Abstract
Objective
The study evaluated the effectiveness of autologous hematopoietic stem cell transplantation (AHSCT) in the treatment of lymphoblastic lymphoma (LL).
Methods
We retrospectively analyzed the data from 41 patients with chemotherapy-sensitive LL who underwent hematopoietic stem cell transplantation (HSCT) from December 1989 to December 2009 in a single institution.
Results
HSCT was conducted as first-line consolidation therapy and salvage therapy in 36 and 5 patients, respectively. The median follow-up was 97.1 months (range, 24.6-173.1 months). The 5-year overall survival (OS) and event-free survival (EFS) rate were 64% and 47% for the initially treated patients, respectively, and were both 20% for the relapsed ones. Bone marrow (BM) involvement and chemotherapy cycles prior to transplantation were identified as significant prognostic factors for EFS in multivariate analysis.
Conclusions
These results confirm that AHSCT is a reasonable option for chemotherapy-sensitive LL patients in first complete remission (CR1).
Keywords: Lymphoblastic lymphoma (LL), high-dose therapy (HDT), hematopoietic stem cell transplantation (HSCT), autologous, allogeneic
Introduction
Lymphoblastic lymphoma (LL) is a relatively infrequent malignancy, constituting approximately 2% of non-Hodgkin’s lymphoma (NHL) (1), which is common among adolescent and young adult males (2). A mass in the anterior mediastinum is common at diagnosis and bone marrow (BM) and/or central nervous system (CNS) involvement is also usually seen during the course of the disease (3).
The results of standard cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP)-based chemotherapy regimens are disappointing (3,4). The intensive protocols for acute lymphoblastic leukemia (ALL) are now proved to be more appropriate for LL with long-term survival rates from 51.0% to 86.5% (5-7). Due to a high chance of complete remission (CR) and subsequent relapse, a high-dose therapy followed by the hematopoietic stem cell transplantation (HDT/HSCT) has also been explored to consolidate remission in LL patients with some supportive evidences (8-12). So far, for adult LL patients, only one prospective randomized trial has been conducted to compare HDT/HSCT with the normal-dose consolidation regimens as post-remission therapy (11). The study suggested that the use of HDT/HSCT in the first remission produced a trend of improved progression-free survival (PFS) rate, whereas it did not improve overall survival (OS) rate. Therefore, more research is needed to evaluate whether HDT/HSCT could improve the survival of the selected appropriate LL patients. The aim of the present study was to assess the effectiveness of autologous hematopoietic stem cell transplantation (AHSCT) in chemotherapy-sensitive LL and to analyze the potential prognostic factors on survival.
Materials and methods
Patients
From December 1989 to December 2009, 120 LL patients received chemotherapy in Cancer Institute/Hospital, Chinese Academy of Medical Sciences (CAMS) & Peking Union Medical College (PUMC), 41 of which were treated with HDT/HSCT when effective remission was achieved. In this retrospective study, we collected the data from the BM and peripheral blood stem cell (PBSC) data registry of our institution.
The study was approved by the Ethics Committee and the Human Research Committee of the CAMS & PUMC. Informed consent from all adult patients and guardians of underage patients was obtained for chemotherapy, radiotherapy and HDT/HSCT related procedures.
The diagnosis of all the patients was confirmed by histological examination, and lymphocyte origins were established by immunohistochemistry. Physical examination, computed tomography (CT) of the neck, chest, abdomen and pelvis, BM aspiration and/or biopsy, and lumbar puncture were involved in staging assessment, and clinical stages were defined according to the Ann Arbor system (13). Before treatment, all patients underwent routine blood test and blood biochemical test. In our center, the patients who were more than 60 years old or had an Eastern Cooperative Oncology Group (ECOG) performance score greater than 2 were considered to be not eligible for HDT/HSCT. The patients who had ALL (more than 25% BM blasts cells), any severe cardiac, pulmonary, hepatic or renal dysfunction, or human immunodeficiency virus (HIV) infection were also excluded from this study.
Induction therapy
CHOP-based protocols, including CHOP, cyclophosphamide, doxorubicin/epirubicin, vincristine, etoposide and prednisone (CHOEP), bleomycin, epirubicin, cyclophosphamide, vincristine and prednisone (BACOP) and prednisone, cyclophosphamide, doxorubicin, etoposide, cytarabine, bleomycin, vincristine and methotrexate (proMACE-CytaBOM), were used for induction before 2004, and fractionated cyclophosphamide, vincristine, adriamycin, and dexamethasone (hyper-CVAD) regimen was taken thereafter. Besides, dexamethasone, ifosfamide, carboplatin and etoposide (DICE) and ifosfamide and topotecan (IT) regimens were also used for rescue. Only one CD20 positive B-LL patient received rituximab during both induction and consolidation period. At least 2 cycles (range, 2-7 cycles) of induction chemotherapy were offered to each patient. CNS prophylaxis included intrathecal 10 mg methotrexate and 5 mg prednisolone or 50 mg cytarabine and 5 mg prednisolone for 6-8 times in 6-12 weeks. For the patients with CNS involvement at diagnosis, the intrathecal treatments above were administered till the results of cerebrospinal fluid test returned to normal level and then additional intrathecal injections should be repeated for 2-4 times.
High-dose therapy (HDT)
High-dose consolidation was performed on the patients who achieved at least partial response (PR) from induction or rescue chemotherapy. The consolidation regimens were divided into two kinds of protocols: one is total body irradiation (TBI)-based therapy, and the other is simple high-dose chemotherapy including the following regimens: CBV (cyclophosphamide 1.8 g/m2, carmustine 450-600 mg/m2 and etoposide 900-1,600 mg/m2), BEAC (carmustine 300 mg/m2, etoposide 800 mg/m2, cytarabine 1,600 mg/m2 and cyclophosphamide 1.8 g/m2), BEAM (carmustine 300 mg/m2, etoposide 800 mg/m2, cytarabine 1,600 mg/m2 and melphalan 140-160 mg/m2), BEAM-R (BEAM and rituximab 375 mg/m2) and ACM (cytarabine 1,600 mg/m2, carboplatin 500-800 mg/m2 and mitoxantrone 50 mg/m2).
Hematopoietic stem cell transplantation (HSCT)
The patients with BM involvement and available human leukocyte antigen (HLA)-identical donors received allogeneic hematopoietic stem cell transplantation (allo-HSCT), and the rest ones accepted AHSCT. During the period of 20 years, graft types changed from BM to PBSC. No ex vivo purging was carried out in this study.
Post-transplantation radiotherapy
Post-transplantation radiotherapy (40-50 Gy) was offered to the patients with mediastinum mass at diagnosis or residual disease at transplantation.
Evaluation of clinical response
Response to treatment was evaluated according to International Workshop Criteria (14), which was based on physical examination and radiological test. CR1 or PR1 was defined as CR or PR from the first-line therapy, and CR2 defined as CR from the rescue therapy.
Follow up and statistical analysis
The SPSS 20.0 software (SPSS Inc., Chicago, USA) was used. Patient characteristics were described as median and range, or proportion. Telephone visitation was used for follow-up inspection. The last follow-up date was November 26th, 2013. OS referred to period between diagnosis and death of any causes or the last-follow up for the surviving patients. Event-free survival (EFS) started from the first day of induction and ended at the date of disease progression or relapse, or death due to any causes, whichever happened first. Survival was analyzed according to the Kaplan-Meier method, and survival variables were compared between groups using the log-rank test. Multivariate analysis with variable significant was performed with the Cox proportional hazard regression model. All P values were 2-sided and P<0.05 was considered statistically significant.
Results
Patient characteristics
Between December 1989 and December 2009, 41 LL patients were enrolled in the study. HDT/HSCT was conducted as consolidation after initial therapy in 36 patients and as salvage therapy in 5 ones. All patients’ clinical characteristics at diagnosis are summarized in Table 1. Median age was 19 years old, and there was a male predominance. There were 34 patients (83%) of T-LL subtype and 7 (17%) of B-LL subtype. There were 18 patients (44%) at stage IV, with pleural or pericardial effusion in 12 patients, BM involvement in 8 patients and CNS involvement in 7 patients. Other extra-nodal involvements included spleen (3 patients), skin or kidney (2 patients each), and adrenal gland, breast, testis or gastrointestinal tract (1 patient each). At diagnosis, 17 (42%) and 11 (27%) patients had elevated plasma lactate dehydrogenase (LDH) levels and β2-micruoglobulin (β2-M) levels, respectively (Table 1).
Table 1. Patients’ characteristics.
| Parameter | Total | CR1 | Other disease status |
|---|---|---|---|
| No. of patients [%] | 41 | 26 [63] | 15 [37] |
| Age (year) | |||
| Median | 19 | 19 | 19 |
| Range | 7-56 | 7-56 | 7-36 |
| Sex, n [%] | |||
| Male | 33 [80] | 19 [73] | 14 [93] |
| Female | 8 [20] | 7 [27] | 1 [7] |
| Immunophenotype, n [%] | |||
| T-lymphoblastic lymphoma | 34 [83] | 22 [85] | 12 [80] |
| B-lymphoblastic lymphoma | 7 [17] | 4 [15] | 3 [20] |
| Ann Arbor Stage at diagnosis, n [%] | |||
| I | 5 [12] | 4 [15] | 1 [7] |
| II | 14 [34] | 10 [39] | 4 [27] |
| III | 4 [10] | 2 [8] | 2 [13] |
| IV | 18 [44] | 10 [39] | 8 [53] |
| B symptom, n [%] | 15 [37] | 8 [31] | 7 [47] |
| ECOG performance status, n [%] | |||
| 0 | 18 [44] | 13 [50] | 5 [33] |
| 1 | 22 [54] | 12 [46] | 10 [67] |
| 2 | 1 [2] | 1 [4] | 0 [0] |
| Mediastinal involvement, n [%] | 30 [73] | 18 [69] | 12 [80] |
| Extra nodal involvement, n [%] | 24 [59] | 15 [58] | 9 [60] |
| BM involvement, n [%] | 8 [19] | 4 [15] | 4 [27] |
| CNS involvement, n [%] | 7 [17] | 3 [12] | 4 [27] |
| Pleural/pericardial effusion, n [%] | 12 [29] | 9 [35] | 3 [20] |
| High LDH at diagnosis, n [%] | 17 [42] | 10 [39] | 7 [47] |
| High β2-M at diagnosis, n [%] | 11 [27] | 10 [39] | 1 [7] |
ECOG, Eastern Cooperative Oncology Group; BM, bone marrow; CNS, central nervous system; LDH, lactate dehydrogenase; β2-M, β2-micruoglobulin.
Before 2004, 24 of the 36 (88%) initially treated patients received CHOP-based induction regimens. And 5 relapsed cases (12%) were rescued by proMACE-CytaBOM (2 patients), or hyper-CVAD, DICE or IT regimen (1 patient each). The median number of prior chemotherapy cycles was 4 (range, 2-7).
At transplantation, there were 26 (63.4%) patients of CR1, 10 (24.3%) of CR2 and 5 (12.2%) of PR1. Hematopoietic stem cell types used included: PBSC in 34 cases, BM in 5 cases, and both PBSC and BM in 2 cases. Post-transplantation radiotherapy was accomplished in 13 patients (Table 2).
Table 2. Transplantation- related factors (N=41).
| Parameter | n (%) or median [range] |
|---|---|
| Disease status at transplantation | |
| CR1 | 26 (63.4) |
| CR2 | 10 (24.3) |
| PR1 | 5 (12.2) |
| No. of prior chemotherapy cycles | 4 [2-7] |
| Graft type | |
| Bone marrow | 5 (12.2) |
| Peripheral blood stem cell | 34 (82.9) |
| Both | 2 (4.9) |
| Graft source | |
| Autologous | 38 (92.7) |
| Allogeneic | 3 (7.3) |
| Purging | 0 (0) |
| Conditioning regimens | |
| TBI based | 14 (34.1) |
| CBV | 5 (12.2) |
| BEAC | 16 (39.0) |
| BEAM | 4 (9.8) |
| BEAM-R | 1 (2.4) |
| ACM | 1 (2.4) |
| Radiotherapy post-HSCT | 13 (31.7) |
CR, complete remission; PR, partial remission; TBI, total body irradiation; CBV, cyclophosphamide, carmustine and etoposide; BEAC, carmustine, etoposide, cytarabine and cyclophosphamide; BEAM, carmustine, etoposide, cytarabine and melphalan; BEAM-R, carmustine, etoposide, cytarabine, melphalan and rituximab; ACM, cytarabine, carboplatin and mitoxantrone; HSCT, hematopoietic stem-cell transplantation.
Remission duration and survival
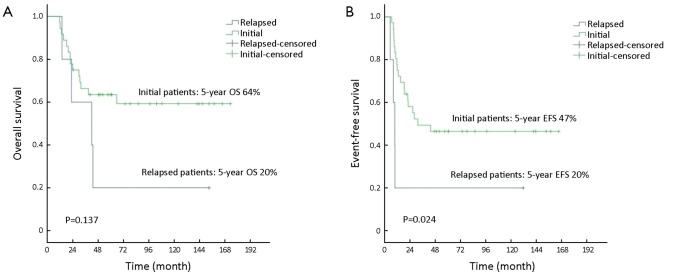
All patients accomplished engraftment successfully, without treatment-related mortality (TRM). With a median follow-up of 97.1 months (range, 24.6-173.1 months) for the surviving patients, the estimated 5-year OS and EFS were 64% and 47% for the 36 initially treated patients, respectively, and they were both 20% for the 5 relapsed patients (Figure 1).
Figure 1.
OS (A) and EFS (B) of initially treated and relapsed patients. OS, overall survival; EFS, event-free survival.
Survival according to status at transplantation
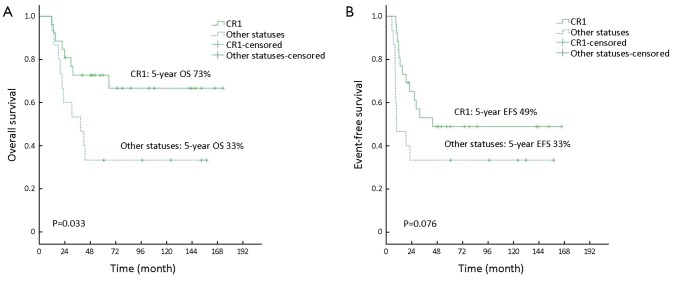
The patients who underwent HDT/HSCT at CR1 had superior outcomes compared to those at other status in terms of 5-year OS (73% vs. 33%, P=0.033), and tended to show better outcomes in 5-year EFS (49% vs. 33%, P=0.076) (Figure 2).
Figure 2.
OS (A) and EFS (B) according to disease status at transplantation: CR1 vs. other statuses. OS, overall survival; EFS, event-free survival; CR1, first complete remission.
Survival of BM involved patients
There were eight patients presenting with disease at BM, of which, three underwent allo-HSCT and remained disease-free during the follow up of 25, 40 and 144 months, respectively, while the other five received AHSCT and all died of disease progression within 25 months after the transplantation.
Prognostic factors
In univariate analysis, there was no statistically significant difference in OS and EFS for any of the following variables: sex, immunophenotype, Ann Arbor stage, B symptom, CNS involvement, pleural/pericardial effusion, high LDH, high β2-M, induction regimens, TBI in consolidation and post-HSCT radiotherapy. BM involvement (P=0.047) and the number of prior chemotherapy cycles (4 cycles as cut-off) (P=0.015) were indicated to be significant predictors for EFS. The only significant factor of OS was CR1 status at transplantation (P=0.033) (Table 3).
Table 3. Univariate analysis of predictive factors influencing survival.
| Prognostic factor | N | 5-year OS |
5-year EFS |
|||
|---|---|---|---|---|---|---|
| % | P | % | P | |||
| Male | 33 | 55 | 0.275 | 45 | 0.916 | |
| T-LBL | 34 | 58 | 0.783 | 41 | 0.574 | |
| Ann Arbor stage IV | 18 | 48 | 0.324 | 33 | 0.188 | |
| B symptom | 15 | 57 | 0.858 | 45 | 0.915 | |
| BM involvement | 8 | 23 | 0.175 | 13 | 0.047* | |
| CNS involvement | 7 | 57 | 0.979 | 57 | 0.475 | |
| Pleural/pericardial effusion | 12 | 58 | 0.88 | 50 | 0.947 | |
| High LDH at diagnosis | 17 | 52 | 0.472 | 41 | 0.627 | |
| High β2-M at diagnosis | 11 | 55 | 0.846 | 36 | 0.403 | |
| Hyper-CVAD regimen | 12 | 67 | 0.453 | 58 | 0.28 | |
| CR1 at transplantation | 26 | 73 | 0.033* | 49 | 0.076 | |
| <4 cycles of prior chemotherapy | 10 | 50 | 0.613 | 20 | 0.015* | |
| TBI in consolidation | 14 | 57 | 0.657 | 36 | 0.41 | |
| Radiotherapy post-HSCT | 13 | 69 | 0.655 | 46 | 0.719 | |
*, P<0.05. T-LBL, T lymphoblastic lymphoma; BM, bone marrow; CNS, central nervous system; LDH, lactate dehydrogenase; β2-M, β2-micruoglobulin.
Multivariate analysis demonstrated that BM involvement [P=0.016; hazard ratio (HR): 3.283; 95% confidence interval (95% CI): 1.250-8.623] and the number of prior chemotherapy cycles (<4 cycles) (P=0.026; HR: 3.201; 95% CI: 1.149-8.913) affected EFS significantly. The patients who underwent transplantation at CR1 status showed a trend towards a longer OS (P=0.070) (Table 4).
Table 4. Multivariate analysis of predictive factors influencing survival.
| Parameter | OS |
EFS |
|||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Bone marrow involvement | 2.011 | 0.652-6.204 | 0.224 | 3.283 | 1.250-8.623 | 0.016 | |
| CR1 at transplantation | 2.465 | 0.928-6.549 | 0.07 | 1.436 | 0.572-3.607 | 0.441 | |
| <4 cycles of prior chemotherapy | 1.191 | 0.376-3.772 | 0.766 | 3.201 | 1.149-8.913 | 0.026 | |
HR, hazard ratio; 95% CI, 95% confidence interval.
Discussion
In this single-center retrospective study, we described the long-term outcomes of a selected series of LL patients treated with HDT/HSCT. Our results confirmed that HDT/HSCT is effective for chemotherapy-sensitive LL patients, and the best timing for transplantation is CR1.
Of all the 41 patients in this study, 23 achieved a long-term disease control, and nearly 80% of them were at CR1 at transplantation. The patients who received HDT/HSCT at CR1 attained a 5-year OS of 73%, which was similar to the most favorable outcomes of adults reported in previous literatures. An intention-to-treat analysis concluded that a planned consolidation with HDT/HSCT in the first response resulted in favorable long-term outcomes, with a 4-year OS of 79% (9). The largest series of HDT/HSCT in LL (n=214) were reported by the European Group for Bone Marrow Transplantation: a 6-year actuarial OS was 63% for the patients transplanted at CR1 (15). Furthermore, the long-term survival seemed superior to those achieved by ALL protocols for adults. The German Multicenter Study Group for adult ALL (GMALL) reported a 7-year OS of 51% in adults with T-LL treated by ALL protocols (6). Another study demonstrated that a 3-year OS of 70% was achieved by hyper-CVAD regimen in adults (7). Besides the effectiveness of HDT/HSCT, the encouraging survival results in our study may have been influenced by the patient selection. Firstly, only the chemotherapy-sensitive patients who achieved at least PR status before transplantation were enrolled in our study. Secondly, the case series contained some children and relatively younger adults, with the median age of 19 years old. Patients younger than 20 years old may have better outcomes (4). Thirdly, all patients except one in our study had an ECOG performance status of 0 or 1 who may have a better tolerance to chemotherapy.
The 5-year EFS of patients at CR1 was 49%, which was likely inferior to the results published in the previous studies assessing HDT/HSCT or protocols for ALL in adults with LL (6,7,9-12). In our study, of the 26 patients in CR1, 17 (65%) were induced by CHOP-based regimens, which were not effective enough for LL later (8,16,17). That may contribute to the relatively poor disease control. In addition, multivariate analysis demonstrated that EFS was significantly affected by the number of prior chemotherapy cycles (4 cycles as cut-off), indicating that intensive chemotherapy protocols and adequate treatment duration were required for ideal disease control. Therefore, efforts had to be made in frontline therapy. An eight-drug intensive induction chemotherapy regimen was reported in children with T-LL, which achieved good outcomes (5-year EFS, 90%). The regimen included L-asparaginase, high-dose methotrexate, moderate cumulative doses of anthracyclines (240 mg/m2) and cyclophosphamide (3 g/m2), and efficacious CNS prevention (18). It was suggested that such intensive multi-drug regimens deserved more exploration in adults.
Of the 23 patients who suffered disease progression after HDT/HSCT, 5 responded to rescue therapies and achieved long survival. For those inherent chemotherapy-sensitive LL patients, second allogeneic or autologous HSCT was reported to be feasible (19,20). A retrospective study with the largest series suggested that a second AHSCT (AHSCT2) should be considered for the patients with relapsed Hodgkin’s lymphoma (HL) or NHL after a prior AHSCT (AHSCT1). The outcomes were encouraging, with a lower TRM than what was reported for allogeneic transplantation in that setting (20). In our series, one patient who relapsed after AHSCT1 underwent AHSCT2 in CR2, and got a long disease-free survival, suggesting that AHSCT2 could be a reasonable option for LL patients and its role remains to be evaluated by more evidences.
In our study, BM involvement was considered as an important prognostic factor for EFS, which was similar to those previous researches (4,9,21). In the patients with BM involvement, those who underwent allo-HSCT achieved obvious longer survival than those who received AHSCT. The finding suggested that for the patients with BM involvement, especially younger patients, allo-HSCT should be the priority. However, Levine et al. summarized their data and found that even though allo-HSCT for LL was associated with fewer relapses than AHSCT, higher TRM offset any potential survival benefits (22). Therefore, further validation was required for the finding.
Conclusions
The present retrospective long-term follow-up study suggests that AHSCT is an effective strategy in the treatment of chemotherapy-sensitive LL patients, and CR1 is the optimal timing for transplantation, resulting in favorable survival outcomes. For the patients with BM involvement, allo-HSCT is superior to AHSCT. Efforts have to be made in frontline therapy and strategies for relapse after AHSCT1 to improve patient survival in the future.
Acknowledgements
Funding: This study was supported in part by grants from the National Technologies Research and Development Program of China during the 9th Five-Year Plan Period (A20199610396-906-01-12), the Ying Dong Fok Foundation for Young College Teacher (B231996001), and Chinese National Major Project for New Drug Innovation (2008ZX09312, 2012ZX09303012).
Disclosure: The authors declare no conflict of interest.
References
- 1.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood 1997;89:3909-18. [PubMed] [Google Scholar]
- 2.Swerdllow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. France: IARC Press, 2008. [Google Scholar]
- 3.Nathwani BN, Diamond LW, Winberg CD, et al. Lymphoblastic lymphoma: a clinicopathologic study of 95 patients. Cancer 1981;48:2347-57. [DOI] [PubMed] [Google Scholar]
- 4.Qin Y, Shi YK, He XH, et al. Comparison of the efficiency of CHOP-based regimen with or without high dose consolidation treatment combined with hematopoietic stem cell transplantation in 63 lymphoblastic lymphoma patients. Zhonghua Zhong Liu Za Zhi (in Chinese)2009;31:469-73. [PubMed] [Google Scholar]
- 5.Katz OB, Ben Barak A, Abrahami G, et al. Treatment of T cell lymphoblastic lymphoma in children and adolescents: Israel Society of Pediatric Hematology Oncology retrospective study. Isr Med Assoc J 2011;13:161-5. [PubMed] [Google Scholar]
- 6.Hoelzer D, Gökbuget N, Digel W, et al. Outcome of adult patients with T-lymphoblastic lymphoma treated according to protocols for acute lymphoblastic leukemia. Blood 2002;99:4379-85. [DOI] [PubMed] [Google Scholar]
- 7.Thomas DA, O'Brien S, Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood 2004;104:1624-30. [DOI] [PubMed] [Google Scholar]
- 8.Bouabdallah R, Xerri L, Bardou VJ, et al. Role of induction chemotherapy and bone marrow transplantation in adult lymphoblastic lymphoma: a report on 62 patients from a single center. Ann Oncol 1998;9:619-25. [DOI] [PubMed] [Google Scholar]
- 9.Song KW, Barnett MJ, Gascoyne RD, et al. Primary therapy for adults with T-cell lymphoblastic lymphoma with hematopoietic stem-cell transplantation results in favorable outcomes. Ann Oncol 2007;18:535-40. [DOI] [PubMed] [Google Scholar]
- 10.Cortelazzo S, Intermesoli T, Oldani E, et al. Results of a lymphoblastic leukemia-like chemotherapy program with risk-adapted mediastinal irradiation and stem cell transplantation for adult patients with lymphoblastic lymphoma. Ann Hematol 2012;91:73-82. [DOI] [PubMed] [Google Scholar]
- 11.Sweetenham JW, Santini G, Qian W, et al. High-dose therapy and autologous stem-cell transplantation versus conventional-dose consolidation/maintenance therapy as postremission therapy for adult patients with lymphoblastic lymphoma: results of a randomized trial of the European Group for Blood and Marrow Transplantation and the United Kingdom Lymphoma Group. J Clin Oncol 2001;19:2927-36. [DOI] [PubMed] [Google Scholar]
- 12.Hunault M, Truchan-Graczyk M, Caillot D, et al. Outcome of adult T-lymphoblastic lymphoma after acute lymphoblastic leukemia-type treatment: a GOELAMS trial. Haematologica 2007;92:1623-30. [DOI] [PubMed] [Google Scholar]
- 13.Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res 1971;31:1860-1. [PubMed] [Google Scholar]
- 14.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999;17:1244. [DOI] [PubMed] [Google Scholar]
- 15.Sweetenham JW, Liberti G, Pearce R, et al. High-dose therapy and autologous bone marrow transplantation for adult patients with lymphoblastic lymphoma: results of the European Group for Bone Marrow Transplantation. J Clin Oncol 1994;12:1358-65. [DOI] [PubMed] [Google Scholar]
- 16.Morel P, Lepage E, Brice P, et al. Prognosis and treatment of lymphoblastic lymphoma in adults: a report on 80 patients. J Clin Oncol 1992;10:1078-85. [DOI] [PubMed] [Google Scholar]
- 17.Liang R, Todd D, Chan TK, et al. Intensive chemotherapy for adult lymphoblastic lymphomas. Cancer Chemother Pharmacol 1991;29:80-2. [DOI] [PubMed] [Google Scholar]
- 18.Reiter A, Schrappe M, Ludwig WD, et al. Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-cell lymphoblastic lymphoma: a BFM group report. Blood 2000;95:416-21. [PubMed] [Google Scholar]
- 19.Freytes CO, Loberiza FR, Rizzo JD, et al. Myeloablative allogeneic hematopoietic stem cell transplantation in patients who experience relapse after autologous stem cell transplantation for lymphoma: a report of the International Bone Marrow Transplant Registry. Blood 2004;104:3797-803. [DOI] [PubMed] [Google Scholar]
- 20.Smith SM, van Besien K, Carreras J, et al. Second autologous stem cell transplantation for relapsed lymphoma after a prior autologous transplant. Biol Blood Marrow Transplant 2008;14:904-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jabbour E, Koscielny S, Sebban C, et al. High survival rate with the LMT-89 regimen in lymphoblastic lymphoma (LL), but not in T-cell acute lymphoblastic leukemia (T-ALL). Leukemia 2006;20:814-9. [DOI] [PubMed] [Google Scholar]
- 22.Levine JE, Harris RE, Loberiza FR, Jr, et al. A comparison of allogeneic and autologous bone marrow transplantation for lymphoblastic lymphoma. Blood 2003;101:2476-82. [DOI] [PubMed] [Google Scholar]