Abstract
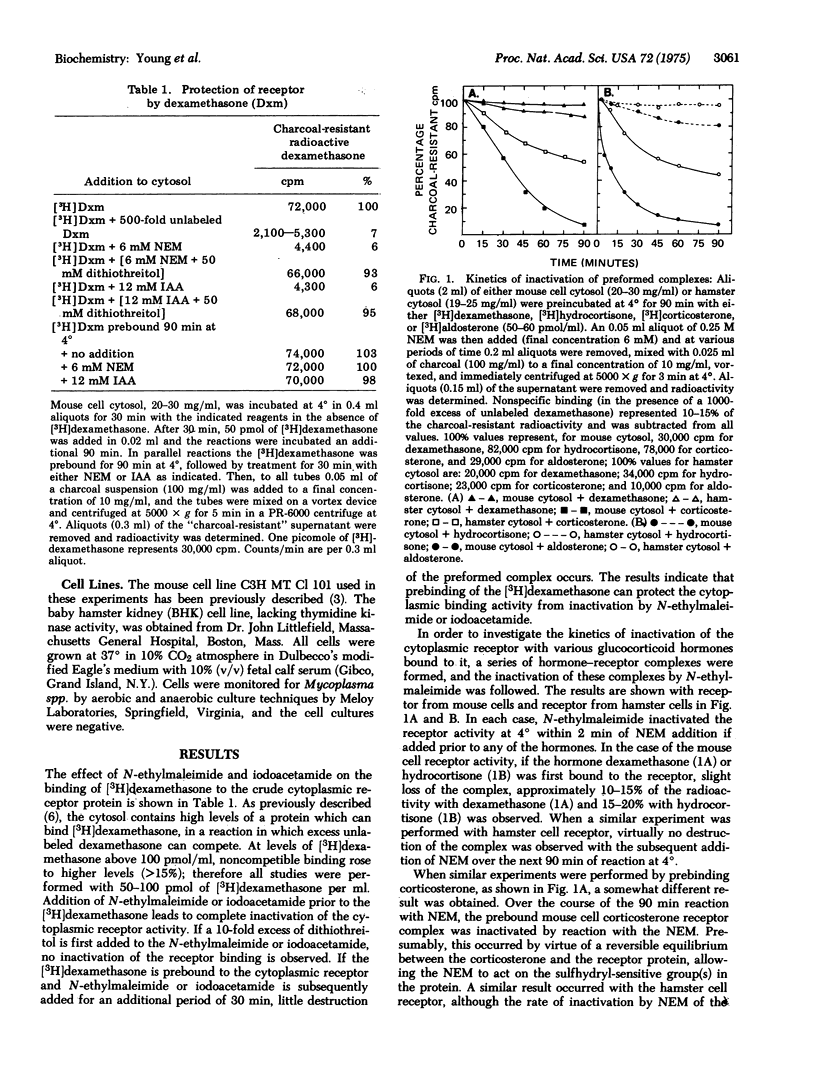
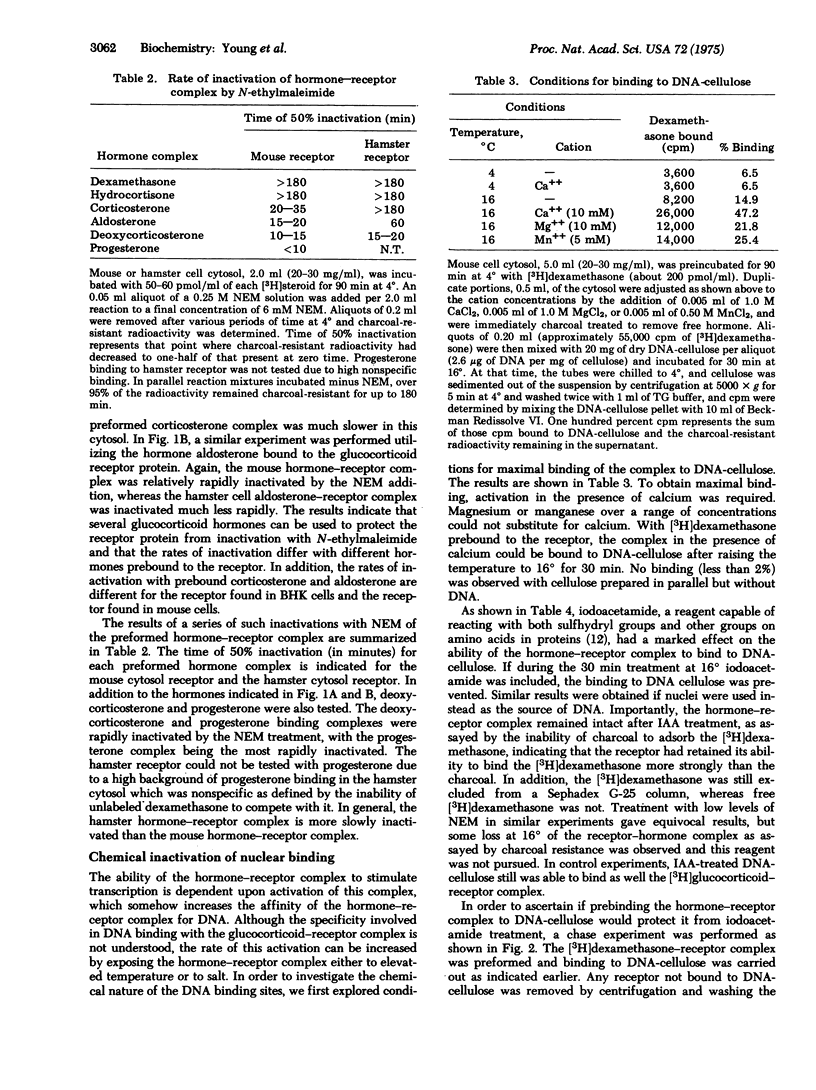
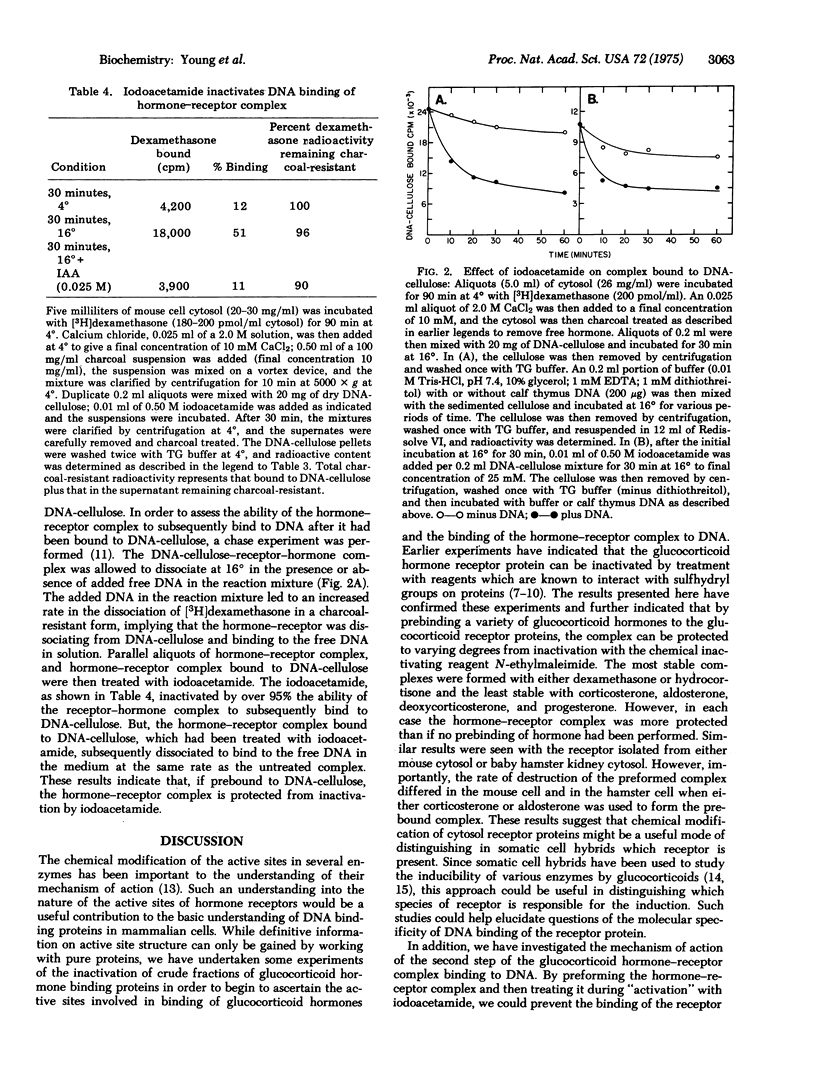
The ffect of N-ethylmaleimide and iodoacetamide on the glucocorticoid receptor activity extracted from the cytosol of either mouse of hamster cells has been investigated. Treatment of mouse or hamster cytosol with N-ethylmaleimide or iodoacetamide rapidly inactivates the [3H]glucocorticoid hormone binding activity of either cytosol. Prebinding the glucocorticoid hormone, dexamethasone, to the cytosol receptor blocks the rapid inactivation of the receptor by N-ethylmaleimide. Treatment of the prebound hormone-receptor complex with iodoacetamide prevents the subsequent binding of the hormone-receptor complex to DNA without causing a dissociation of the complex. Although the conclusions may be limited by the lack of purity of the receptor, the results suggest that a sulfhydryl group is involved in the binding of glucocorticoid hormones to the receptor protein. In addition, the results suggest that iodoacetamide is inactivating a separate chemical site which is necessary for the binding of the hormone-receptor complex to DNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andre J., Rochefort H. In vitro binding of the estrogen receptor to DNA: absence of saturation at equilibrium. FEBS Lett. 1975 Feb 15;50(3):319–323. doi: 10.1016/0014-5793(75)80519-9. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Koprowski H., Litwack G. Regulation of the corticosteroid inducibility of tyrosine aminotransferase in interspecific hybrid cells. Nature. 1974 Jun 28;249(460):839–841. doi: 10.1038/249839a0. [DOI] [PubMed] [Google Scholar]
- Gardner D. G., Wittliff J. L. Characterization of a distinct glucocorticoid-binding protein in the lactating mammary gland of the rat. Biochim Biophys Acta. 1973 Oct 5;320(3):617–627. doi: 10.1016/0304-4165(73)90141-4. [DOI] [PubMed] [Google Scholar]
- Gehring U., Tomkins G. M. A new mechanism for steroid unresponsiveness: loss of nuclear binding activity of a steroid hormone receptor. Cell. 1974 Nov;3(3):301–306. doi: 10.1016/0092-8674(74)90145-7. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., DeSombre E. R. Mechanism of action of the female sex hormones. Annu Rev Biochem. 1972;41:203–230. doi: 10.1146/annurev.bi.41.070172.001223. [DOI] [PubMed] [Google Scholar]
- Litman R. M. A deoxyribonucleic acid polymerase from Micrococcus luteus (Micrococcus lysodeikticus) isolated on deoxyribonucleic acid-cellulose. J Biol Chem. 1968 Dec 10;243(23):6222–6233. [PubMed] [Google Scholar]
- Mayer M., Kaiser N., Milholland R. J., Rosen F. Cortisol binding in rat skeletal muscle. J Biol Chem. 1975 Feb 25;250(4):1207–1211. [PubMed] [Google Scholar]
- McGrath C. M. Replication of mammary tumor virus in tumor cell cultures: dependence on hormone-induced cellular organization. J Natl Cancer Inst. 1971 Aug;47(2):455–467. [PubMed] [Google Scholar]
- Parks W. P., Ransom J. C., Young H. A., Scolnick E. M. Mammary tumor virus induction by glucocorticoids. Characterization of specific transcriptional regulation. J Biol Chem. 1975 May 10;250(9):3330–3336. [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Kozikowski E. H. Dexamethasone stimulation of murine mammary tumor virus expression: a tissue culture source of virus. Science. 1974 Apr 12;184(4133):158–160. doi: 10.1126/science.184.4133.158. [DOI] [PubMed] [Google Scholar]
- Schaumburg B. P. Investigations on the glucocorticoid-binding protein from rat thymocytes. II. Stability, kinetics and specificity of binding of steroids. Biochim Biophys Acta. 1972 Jan 28;261(1):219–235. doi: 10.1016/0304-4165(72)90333-9. [DOI] [PubMed] [Google Scholar]
- Schutz G., Killewich L., Chen G., Feigelson P. Control of the mRNA for hepatic tryptophan oxygenase during hormonal and substrate induction. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1017–1020. doi: 10.1073/pnas.72.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley C. H., Tomkins G. M. Mechanisms of steroid resistance. Cell. 1974 Aug;2(4):221–227. doi: 10.1016/0092-8674(74)90014-2. [DOI] [PubMed] [Google Scholar]
- Thompson E. B., Gelehrter T. D. Expression of tyrosine aminotransferase activity in somatic-cell heterokaryons: evidence for negative control of enzyme expression. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2589–2593. doi: 10.1073/pnas.68.10.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F. Chemical approaches to the properties of active sites of enzymes. Annu Rev Biochem. 1969;38:733–794. doi: 10.1146/annurev.bi.38.070169.003505. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Orth D. N., Toft D. O. Glucocorticoid receptors in pituitary tumor cells. I. Cytosol receptors. J Biol Chem. 1973 Nov 25;248(22):7625–7630. [PubMed] [Google Scholar]
- Yamamoto K. R., Stampfer M. R., Tomkins G. M. Receptors from glucocorticoid-sensitive lymphoma cells and two clases of insensitive clones: physical and DNA-binding properties. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3901–3905. doi: 10.1073/pnas.71.10.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H. A., Scolnick E. M., Parks W. P. Glucocorticoid-receptor interaction and induction of murine mammary tumor virus. J Biol Chem. 1975 May 10;250(9):3337–3343. [PubMed] [Google Scholar]


