Abstract
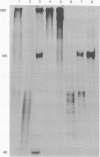
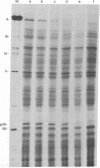
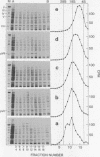
Escherichia coli ribonuclease III cleaves adenovirus messenger RNA and mammalian 28S and 18S ribosomal RNA. Fragmentation is not random, but in each case a specific collection of products is generated. This points to the potential use of the enzyme as a tool for specific fragmentation of RNA. Cleavage by RNase III abolishes the capability of adenovirus messenger RNA to direct cell-free synthesis of virus polypeptides.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S. Biosynthesis of transfer RNA in Escherichia coli. Cell. 1975 Jan;4(1):21–29. doi: 10.1016/0092-8674(75)90129-4. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W., Lewis J. B., Atkins J. F., Gesteland R. F. Cell-free synthesis of adenovirus 2 proteins programmed by fractionated messenger RNA: a comparison of polypeptide products and messenger RNA lengths. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2756–2760. doi: 10.1073/pnas.71.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns A. J.M., De Abreu R. A., Van Kraaikamp M., Benedetti E. L., Bloemendal H. Synthesis of lens protein in vitro V. Isolation of messenger-like RNA from lens by high resolution zonal centrifugation. FEBS Lett. 1971 Oct 15;18(1):159–163. doi: 10.1016/0014-5793(71)80434-9. [DOI] [PubMed] [Google Scholar]
- Both G. W., Banerjee A. K., Shatkin A. J. Methylation-dependent translation of viral messenger RNAs in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1189–1193. doi: 10.1073/pnas.72.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Altman S. Characterization of ribonuclease NU cleavage sites in a bacteriophage phi80-induced ribonucleic acid. J Biol Chem. 1975 Feb 25;250(4):1460–1463. [PubMed] [Google Scholar]
- Crouch R. J. Ribonuclease 3 does not degrade deoxyribonucleic acid-ribonucleic acid hybrids. J Biol Chem. 1974 Feb 25;249(4):1314–1316. [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle H. Buffer combinations for mammalian cell culture. Science. 1971 Oct 29;174(4008):500–503. doi: 10.1126/science.174.4008.500. [DOI] [PubMed] [Google Scholar]
- Eron L., Callahan R., Westphal H. Cell-free synthesis of adenovirus coat proteins. J Biol Chem. 1974 Oct 10;249(19):6331–6338. [PubMed] [Google Scholar]
- Eron L., Wesphal H., Callahan R. In vitro synthesis of adenovirus core proteins. J Virol. 1974 Aug;14(2):375–383. doi: 10.1128/jvi.14.2.375-383.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L., Westphal H. Cell-free translation of highly purified adenovirus messenger RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3385–3389. doi: 10.1073/pnas.71.9.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuke M. Introduction of specific cleavages into RNAs of RNA bacteriophages for determination of base sequences. Proc Natl Acad Sci U S A. 1974 Mar;71(3):742–745. doi: 10.1073/pnas.71.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill P., Kabat D. Unexpectedly large size of globin messenger ribonucleic acid. Proc Natl Acad Sci U S A. 1971 Jan;68(1):72–75. doi: 10.1073/pnas.68.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh S., Nikolaev N., Battaner E., Birge C. H., Schlessinger D. Escherichia coli RNAse 3 cleaves HeLa cell nuclear RNA. Biochem Biophys Res Commun. 1974 Aug 5;59(3):972–978. doi: 10.1016/s0006-291x(74)80075-6. [DOI] [PubMed] [Google Scholar]
- Gralla J., DeLisi C. mRNA is expected to form stable secondary structures. Nature. 1974 Mar 22;248(446):330–332. doi: 10.1038/248330a0. [DOI] [PubMed] [Google Scholar]
- Kramer R. A., Rosenberg M., Steitz J. A. Nucleotide sequences of the 5' and 3' termini of bacteriophage T7 early messenger RNAs synthesized in vivo: evidence for sequence specificity in RNA processing. J Mol Biol. 1974 Nov 15;89(4):767–776. doi: 10.1016/0022-2836(74)90051-5. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Anderson C. W., Atkins J. F., Gesteland R. F. The origin and destiny of adenovirus proteins. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):581–590. doi: 10.1101/sqb.1974.039.01.072. [DOI] [PubMed] [Google Scholar]
- Nikolaev N., Schlessinger D., Wellauer P. K. 30 S pre-ribosomal RNA of Escherichia coli and products of cleavage by ribonuclease III: length and molecular weight. J Mol Biol. 1974 Jul 15;86(4):741–747. doi: 10.1016/0022-2836(74)90350-7. [DOI] [PubMed] [Google Scholar]
- Nikolaev N., Silengo L., Schlessinger D. A role for ribonuclease 3 in processing of ribosomal ribonucleic acid and messenger ribonucleic acid precursors in Escherichia coli. J Biol Chem. 1973 Nov 25;248(22):7967–7969. [PubMed] [Google Scholar]
- Oberg B., Saborio J., Persson T., Everitt E., Philipson L. Identification of the in vitro translation products of adenovirus mRNA by immunoprecipitation. J Virol. 1975 Jan;15(1):199–207. doi: 10.1128/jvi.15.1.199-207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partington G. A., Kemp D. J., Rogers G. E. Isolation of feather keratin mRNA and its translation in a rabbit reticulocyte cell-free system. Nat New Biol. 1973 Nov 14;246(150):33–36. doi: 10.1038/newbio246033a0. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dunn J. J. Ribonucleic acid processing activity of Escherichia coli ribonuclease III. J Biol Chem. 1975 Apr 25;250(8):3050–3056. [PubMed] [Google Scholar]
- Robertson H. D., Hunter T. Sensitive methods for the detection and characterization of double helical ribonucleic acid. J Biol Chem. 1975 Jan 25;250(2):418–425. [PubMed] [Google Scholar]
- Robertson H. D., Mathews M. B. Double-stranded RNA as an inhibitor of protein synthesis and as a substrate for a nuclease in extracts of Krebs II ascites cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):225–229. doi: 10.1073/pnas.70.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Webster R. E., Zinder N. D. Purification and properties of ribonuclease III from Escherichia coli. J Biol Chem. 1968 Jan 10;243(1):82–91. [PubMed] [Google Scholar]
- Rosenberg M., Kramer R. A., Steitz J. A. T7 early messenger RNAs are the direct products of ribonuclease III cleavage. J Mol Biol. 1974 Nov 15;89(4):777–782. doi: 10.1016/0022-2836(74)90052-7. [DOI] [PubMed] [Google Scholar]
- Schweitz H., Ebel J. P. A study of the mechanism of action of E. coli ribonuclease 3. Biochimie. 1971;53(5):585–593. doi: 10.1016/s0300-9084(71)80014-7. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Williamson A. R. Isolation of messenger RNA coding for mouse heavy-chain immunoglobulin. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1127–1131. doi: 10.1073/pnas.70.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Swan D., Aviv H., Leder P. Purification and properties of biologically active messenger RNA for a myeloma light chain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1967–1971. doi: 10.1073/pnas.69.7.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Secondary structure maps of RNA: processing of HeLa ribosomal RNA. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2827–2831. doi: 10.1073/pnas.70.10.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal H., Eron L., Ferdinand F. J., Callahan R., Lai S. P. Analysis of adenovirus type 2 gene functions by cell-free translation of viral messenger RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):575–579. doi: 10.1101/sqb.1974.039.01.071. [DOI] [PubMed] [Google Scholar]
- Winicov I., Perry R. P. Characterization of a nucleolar endonuclease possibly involved in ribosomal ribonucleic acid maturation. Biochemistry. 1974 Jul 2;13(14):2908–2914. doi: 10.1021/bi00711a021. [DOI] [PubMed] [Google Scholar]
- Zelenka P., Piatigorsky J. Isolation and in vitro translation of delta-crystallin mRNA from embryonic chick lens fibers. Proc Natl Acad Sci U S A. 1974 May;71(5):1896–1900. doi: 10.1073/pnas.71.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]