator site in transfer RNA? Proc Natl Acad Sci U S A. 1972 Oct;69(10):3063–3067. doi: 10.1073/pnas.69.10.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOARD D. E., OTT D. G. CONVERSION OF MONO- AND OLIGODEOXYRIBONUCLEOTIDES TO 5-TRIPHOSPHATES. J Am Chem Soc. 1965 Apr 20;87:1785–1788. doi: 10.1021/ja01086a031. [DOI] [PubMed] [Google Scholar]
- Kisselev L. L., Favorova O. O. Aminoacyl-tRNA synthetases: sone recent results and achievements. Adv Enzymol Relat Areas Mol Biol. 1974;40(0):141–238. doi: 10.1002/9780470122853.ch5. [DOI] [PubMed] [Google Scholar]
- Maelicke A., Sprinzl M., von der Haar F., Khwaja T. A., Cramer F. Structural studies on phenylalanine transfer ribonucleic acid from yeast with the spectroscopic label formycin. Eur J Biochem. 1974 Apr 16;43(3):617–625. doi: 10.1111/j.1432-1033.1974.tb03449.x. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Chládek S., Robilard G., Bierbaum J. Enzymatic acylation of oxidized-reduced transfer ribonucleic acid by Escherichia coli, yeast, and rat liver synthetases occurs almost exclusively at the 2'-hydroxyl. Biochemistry. 1974 Dec 17;13(26):5425–5432. doi: 10.1021/bi00723a029. [DOI] [PubMed] [Google Scholar]
- Roe B., Marcu K., Dudock B. The isolation and sequence analysis of transfer RNA: the use of plaskon chromatography (RPC-5). Biochim Biophys Acta. 1973 Aug 10;319(1):25–36. doi: 10.1016/0005-2787(73)90037-3. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Cramer F. Accepting site for aminoacylation of tRNAphe from yeast. Nat New Biol. 1973 Sep 5;245(140):3–5. doi: 10.1038/newbio245003a0. [DOI] [PubMed] [Google Scholar]
- Sprinzl M. On the structure of phenylalanine tRNA from yeast. Spin-label studies. Eur J Biochem. 1974 Dec 2;49(3):595–605. doi: 10.1111/j.1432-1033.1974.tb03863.x. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Scheit K. H., Sternbach H., von der Haar F., Cramer F. In vitro in corporation of 2'-deoxyadenosine and 3'-deoxyadenosine into yeast tRNA Phe using t-RNA nucleotidyl transferase, and properties of tRNA Phe -C-C-2'dA and tRNA Phe -C-C-3'dA. Biochem Biophys Res Commun. 1973 Apr 16;51(4):881–887. doi: 10.1016/0006-291x(73)90009-0. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., von der Haar F., Schlimme E., Sternbach H., Cramer F. Incorporation of 5-iodocytidine into yeast tRNAphe with tRNA nucleotidyl transferase in vitro. Eur J Biochem. 1972 Feb 15;25(2):262–266. doi: 10.1111/j.1432-1033.1972.tb01692.x. [DOI] [PubMed] [Google Scholar]
- Sternbach H., von der Haar F., Schlimme E., Gaertner E., Cramer F. Isolation and properties of tRNA nucleotidyl transferase from yeast. Eur J Biochem. 1971 Sep 24;22(2):166–172. doi: 10.1111/j.1432-1033.1971.tb01528.x. [DOI] [PubMed] [Google Scholar]
- Tal J., Deutscher M. P., Littauer U. Z. Biological activity of Escherichia coli tRNA Phe modified in its C-C-A terminus. Eur J Biochem. 1972 Aug 4;28(4):478–491. doi: 10.1111/j.1432-1033.1972.tb01935.x. [DOI] [PubMed] [Google Scholar]
- Von Der Haar F., Gaertner E. Phenylalanyl-tRNA synthetase from baker's yeast: role of 3'-terminal adenosine of tRNA-Phe in enzyme-substrate interaction studied with 3'-modified tRNA-Phe species. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1378–1382. doi: 10.1073/pnas.72.4.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M., Kato T., Takenishi T. A novel method for phosphorylation of nucleosides to 5'-nucleotides. Tetrahedron Lett. 1967 Dec;50:5065–5068. doi: 10.1016/s0040-4039(01)89915-9. [DOI] [PubMed] [Google Scholar]
- ZAMECNIK P. C. Unsettled questions in the field of protein synthesis. Biochem J. 1962 Nov;85:257–264. doi: 10.1042/bj0850257. [DOI] [PMC free article] [PubMed] [Google Scholar]


