Abstract
The pentose phosphate pathway (PPP), which branches from glycolysis at the first committed step of glucose metabolism, is required for the synthesis of ribonucleotides and is a major source of NADPH. NADPH is required for and consumed during fatty acid synthesis and the scavenging of reactive oxygen species. Therefore, the PPP plays a pivotal role in helping glycolytic cancer cells to meet their anabolic demands and combat oxidative stress. Recently, several neoplastic lesions were shown to have evolved to facilitate the flux of glucose into the pentose phosphate pathway. This review summarizes the fundamental functions of the PPP, its regulation in cancer cells, and its importance in cancer cell metabolism and survival.
Introduction to the pentose phosphate pathway
The pentose phosphate pathway (PPP) also known as the hexose monophosphate shunt or phosphogluconate pathway, branches from glycolysis at the first committed step, which is catalyzed by hexokinase and consumes glucose-6-phosphate as a primary substrate (Fig. 1). The PPP gained significant attention approximately 90 years ago due to the revelation that hemolytic anemia, which is induced by oxidant agents, Fava beans and certain drugs such as antimalarial drugs and sulfa antibiotics is correlated with the lack of reduced glutathione (GSH)1. Subsequently, it was found that individuals who are susceptible to hemolytic anemia display genetically inherited reduced activity of glucose-6-phosphate dehydrogenase (G6PDH), which catalyzes the first committed step in the PPP2. In red blood cells the PPP is the exclusive source of NADPH, which is required for the generation of reduced GSH, a major scavenger of reactive oxygen species (ROS). Therefore, attenuated PPP activity renders red blood cells more vulnerable to oxidants and reagents that interfere with the PPP2. In the 1930s, Otto Warburg first discovered that NADP+ is required for the oxidation of glucose-6-phophate, which is the first committed step of PPP. However, it was the seminal works of Frank Dickens, Bernard Horecker, Fritz Lipmann and Efraim Racker in the 1950s that fully elucidated the entire pentose phosphate pathway3. Taken together, these studies revealed that in addition to its principal function of generating phosphopentoses and ribonucleotides, the PPP is a major source of NADPH, and it plays a pivotal role in the cellular redox state.
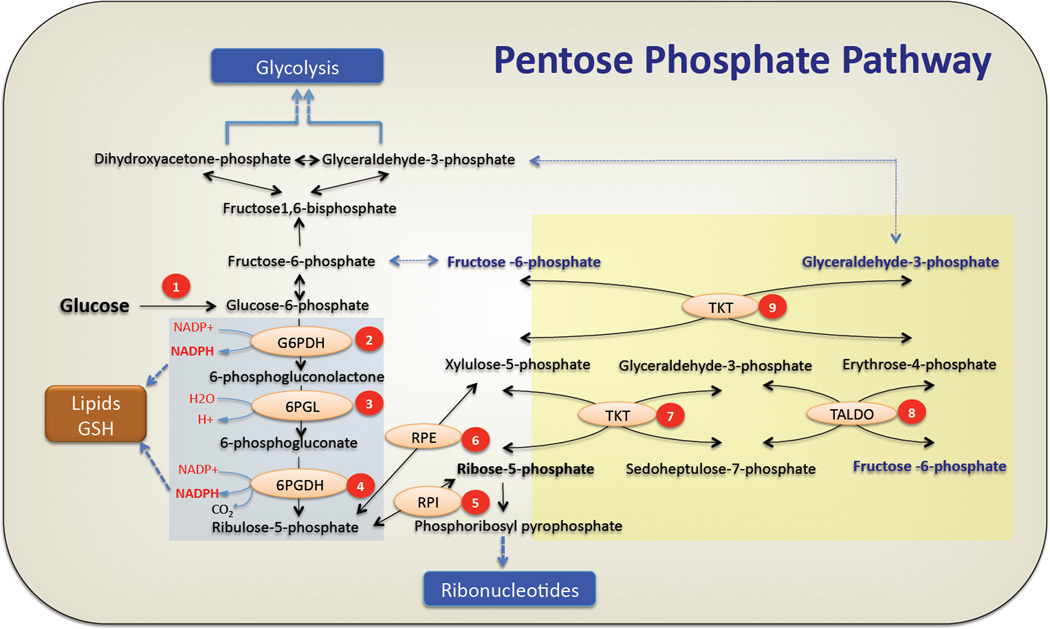
Figure 1. The pentose phosphate pathway.
The oxidative arm of pentose phosphate pathway is initiated by conversion of glucose to glucose-6-phosphate (G6P) by hexokinases. (1) Glucose-6 Phosphate Dehydrogenase (G6PDH) oxidizes glucose-6 phosphate to form 6-phosphogluconolactone while reducing NADP+ to NADPH. (2) Hydrolysis of 6-phosphogluconolactone by 6-Phosphogluconase (6PGL) generates 6-phosphogluconate(6PG). (3) Formation of ribulose-5-phosphate occurs by oxidative decarboxylation of 6-phosphogluconate by 6PGDH. In this step the second molecule of NADPH is generated. NADPH produced in oxidative PPP can be utilized for lipid biosynthesis or ROS detoxification (GSH) (see text for deatails). Ribulose 5 phoshate (Ru5P) undergoes (5) an isomerization by Ribulose-5-Phoshate Isomerase (RPI) or (6) epimerization reaction by Ribulose-5-Phoshate Epimerase (RPE) to generate ribose-5-phosphate(R5P) or xylulose-5-phosphate(Xu5P) respectively. Ribose-5-phosphate is converted to phosphoribosyl-pyrophosphate that serves as the backbone for ribonucleotide synthesis. In the nonoxidative PPP, (7) Transketolase transfers two carbon units from xylulose-5-phosphate to ribose-5-phosphate to generate sedoheptulose-7-phosphate (S7P) and glyceraldehyde-3-phosphate (G3P). (8) Transaldolase transfers three carbon units from sedoheptulose-7-phosphate to glyceraldehyde-3-phosphate to generate erythrose-4-phosphate and the first molecule of fructose-6-phosphate. (9) In a second transketolase reaction, two carbon units from xylulose-5-phosphate are transferred to erythrose-4-phosphate to yield a second molecule of fructose-6-phosphate and glyceraldehyde-3-phosphate. Fructose-6-phosphate (F6P) can either be used for glycolysis or be converted back to G6P to replenish the oxidative PPP, while G3P can be utilized in glycolysis, depending on the cellular requirements (see text for details). The oxidative and nonoxidative branches of the PPP are highlighted by a blue and yellow backgrounds respectively.
The PPP is composed of two phases or branches: the oxidative branch, and the nonoxidative branch. The oxidative branch, which generates NADPH and ribonucleotides, has three irreversible reactions. In the first reaction, glucose-6-phophate (G6P) is dehydrogenated by G6PDH to yield NADPH and 6-phosphogluconlactone, which is subsequently hydrolyzed by phosphogluconolactonase (6PGL) into 6-phosphogluconate. The third reaction is the oxidative decarboxylation of 6-phosphogluconate, which is catalyzed by 6-phosphogluconate dehydrogenase (6PGDH), to yield a second NADPH and ribulose-5-phosphate (Ru5P), which is then converted to ribose-5-phosphate (R5P) (Fig. 1). The nonoxidative branch consists of a series of reversible reactions that recruit additional glycolytic intermediates, such as fructose-6-phosphate (F6P) and glyceraldehyde-3-phosphate (G3P), which can be converted into pentose phosphates and vice versa (Fig. 1)3–5.
Enzymes in the PPP are subjected to allosteric regulation by their own catalytic products and other metabolites6 (discussed later). The reversible nature of the nonoxidative PPP branch and the allosteric regulation of enzymes in this pathway enable the PPP to adapt to the metabolic demands of cells, operating in different modes. For instance, in cells for which maintaining redox homeostasis is more important than nucleic acid synthesis, the PPP is tailored to accelerate the oxidative branch and to direct the nonoxidative branch towards re-synthesizing F6P from pentose phosphate, which is then converted back to G6P to replenish the oxidative branch (Fig. 1). In rapidly dividing cells, most of the pentose phosphates that are incorporated into DNA are derived from the PPP7. Therefore, the PPP is diverted toward the generation of pentose phosphates from both G6P in the oxidative branch and F6P and G3P in the nonoxidative branch8,9. Thus, the different modes of the PPP could influence the flux of glucose in glycolysis, and vice versa.
The PPP is especially critical for cancer cells because it generates not only pentose phosphates to supply their high rate of nucleic acid synthesis, but also provides NADPH, which is required for both the synthesis of fatty acids and cell survival under stress conditions. In recent years, accumulating data have indicated that neoplastic lesions in cancer cells modulate the flux of the PPP either directly or indirectly. In the following sections we will review the enzymatic machineries, which drive the PPP and how the two modes of the PPP are coordinated in response to the dynamic tumor microenvironment and intracellular demands to promote cancer cell proliferation, growth and survival. Finally we will discuss the challenges of inhibiting the PPP as a potential strategy for cancer therapy.
The enzymes that execute the PPP
The enzymes that execute the PPP could determine the rate of the PPP and the relative activities of the oxidative and the nonoxidative branches. Since these enzymes are subject to regulation and modification by multiple mechanisms that could change their activities in cancer cells it is important to understand how the individual enzymes are structured and how their activities are regulated.
Glucose-6-Phosphate Dehydrogenase
Glucose-6-Phosphate Dehydrogenase (G6PDH) catalyzes the rate-limiting step in the oxidative branch of PPP that generates the first molecule of NADPH, therefore its expression and activity are tightly regulated. G6PDH exists as either an inactive monomer or an active dimer. A higher order complex, such as a tetramer (composed of two dimers), has also been reported10. Relatively high levels of this enzyme are present in many normal metabolizing tissues, including the liver, adipose, and mammary and adrenal glands11–14. Tumor cells also express relatively high levels of this enzyme15. In terms of activity regulation, the NADP+/NADPH ratio is one of the main modulators of this enzyme. NADPH negatively regulates the activity of G6PDH, whereas NADP+ is required for its enzymatic activity and for its proper conformation16. Hence, in cells with high NADPH consumption, such as cancer cells, the activity of this enzyme is also high17. Normal resting cells with low NADPH consumption have a relatively low NADP+/NADPH ratio, possibly explaining why G6PDH activity is relatively low despite high expression levels in some tissues.
G6PDH is regulated by several extracellular stimuli and signaling pathways that regulate its expression and modulate its activity via posttranslational mechanisms. It was reported that growth factors such as platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) induce the release of bound G6PDH (with structural elements) to a soluble fraction, which is associated with an increase in its activity18,19. This effect appears to be mediated by phosphatidylinositol-3-kinase (PI3K) and Ras. In addition, the tyrosine kinase Src can directly phosphorylate G6PDH and induce its translocation20. Thus, pro-oncogenic signaling pathways that are often hyperactivated in cancer cells accelerate the PPP by positively regulating G6PDH (Fig. 2). Notably, the gene encoding G6PDH is on the X chromosome, and therefore is subject to X inactivation in females. However, occasionally the two alleles are expressed in females as a result of DNA demethylation21, and thus methylation and demethylation can regulate the expression of G6PDH, which could potentially affect its expression in cancer cells. In certain cell types, cyclic AMP (c-AMP) downregulates G6PDH activity both directly and indirectly. c-AMP activates protein kinase A (PKA), which directly phosphorylates G6PDH on serine and threonine residues and inhibits G6PDH activity. c-AMP also inhibits transcription of the gene encoding G6PDH via a c-AMP response element within the promoter region of the gene22–24.
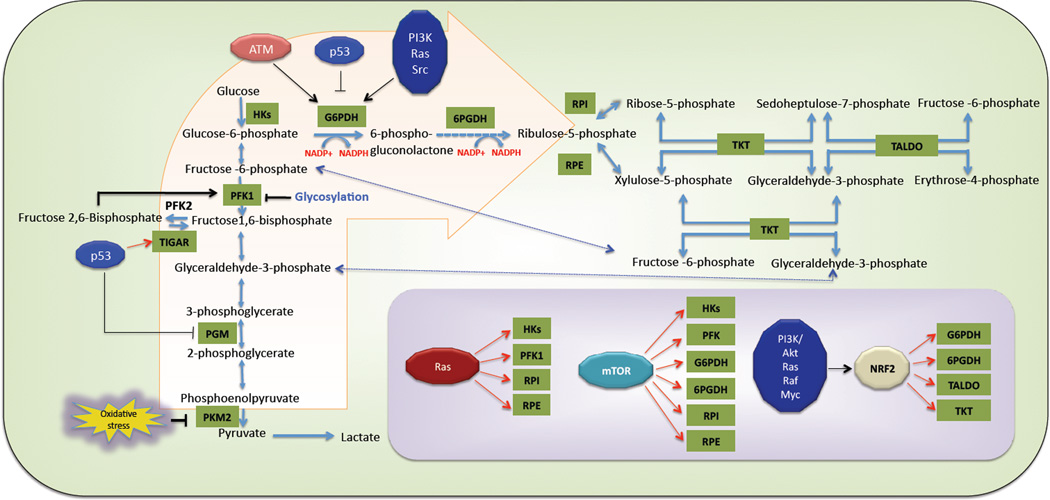
Figure 2. Oncogenic regulation of the pentose phosphate pathway.
By transcriptional upregulation of TIGAR, p53 increases the flux of glucose to the PPP. By contrast, p53 was shown to inhibit G6PDH activity through protein-protein interaction. Oxidation and glycosylation, two post-translational modifications, inhibit the glycolytic enzymes PKM2 and PFK1, respectively, and re-route the glycolytic flux towards oxidative PPP. ATM, Ras, PI3K/Akt and Src positively influence G6PDH activity through several mechanisms. In addition, several oncoproteins modulate the PPP by transcriptional up-regulation of several enzymes that affect the PPP directly or indirectly (Inset) (Transcriptional regulations are showed by red arrows. (For details see text). Abbreviations: HKs: Hexokinases, PFK1: Phosphofructo Kinase-1, TIGAR: TP53-inducible glycolysis and apoptosis regulator, PGM: Phosphoglycerate Mutase, PKM2: Pyruvate Kinase Muscle isoform-2, ATM: Ataxia Telangiectasia Mutated, G6PDH: Glucose-6 Phosphate Dehydrogenase, 6PGDH: 6-Phosphogluconate Dehydrogenase, RPI: Ribulose-5-Phoshate Isomerase, RPE: Ribulose-5-Phoshate Epimerase, TKT: Transketolase, TALDO: Transaldolase, mTOR: Mammalian target of rapamycin, NRF2: Nuclear factor erythroid 2–related factor 2.
As will be discussed later, several oncoproteins and tumor suppressors modulate the expression and activity of G6PDH, thus influencing the metabolic demands and survival of cancer cells.
6-Phosphogluconolactonase
The conversion of 6-phosphogluconolactone to 6-phosphogluconate was initially believed to be a non-enzymatic reaction. 6-phosphogluconolactone is very unstable, and hydrolysis of lactone occurs at neutral pH. Because this non-enzymatic hydrolysis occurs at a slower rate, an enzymatic reaction was postulated, and 6-Phosphogluconolactonase (6PGL) was discovered as an enzyme that hydrolyses 6-phosphogluconolactone to 6-phosphogluconate25. Although this enzyme has not been well studied, a mutation in this enzyme in erythrocytes was reported to cause hemolytic anemia in a particular group of subjects26.
6-Phosphogluconate Dehydrogenase
The next step in the oxidative PPP generates the second molecule of NADPH and Ribulose-5-phosphate (Ru5P), and it is catalyzed by 6-Phosphogluconate Dehydrogenase (6PGDH). In lung cancer cells, 6PGDH is critical for proliferation and tumorigenic potential27. Interestingly, genetic silencing of 6PGDH resulted in p53 accumulation and senescence in lung cancer cells. This is also accompanied by increased oxygen consumption, resulting accumulation of ROS, that might also contribute to senescence. Surprisingly, the level of NADPH did not change, although upstream metabolites in the oxidative branch of PPP, 6-phosphogluconate and 6-phosphogluconolactone, did accumulate. It is possible that the absence of 6PGDH resulted in a temporal increase in the NADP+/NADPH ratio that induced G6PDH activity, thus generating more NADPH in the first reaction of the PPP and compensating for the reduced NADPH resulting from the loss of 6PGDH.
Ribulose-5-phosphate isomerase and Ribulose-5-phosphate epimerase
Ribulose-5-phosphate is converted to Ribose-5-phosphate (R5P) and Xylulose-5 phosphate (Xu5P) by Ribulose-5-phosphate isomerase (RPI) and Ribulose-5-phosphate epimerase (RPE), respectively (Fig. 1). R5P is the critical metabolite precursor for de novo ribonucleotide synthesis in proliferating cancer cells. Xu5P, in addition to its role in the PPP, was reported to affect glycolysis. Xu5P increases the level of fructose 2,6 bisphosphate (F-2,6BP), which activates phospho-fructose kinase 1 (PFK1). Xu5P exerts its effect on the intracellular levels of F-2,6BP via the activation of Protein phosphatase-2A (PP2A), which dephosphorylates fructose 6-phosphate 2-kinase/fructose 2,6-bisphosphatase28. Recent reports have demonstrated that RPI and RPE play important roles in oncogenic K-Ras induced pancreatic cancer29.
Transketolase and Transaldolase
Transketolase (TKT) and transaldolase (TALDO) are the two major enzymes that mediate the nonoxidative PPP. Due to the reversible nature of these enzymes, they can determine the direction of metabolite flux in the PPP in a temporal manner. TALDO is a thiamine pyrophosphate cofactor-containing enzyme that functions as a homodimer30. During oxidative stress, NADPH is needed for the generation of reduced glutathione. Under these conditions, transketolase converts R5P and Xu5P to produce glyceraldehyde-3-phosphate (G3P) and sedoheptulose-7 phosphate (S7P), while transaldolase transfers a C3 unit from S7P to G3P to form erythrose-4-phosphate (E4P) and fructose-6-phosphate (F6P) (Fig. 1B). A second transketolase reaction occurs between Xu5P and E4P to generate F6P and G3P. F6P can then be converted back to G6P to replenish the oxidative PPP to generate additional NADPH, while G3P can be utilized in the subsequent steps of glycolysis (Fig. 1). In rapidly dividing cancer cells, when the metabolic need for nucleotides exceeds that of NADPH, TKT and TALDO catalyze the reverse reactions and divert G3P and F6P from glycolysis to the nonoxidative PPP to generate additional ribonucleotides. Cancer cells predominantly employ the nonoxidative PPP to generate ribonucleotides de novo for the synthesis of RNA and DNA. 13C tracing experiments demonstrated that in rapidly proliferating cancer cells, approximately 80% of ribonucleotides are derived from the nonoxidative PPP31.
Cancer cells can accelerate the nonoxidative PPP by elevating the expression of the enzymes in this branch of the PPP. For instance, cancer cells can elevate TKT expression32 and induce expression of a transketolase-like-1 (TKTL1) gene33. TKTL1 is highly expressed in various cancers, and its expression is correlated with poor prognosis in colon and urothelial cancer34. However, although it is widely accepted that TKTL1 possesses transketolase activity, this assumption was questioned by some studies35.TALDO expression is increased in liver tumors36. Interestingly, deletion of TALDO in mice elicits hepatocellular carcinoma (HCC)37. This observation can be explained by a redox imbalance due to the failure to recycle PPP metabolites for the generation of a sufficient amount of NADPH, which would subsequently expose the liver to oxidative damage and pro-tumorigenic inflammation. In summary, in cancer cells the expression and activities of the PPP enzymes are regulated according to the adaptive microenvironment. In addition, the increased glycolytic flux in cancer could indirectly affect the PPP (BOX1). These regulations are vital for the survival and proliferation of tumor cells.
BOX-1 Crosstalk between the PPP and glycolysis.
Increasing the glycolytic flux in cancer cells might also indirectly impact the nonoxidative PPP. For instance, hexokinase-2 (HK2), which is highly expressed in cancer cells, is required for increased ribonucleotide synthesis via the nonoxidative PPP47. Finally, the elevated glycolytic flux in hypoxic cancer cells increases the abundance of F6P and G3P, which can be utilized in the nonoxidative PPP.
As described earlier, the PPP interacts with glycolysis through the nonoxidative branch. In addition, several glycolytic enzymes regulate the flux of glucose to the oxidative branch of PPP in a context dependent manner. For instance, pyruvate kinase isoform-2 (PKM2) is posttranslationally modified on cysteine residues by oxidative stress. This modification inactivates PKM2 and attenuates the glucose flux and thus more G6P could be diverted to the oxidative branch of the PPP to generate NADPH to neutralize ROS73. Additionally, the rate limiting glycolytic enzyme, Phosphofructokinase-1 (PFK1), is modified by O-GlcNAcylation on a serine residue under hypoxic conditions, which inhibits its enzymatic activity74. This posttranslational modification is a fail-safe mechanism adopted by tumor cells to redirect glucose metabolism for NADPH production during hypoxia through the oxidative branch of PPP. Balancing glycolytic and PPP flux is also important for prostate cancer. RNAi screening of metabolic enzymes in prostate cancer cells revealed that 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 (PFKFB4), an isoform of Phosphofructokinase-2 (PFK2), plays a critical role in cell survival and tumorigenicity75. Silencing PFKFB4 increases F2,6-BP, an allosteric activator of PFK1, diverting glucose flux towards glycolysis. Thus, by modulating PFK1 activity, PFKFB4 can maintain a homeostatic balance between glycolysis and the PPP. Finally, phosphoglycerate mutase-1 (PGAM1), a glycolytic enzyme that converts 3-phosphoglycerate (3PG) to 2-phosphoglycerate (2PG), is overexpressed in cancer cells. Because 3-PG is an inhibitor of the oxidative PPP enzyme 6PGDH, which generates R5P, overexpression of PGAM1 indirectly increases oxidative PPP. Similarly, knockdown of PGAM1 decreased oxidative PPP and subsequent glucose incorporation into RNA synthesis39. Therefore, in cancer cells, glycolytic enzymes regulate both the oxidative and the nonoxidative PPP based on demand.
Oncogenic regulation of the PPP
To fulfill their requirements for nucleic acid synthesis, NADPH generation, fatty acid synthesis, and cell survival, rapidly dividing cancer cells have evolved mechanisms that regulate the PPP. As indicated earlier, hyperactivation of pro-oncogenic signaling pathways such as PI3K/Akt, Ras, and Src might promote G6PDH activation by posttranslational mechanisms. However, several other mechanisms described below by which tumor suppressor proteins and oncoproteins influence the PPP have been reported in recent years.
The tumor suppressor p53
Several studies implicate the tumor suppressor p53 in the regulation of the PPP. As a transcription factor, p53 binds to the promoter region of several genes involved in various control points of the PPP. p53 directly binds to the promoters of the glucose transporter genes GLUT1 and GLUT4 and inhibits their expression38. Hence, the loss of p53 increases glucose uptake in cancer cells, which supports both glycolysis and the PPP. p53 could also indirectly inhibit the oxidative PPP through the suppression of expression of the glycolytic enzyme PGAM139,40. As was indicated in BOX1 PGAM1 increases the oxidative PPP by decreasing the level of 3PG, which otherwise inhibits 6PGDH39.
TIGAR (TP53-induced glycolysis and apoptosis regulator), which is transcriptionally induced by p53, inhibits glycolysis, redirecting the metabolites to the PPP. TIGAR appears to function like F2,6 bisphosphatase (F2,6BPase), which lowers the level of F2,6BP. The gatekeeper glycolytic enzyme, PFK1, is allosterically activated by F2,6BP, and thus the induction of TIGAR expression by p53 reduces the activity of PFK1 and the flux into glycolysis. Consequently, glucose is diverted to the PPP to increase NADPH and decrease ROS41. The accumulation of F6P caused by TIGAR could also increase the flux of F6P into the nonoxidative PPP to help generate the nucleotides required for DNA repair after DNA damage (Fig. 2).
TIGAR also inhibits ROS by binding to HK2 and promoting the activity of HK2 at the mitochondria during hypoxia. This binding is independent of the bisphosphatase activity of TIGAR, suggesting that TIGAR is a positive regulator of the PPP in both a phosphatase-dependent and -independent manner42. In an in vivo study utilizing TIGAR conditional knockout mice, TIGAR loss impaired tumor formation in intestinal colon cancer generated by the loss of the tumor suppressor APC. Intestinal regeneration and adenoma formation were suppressed by elevated ROS due to decreased PPP activity. Interestingly, treatment with the antioxidant N-acetyl cysteine (NAC), or a supplement of nucleosides, rescued the reduced growth and proliferation induced by the loss of TIGAR42.
In addition to TIGAR another p53 target, the pro-apoptotic protein NOXA, was implicated in increasing flux through the PPP43. In myeloid and lymphoid cancer cells NOXA plays a dual role depending on the availability of glucose. In the absence of glucose, NOXA functions as a pro-apoptotic protein. Intriguingly, in the presence of glucose, NOXA promotes cell survival by facilitating the first step in the PPP to accelerate the generation of NADPH, although the exact mechanism remains unknown.
In response to DNA damage or oxidative stress the activity of the ataxia telangiectasia mutated (ATM) kinase is elevated, which in turn activates p53. Interestingly, ATM activates the PPP, independently of p53, through a posttranslational mechanism44. ATM activation mediates the interaction between heat shock protein Hsp27 and G6PDH. This interaction increases the activity of G6PDH and subsequently increases the level of NADPH and nucleotides generated by the PPP (Fig. 2). This may explain why ATM-deficient cells and cells derived from patients with ataxia telangiectasia, who have a mutated inactive ATM gene, have high levels of ROS.
Contrary to the reported positive effects of p53 and ATM on the oxidative PPP, other reports suggest that p53 inhibits the PPP, as the loss of p53 increases NADPH production45. According to this report, most cytoplasmic p53 is associated with G6PDH, and this interaction suppresses G6PDH activity by inhibiting the assembly of G6PDH monomers into active dimers. Although cancer-associated p53 mutants are capable of binding G6PDH, they are impaired in their ability to inhibit G6PDH activity. However, because only 10% of G6PDH binds cytoplasmic p53, it is not clear how this inhibition is exerted. In contrast to p53, the p53-related protein p73, which promotes cell proliferation, induces the expression of G6PDH and facilitates the PPP46.
Perhaps the two seemingly contradicting effects of p53 on the PPP could be explained by its two activities; namely, as an inducer of cell cycle arrest and/or apoptosis. p53-mediated cell cycle arrest in response to DNA damage enables cells to repair the damage before re-entering the cell cycle. Under these conditions, the positive effect of p53 on the PPP maintains cell survival while generating nucleotides for DNA repair. If the cells are unable to repair the damage, p53 activation induces cell death. Under these conditions, inhibition of the PPP by p53 accelerates cell death by decreasing NADPH levels and consequently increasing intracellular levels of ROS.
Oncogenic Ras
Activating mutations in RAS genes occur in many human cancers. Human cancers that frequently display activating mutations of K-Ras include lung, pancreatic and colon cancers. Studies on the metabolic consequences of K-Ras activation in a mouse model of pancreatic cancer revealed that the nonoxidative PPP is substantially activated, whereas the oxidative branch is unaffected29. Therefore, these cells generate nucleotides primarily through the nonoxidative PPP. The elevated nonoxidative PPP is accompanied by the transcriptional upregulation of the genes encoding RPI and RPE, without any significant change in the expression of enzymes in the oxidative PPP. Thus, these pancreatic cancer cells depend on RPI and RPE to generate the ribonucleotides required for nucleic acid biosynthesis.
A high glucose flux is required to generate G6P to maintain and facilitate the oxidative and nonoxidative PPP in cancer cells, which can be achieved by the induction of HK2 expression by oncogenic Ras47. Genetic ablation of HK2 in K-Ras-induced mouse models of lung cancer reduced tumor burden47. HK2 deficiency impaired glucose dependent ribonucleotide synthesis via the nonoxidative PPP while maintaining NADPH production by the oxidative PPP, suggesting that the elevated nonoxidative PPP in K-Ras-dependent cancer is also mediated by the increased expression of HK247.
mTORC1
The mammalian target of rapamycin complex 1 (mTORC1) is frequently activated in cancer cells due to activation of PI3K/Akt signaling and other mechanisms. Gene expression and metabolic profiles revealed that mTORC1 activation causes a significant upregulation of the oxidative PPP by elevating the activity of the transcription factor sterol regulatory element-binding protein (SREBP)48. The SREBP transcription factors are normally inserted in the endoplasmic reticulum in an inactive form. They are activated by trafficking to the Golgi where they are processed and cleaved into active forms, which subsequently translocate to the nucleus. Activation of mTORC1 elevates and activates SREBP1 and SREBP2 by multiple mechanisms49, and transcription of the gene encoding G6PDH is elevated by SREBP1 in an mTORC1-dependent manner48. Elevation of the oxidative PPP by mTORC1 provides NADPH for fatty acid synthesis, which is also positively regulated by SREBPs downstream of mTORC1.
Nrf2
Nrf2 (nuclear factor erythroid 2 (NF-E2)-related factor 2) is a transcription factor that is regulated by oxidative stress or xenobiotic stress. Normally it is associated with Keap1 (Kelch-like erythroid cell-derived protein with CNC homology (ECH) associated protein 1) in the cytoplasm, which mediates Nrf2 ubiquitylation and degradation. Under oxidative stress or xenobiotic stress and increased ROS levels, Nrf2 dissociates from Keap1 and translocates to the nucleus where it binds antioxidant response elements (ARE) and activates the transcription of associated genes50.
Recent studies indicate that Nrf2, through its binding to ARE, elevates transcription of the PPP enzymes G6PDH, 6PGDH, TKT, and TALDO. Thus, Nrf2 activates both the oxidative and nonoxidative PPP to increase NADPH and nucleotide production51. The expression of Nrf2 is elevated by oncogenic K-Ras and B-Raf as well as overexpression of Myc, and its level is sustained in cancer cells that display hyperactivation of the PI3K/Akt signaling pathway52,53. In addition, activating mutations in Nrf2 and inactivating mutations in Keap1 have been identified in several human cancers. These mutations interfere with the Nrf2-Keap1 interaction and constitutively activate Nrf2. These mutations were observed in many human cancers, including squamous cell carcinoma, non-small cell lung cancer, hepatocellular carcinoma, renal cell carcinoma, and melanoma (reviewed in54). Epigenetic silencing of Keap1 and activation of Nrf2 were observed in lung cancer and glioblastoma, and they are associated with poor prognosis55,56. Hence, multiple mechanisms that activate Nrf2 in cancer cells, lead to the elevation of both branches of the PPP.
In summary, a plethora of mechanisms elicited by the activation of oncoproteins or the inactivation of tumor suppressors regulate the expression and activity of enzymes that govern both modes of the PPP. These mechanisms enable cancer cells to adapt to anabolic demands that require rapid DNA, RNA, and lipid biosynthesis and to oxidative cellular stress imposed by microenvironment.
The requirement of the PPP for cancer cell survival and metastasis
Perhaps the most appreciated role of the PPP in the tumorigenic process is protection from cell death. In a tumor environment, reactive oxygen species, which are generated by accelerated metabolism, hypoxia or DNA damage, must be managed to maintain a high proliferative advantage for cancer cells. As a consequence of accelerated metabolism, cancer cells usually display higher levels of intracellular ROS than normal cells. The relatively high level of ROS in cancer cells may be a double-edged sword (reviewed in57). Elevated levels of ROS may increase the rate of pro-oncogenic mutations and facilitate pro-tumorigenic signaling pathways. However, high levels of ROS may render cancer cells more vulnerable to energetic and oxidative stress. Thus, mechanisms evolved to facilitate the oxidative PPP in cancer cells to generate a relatively high level of NADPH to combat ROS. However, the PPP depends on the availability of glucose, and if sufficient glucose is not available then reduced NADPH levels may increase intracellular ROS above a certain threshold level that might elicit cell death. This could occur during the initial stages of solid tumor development, when cells migrate to the lumen. Under these conditions, tumor cells cannot utilize glucose and undergo energetic stress58. In the absence of the oxidative PPP, cells can die during this process. Thus, under these conditions alternative mechanisms to generate NADPH that do not depend on the immediate supply of glucose are induced. These mechanisms are largely mediated by the activation of AMPK59,60. AMPK, through the inhibition of ACC1 and ACC2, inhibits fatty acid synthesis thereby inhibiting the consumption of NADPH, while elevating fatty acid oxidation to increase the generation of NADPH by malic enzyme (ME) and Isocitrate-dehydrogenase 1 (IDH1) In the absence of this compensation mechanism, which is induced by AMPK, tumor cells may die during solid tumor formation. During metastasis, when cancer cells detach from the primary tumor site and migrate to the metastatic site, they may also undergo a similar energetic stress. Therefore, the survival of metastatic cells during migration could depend on the level of NADPH generated by either the oxidative PPP or alternative mechanisms induced by AMPK.
Various changes, including growth factor signaling, matrix remodeling, and adhesion properties, accompany cancer cell metastasis. Therefore, metastatic cancer cells should be versatile and more adaptable than other cells during and following migration to the secondary site. Some of the adaptive changes are metabolic changes, including both the oxidative and nonoxidative PPP. For instance, in metastatic renal cancers, both the oxidative and nonoxidative PPP are over-active61. This study reported a greater increase in the nonoxidative PPP compared to the oxidative PPP due to the increase in TKT activity and TKTL1 overexpression in metastatic cancer cells, which may fulfill the requirement for glycolytic intermediates and ribonucleotides in aggressive cancer cells. Other studies demonstrated that, during the progression of mammary gland tumorigenesis, no significant changes in the PPP occurred between early stage and invasive tumors62. However, increased PPP was reported in the brain metastasis of breast cancer63. Therefore, the association of PPP with metastasis seems to be both context-dependent and metastatic site-dependent.
Concluding remarks
The elevated PPP in cancer cells may distinguish cancer cells from normal cells; thus, targeting the PPP for cancer therapy might be appealing. The elevated PPP in cancer cells generates high NADPH levels to reduce ROS while simultaneously generating high levels of nucleotides for DNA synthesis and repair. These activities of the PPP may provoke resistance to certain cancer therapies that increase oxidative stress or DNA damage. Furthermore, PPP activity is increased in response to oxidative stress64, ionizing radiation65 or chemotherapies66, which elicit high ROS levels and provoke an adaptive response by augmenting the PPP. In multiple cancer cell lines, it has been documented that the acquisition of drug resistance is accompanied by elevation of the oxidative PPP. Sustained high levels of G6PDH and GSH are hallmarks of elevated oxidative PPP following drug resistance67–69. Drug resistance could be reversed by treatment with DHEA or 6-AN, which inhibit the first and the second step in the oxidative PPP, respectively70.
The nonoxidative PPP does not participate in ROS detoxification elicited by chemotherapy. However, resistance to certain DNA damaging agents, such as 5-fluorouracyl (5-FU), is associated with elevated nonoxidative PPP, and colon cancer cells resistant to 5-FU exhibit elevated expression of transketolase71. Thus, targeting the PPP for cancer therapy may be challenging (BOX2). However, in contrast to the expected resistance exerted by the elevated PPP in response to certain drugs, the PPP may sensitize cells to other therapeutic drugs. Indeed, it appears that the high levels of NADPH generated by a hyperactive PPP sensitize cells to anthracyclines. Anthracyclines are a class of antibiotics used in cancer therapy, and the most commonly used member of this class is adriamycin, also known as doxorubicin. Anthracyclines are metabolized by cytochrome p450 reductase to produce free radicals, which induce cytotoxicity72. Because NADPH is a cofactor that is required for this activity of cytochrome p450, the high levels of NADPH generated by the PPP may sensitize cancer cells to doxorubicin. Consistently, adriamycin/doxorubicin-resistant MCF-7 cells display reduced G6PDH and PPP activity compared to sensitive cells66.
BOX-2 Outstanding questions.
What are the exact mechanisms by which oncogenic Ras or other oncogenic signals elevate the nonoxidative PPP to generate ribonucleotides?
Unlike the oxidative PPP, the nonoxidative PPP is not a thermodynamically regulated process because all the pathways are reversible. However, a recent metabolomics study with genetic deletion experiments in yeast discovered SBH17, a phosphatase that converts sedoheptulose 1,7 bisphosphate to sedoheptulose 7-phosphate76. This reaction results in a net loss of high-energy phosphate bonds, which drives the flux towards riboneogenesis. This route is favored according to the metabolic necessities of cells. It remains to be determined if an orthologue of SBH17 is expressed in mammalian cells and is induced in cancer cells.
Is the depletion of NADPH by the inhibition of the oxidative PPP elicits compensatory alternative mechanisms that generate NADPH59,60.
Is it possible that the inhibition of the oxidative PPP could increase intracellular ROS, which in turn selectively eradicates cancer cells57?
Is it possible to inhibit the PPP at the organismal level to selectively inhibit tumorigenesis without significant adverse physiological Manuscript consequences?
Since patients with G6PDH deficiency are hypersensitive to hemolytic anemia, it remains to be determined if targeting the oxidative PPP for cancer therapy could have similar consequences.
In summary, more work is required to determine the targetable Achilles’ heel of the hyperactive PPP in cancer cells. As discussed in this review, cancer cells have acquired multiple mechanisms to deregulate the oxidative and nonoxidative PPP based on demand. It is likely that future studies will uncover additional mechanisms by which cancer cells hijack this important pathway to aid their survival and proliferation (BOX2).
Highlights.
PPP is critical for cancer cell survival and ribonucleotide and lipid biosynthesis.
Cancer cells modulate the PPP to maintain anabolic demands and redox homeostasis.
Targeting the PPP for cancer therapy is challenging.
Further studies on PPP regulation in cancer cells are required for targeting the PPP.
Acknowledgments
Work in N.H. laboratory is supported by NIH grants R01AG016927 and R01CA090764, and by VA Merit Award BX000733.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cordes W. Annual report. Vol. 15. Boston, MA: United Fruit Co.; 1926. Experiences with plasmochin in malaria; pp. 66–71. [Google Scholar]
- 2.Alving AS, et al. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science. 1956;124(3220):484–485. doi: 10.1126/science.124.3220.484-a. [DOI] [PubMed] [Google Scholar]
- 3.Horecker BL. The pentose phosphate pathway. J Biol Chem. 2002;277(50):47965–47971. doi: 10.1074/jbc.X200007200. [DOI] [PubMed] [Google Scholar]
- 4.Kruger NJ, von Schaewen A. The oxidative pentose phosphate pathway: structure and organisation. Curr Opin Plant Biol. 2003;6(3):236–246. doi: 10.1016/s1369-5266(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 5.Riganti C, et al. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med. 2012;53(3):421–436. doi: 10.1016/j.freeradbiomed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Eggleston LV, Krebs HA. Regulation of the pentose phosphate cycle. Biochem J. 1974;138(3):425–435. doi: 10.1042/bj1380425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rais B, et al. Oxythiamine and dehydroepiandrosterone induce a G1 phase cycle arrest in Ehrlich's tumor cells through inhibition of the pentose cycle. FEBS Lett. 1999;456(1):113–118. doi: 10.1016/s0014-5793(99)00924-2. [DOI] [PubMed] [Google Scholar]
- 8.Boros LG, et al. Inhibition of the oxidative and nonoxidative pentose phosphate pathways by somatostatin: a possible mechanism of antitumor action. Med Hypotheses. 1998;50(6):501–506. doi: 10.1016/s0306-9877(98)90271-7. [DOI] [PubMed] [Google Scholar]
- 9.Boros LG, et al. Nonoxidative pentose phosphate pathways and their direct role in ribose synthesis in tumors: is cancer a disease of cellular glucose metabolism? Med Hypotheses. 1998;50(1):55–59. doi: 10.1016/s0306-9877(98)90178-5. [DOI] [PubMed] [Google Scholar]
- 10.Cohen P, Rosemeyer MA. Subunit interactions of glucose-6-phosphate dehydrogenase from human erythrocytes. Eur J Biochem. 1969;8(1):8–15. doi: 10.1111/j.1432-1033.1969.tb00488.x. [DOI] [PubMed] [Google Scholar]
- 11.Hilf R, et al. Multiple molecular forms of glucose-6-phosphate dehydrogenase in normal, preneoplastic, and neoplastic mammary tissues of mice. Cancer Res. 1975;35(8):2109–2116. [PubMed] [Google Scholar]
- 12.Okano K, et al. Histochemical comparison of oxidative enzymes in adrenal glands of mammals. Histochemie. 1965;4(6):494–501. doi: 10.1007/BF00281902. [DOI] [PubMed] [Google Scholar]
- 13.Park J, et al. Overexpression of glucose-6-phosphate dehydrogenase is associated with lipid dysregulation and insulin resistance in obesity. Mol Cell Biol. 2005;25(12):5146–5157. doi: 10.1128/MCB.25.12.5146-5157.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudack D, et al. Rat liver glucose 6-phosphate dehydrogenase. Regulation by carbohydrate diet and insulin. J Biol Chem. 1971;246(5):1249–1254. [PubMed] [Google Scholar]
- 15.Jonas SK, et al. Increased activity of 6-phosphogluconate dehydrogenase and glucose-6-phosphate dehydrogenase in purified cell suspensions and single cells from the uterine cervix in cervical intraepithelial neoplasia. Br J Cancer. 1992;66(1):185–191. doi: 10.1038/bjc.1992.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Au SW, et al. Human glucose-6-phosphate dehydrogenase: the crystal structure reveals a structural NADP(+) molecule and provides insights into enzyme deficiency. Structure. 2000;8(3):293–303. doi: 10.1016/s0969-2126(00)00104-0. [DOI] [PubMed] [Google Scholar]
- 17.Ayala A, et al. The role of NADPH in the regulation of glucose-6-phosphate and 6-phosphogluconate dehydrogenases in rat adipose tissue. Mol Cell Biochem. 1991;105(1):1–5. doi: 10.1007/BF00230368. [DOI] [PubMed] [Google Scholar]
- 18.Stanton RC, et al. Rapid release of bound glucose-6-phosphate dehydrogenase by growth factors. Correlation with increased enzymatic activity. J Biol Chem. 1991;266(19):12442–12448. [PubMed] [Google Scholar]
- 19.Tian WN, et al. Signal transduction proteins that associate with the platelet-derived growth factor (PDGF) receptor mediate the PDGF-induced release of glucose-6-phosphate dehydrogenase from permeabilized cells. J Biol Chem. 1994;269(20):14798–14805. [PubMed] [Google Scholar]
- 20.Pan S, et al. Glucose 6-phosphate dehydrogenase is regulated through c-Src-mediated tyrosine phosphorylation in endothelial cells. Arterioscler Thromb Vasc Biol. 2009;29(6):895–901. doi: 10.1161/ATVBAHA.109.184812. [DOI] [PubMed] [Google Scholar]
- 21.Toniolo D, et al. Expression of the G6PD locus on the human X chromosome is associated with demethylation of three CpG islands within 100 kb of DNA. EMBO J. 1988;7(2):401–406. doi: 10.1002/j.1460-2075.1988.tb02827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, et al. High glucose inhibits glucose-6-phosphate dehydrogenase via cAMP in aortic endothelial cells. J Biol Chem. 2000;275(51):40042–40047. doi: 10.1074/jbc.M007505200. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, et al. Diabetes causes inhibition of glucose-6-phosphate dehydrogenase via activation of PKA, which contributes to oxidative stress in rat kidney cortex. Am J Physiol Renal Physiol. 2005;289(5):F1040–F1047. doi: 10.1152/ajprenal.00076.2005. [DOI] [PubMed] [Google Scholar]
- 24.Leopold JA, et al. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat Med. 2007;13(2):189–197. doi: 10.1038/nm1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brodie AF, Lipmann F. Identification of a gluconolactonase. J Biol Chem. 1955;212(2):677–685. [PubMed] [Google Scholar]
- 26.Beutler E, et al. 6-Phosphogluconolactonase deficiency, a hereditary erythrocyte enzyme deficiency: possible interaction with glucose-6-phosphate dehydrogenase deficiency. Proc Natl Acad Sci U S A. 1985;82(11):3876–3878. doi: 10.1073/pnas.82.11.3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sukhatme VP, Chan B. Glycolytic cancer cells lacking 6-phosphogluconate dehydrogenase metabolize glucose to induce senescence. FEBS Lett. 2012;586(16):2389–2395. doi: 10.1016/j.febslet.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura M, Uyeda K. Purification and characterization of a novel xylulose 5-phosphate-activated protein phosphatase catalyzing dephosphorylation of fructose-6-phosphate,2-kinase:fructose-2,6-bisphosphatase. J Biol Chem. 1995;270(44):26341–26346. doi: 10.1074/jbc.270.44.26341. [DOI] [PubMed] [Google Scholar]
- 29.Ying H, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149(3):656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindqvist Y, et al. Three-dimensional structure of transketolase, a thiamine diphosphate dependent enzyme, at 2.5 A resolution. EMBO J. 1992;11(7):2373–2379. doi: 10.1002/j.1460-2075.1992.tb05301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boros LG, et al. Oxythiamine and dehydroepiandrosterone inhibit the nonoxidative synthesis of ribose and tumor cell proliferation. Cancer Res. 1997;57(19):4242–4248. [PubMed] [Google Scholar]
- 32.Liu H, et al. Fructose induces transketolase flux to promote pancreatic cancer growth. Cancer Res. 2010;70(15):6368–6376. doi: 10.1158/0008-5472.CAN-09-4615. [DOI] [PubMed] [Google Scholar]
- 33.Coy JF, et al. Mutations in the transketolase-like gene TKTL1: clinical implications for neurodegenerative diseases, diabetes and cancer. Clin Lab. 2005;51(5–6):257–273. [PubMed] [Google Scholar]
- 34.Langbein S, et al. Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer. 2006;94(4):578–585. doi: 10.1038/sj.bjc.6602962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meshalkina LE, et al. Is transketolase-like protein, TKTL1, transketolase? Biochim Biophys Acta. 2013;1832(3):387–390. doi: 10.1016/j.bbadis.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Heinrich PC, et al. Behavior of transaldolase (EC 2.2.1.2) and transketolase (EC 2.2.1.1) Activities in normal, neoplastic, differentiating, and regenerating liver. Cancer Res. 1976;36(9 pt.1):3189–3197. [PubMed] [Google Scholar]
- 37.Hanczko R, et al. Prevention of hepatocarcinogenesis and increased susceptibility to acetaminophen-induced liver failure in transaldolase-deficient mice by N-acetylcysteine. J Clin Invest. 2009;119(6):1546–1557. doi: 10.1172/JCI35722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartzenberg-Bar-Yoseph F, et al. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res. 2004;64(7):2627–2633. doi: 10.1158/0008-5472.can-03-0846. [DOI] [PubMed] [Google Scholar]
- 39.Hitosugi T, et al. Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth. Cancer Cell. 2012;22(5):585–600. doi: 10.1016/j.ccr.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kondoh H, et al. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005;65(1):177–185. [PubMed] [Google Scholar]
- 41.Bensaad K, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126(1):107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 42.Cheung EC, et al. Mitochondrial localization of TIGAR under hypoxia stimulates HK2 and lowers ROS and cell death. Proc Natl Acad Sci U S A. 2012;109(50):20491–20496. doi: 10.1073/pnas.1206530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowman XH, et al. The proapoptotic function of Noxa in human leukemia cells is regulated by the kinase Cdk5 and by glucose. Mol Cell. 2010;40(5):823–833. doi: 10.1016/j.molcel.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 44.Cosentino C, et al. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011;30(3):546–555. doi: 10.1038/emboj.2010.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang P, et al. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol. 2011;13(3):310–316. doi: 10.1038/ncb2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du W, et al. TAp73 enhances the pentose phosphate pathway and supports cell proliferation. Nat Cell Biol. 2013;15(8):991–1000. doi: 10.1038/ncb2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patra KC, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24(2):213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duvel K, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39(2):171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quinn WJ, 3rd, Birnbaum MJ. Distinct mTORC1 pathways for transcription and cleavage of SREBP-1c. Proc Natl Acad Sci U S A. 2012;109(40):15974–15975. doi: 10.1073/pnas.1214113109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taguchi K, et al. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16(2):123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 51.Mitsuishi Y, et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22(1):66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 52.DeNicola GM, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475(7354):106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitsuishi Y, et al. The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism. Front Oncol. 2012;2:200. doi: 10.3389/fonc.2012.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27(20):2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muscarella LA, et al. Regulation of KEAP1 expression by promoter methylation in malignant gliomas and association with patient's outcome. Epigenetics. 2011;6(3):317–325. doi: 10.4161/epi.6.3.14408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muscarella LA, et al. Frequent epigenetics inactivation of KEAP1 gene in non-small cell lung cancer. Epigenetics. 2011;6(6):710–719. doi: 10.4161/epi.6.6.15773. [DOI] [PubMed] [Google Scholar]
- 57.Nogueira V, Hay N. Molecular pathways: reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin Cancer Res. 2013;19(16):4309–4314. doi: 10.1158/1078-0432.CCR-12-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schafer ZT, et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461(7260):109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeon SM, et al. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485(7400):661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jeon SM, Hay N. The dark face of AMPK as an essential tumor promoter. Cell Logist. 2012;2(4):197–202. doi: 10.4161/cl.22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langbein S, et al. Metastasis is promoted by a bioenergetic switch: new targets for progressive renal cell cancer. Int J Cancer. 2008;122(11):2422–2428. doi: 10.1002/ijc.23403. [DOI] [PubMed] [Google Scholar]
- 62.Lu X, et al. Metabolomic changes accompanying transformation and acquisition of metastatic potential in a syngeneic mouse mammary tumor model. J Biol Chem. 2010;285(13):9317–9321. doi: 10.1074/jbc.C110.104448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen EI, et al. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 2007;67(4):1472–1486. doi: 10.1158/0008-5472.CAN-06-3137. [DOI] [PubMed] [Google Scholar]
- 64.Przybytkowski E, Averill-Bates DA. Correlation between glutathione and stimulation of the pentose phosphate cycle in situ in Chinese hamster ovary cells exposed to hydrogen peroxide. Arch Biochem Biophys. 1996;325(1):91–98. doi: 10.1006/abbi.1996.0011. [DOI] [PubMed] [Google Scholar]
- 65.Tuttle S, et al. Glucose-6-phosphate dehydrogenase and the oxidative pentose phosphate cycle protect cells against apoptosis induced by low doses of ionizing radiation. Radiat Res. 2000;153(6):781–787. doi: 10.1667/0033-7587(2000)153[0781:gpdato]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 66.Yeh GC, et al. Adriamycin resistance in human tumor cells associated with marked alteration in the regulation of the hexose monophosphate shunt and its response to oxidant stress. Cancer Res. 1987;47(22):5994–5999. [PubMed] [Google Scholar]
- 67.Ferretti A, et al. Pentose phosphate pathway alterations in multi-drug resistant leukemic T-cells 31P NMR and enzymatic studies. Anticancer Res. 1993;13(4):867–872. [PubMed] [Google Scholar]
- 68.Friesen C, et al. A critical role of glutathione in determining apoptosis sensitivity and resistance in leukemia cells. Cell Death Differ. 2004;11(Suppl 1):S73–S85. doi: 10.1038/sj.cdd.4401431. [DOI] [PubMed] [Google Scholar]
- 69.Gessner T, et al. Elevated pentose cycle and glucuronyltransferase in daunorubicin-resistant P388 cells. Cancer Res. 1990;50(13):3921–3927. [PubMed] [Google Scholar]
- 70.Lai GM, et al. Contribution of glutathione and glutathione-dependent enzymes in the reversal of adriamycin resistance in colon carcinoma cell lines. Int J Cancer. 1991;49(5):688–695. doi: 10.1002/ijc.2910490511. [DOI] [PubMed] [Google Scholar]
- 71.Shin YK, et al. Upregulation of glycolytic enzymes in proteins secreted from human colon cancer cells with 5-fluorouracil resistance. Electrophoresis. 2009;30(12):2182–2192. doi: 10.1002/elps.200800806. [DOI] [PubMed] [Google Scholar]
- 72.Bachur NR, et al. NADPH cytochrome P-450 reductase activation of quinone anticancer agents to free radicals. Proc Natl Acad Sci U S A. 1979;76(2):954–957. doi: 10.1073/pnas.76.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anastasiou D, et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334(6060):1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yi W, et al. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337(6097):975–980. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ros S, et al. Functional metabolic screen identifies 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 as an important regulator of prostate cancer cell survival. Cancer Discov. 2012;2(4):328–343. doi: 10.1158/2159-8290.CD-11-0234. [DOI] [PubMed] [Google Scholar]
- 76.Clasquin MF, et al. Riboneogenesis in yeast. Cell. 2011;145(6):969–980. doi: 10.1016/j.cell.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]