Abstract
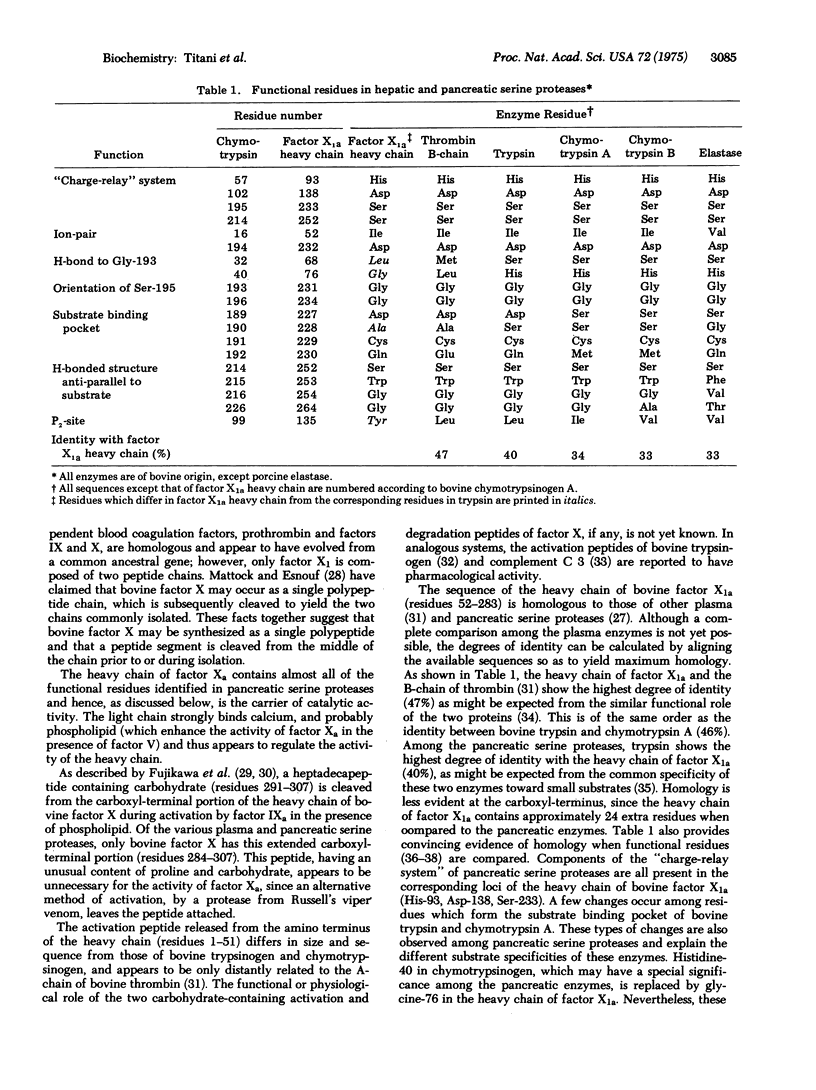
The amino-acid sequence of the heavy chain of bovine blood coagulation factor X1 (Stuart factor) isolated before and after activation has been determined. Sequence analysis was performed on fragments obtained by cleavage with cyanogen bromide and by tryptic digestion. Comparison of the complete sequence with those of other hepatic and pancreatic serine proteases demonstrates homology of the heavy chain of activated factor X1 (factor X1a) with the B chain of bovine thrombin as well as with bovine trypsin, chymotrypsins A and B, and porcine elastase. The activation peptide cleaved near the amino terminus by a protease from Russell's viper venom differs in both size and sequence from those of other serine proteases. With three exceptions, all of the residues which are important in the catalytic functions of trypsin and chymotrypsin occur in corresponding loci in the heavy chain of factor Xa. These finding suggest that the three-dimensional structure of the heavy chain is similar to that of the pancreatic serine proteases and that these enzymes have evolved from a common ancestral gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abita J. P., Moulin A., Lazdunski M., Hage G., Palasciano G., Brasca A., Tiscornia O. A physiological inhibitor of gastric secretion, the activation peptide of trypsinogen. FEBS Lett. 1973 Aug 15;34(2):251–255. doi: 10.1016/0014-5793(73)80805-1. [DOI] [PubMed] [Google Scholar]
- Davie E. W., Fujikawa K. Basic mechanisms in blood coagulation. Annu Rev Biochem. 1975;44:799–829. doi: 10.1146/annurev.bi.44.070175.004055. [DOI] [PubMed] [Google Scholar]
- ESNOUF M. P., WILLIAMS W. J. The isolation and purification of a bovine-plasma protein which is a substrate for the coagulant fraction of Russell's-viper venom. Biochem J. 1962 Jul;84:62–71. doi: 10.1042/bj0840062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Enfield D. L., Ericsson L. H., Fujikawa K., Titani K., Walsh K. A., Neurath H. Bovine factor IX (Christmas factor). Further evidence of homology with factor X (Stuart factor) and prothrombin. FEBS Lett. 1974 Oct 1;47(1):132–135. doi: 10.1016/0014-5793(74)80442-4. [DOI] [PubMed] [Google Scholar]
- Enfield D. L., Ericsson L. H., Walsh K. A., Neurath H., Titani K. Bovine factor X1 (Stuart factor). Primary structure of the light chain. Proc Natl Acad Sci U S A. 1975 Jan;72(1):16–19. doi: 10.1073/pnas.72.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser K. J., Pulsen K., Haber E. Specific cleavage between variable and constant domains of rabbit antibody light chains by dilute acid hydrolysis. Biochemistry. 1972 Dec 19;11(26):4974–4977. doi: 10.1021/bi00776a016. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Coan M. H., Enfield D. L., Titani K., Ericsson L. H., Davie E. W. A comparison of bovine prothrombin, factor IX (Christmas factor), and factor X (Stuart factor). Proc Natl Acad Sci U S A. 1974 Feb;71(2):427–430. doi: 10.1073/pnas.71.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa K., Coan M. H., Legaz M. E., Davie E. W. The mechanism of activation of bovine factor X (Stuart factor) by intrinsic and extrinsic pathways. Biochemistry. 1974 Dec 17;13(26):5290–5299. doi: 10.1021/bi00723a006. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Legaz M. E., Davie E. W. Bovine factor X 1 (Stuart factor). Mechanism of activation by protein from Russell's viper venom. Biochemistry. 1972 Dec 19;11(26):4892–4899. doi: 10.1021/bi00776a003. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Legaz M. E., Davie E. W. Bovine factors X 1 and X 2 (Stuart factor). Isolation and characterization. Biochemistry. 1972 Dec 19;11(26):4882–4891. doi: 10.1021/bi00776a002. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Legaz M. E., Kato H., Davie E. W. The mechanism of activation of bovine factor IX (Christmas factor) by bovine factor XIa (activated plasma thromboplastin antecedent). Biochemistry. 1974 Oct 22;13(22):4508–4516. doi: 10.1021/bi00719a006. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Homologies in serine proteinases. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):77–87. doi: 10.1098/rstb.1970.0010. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Titani K., Neurath H., Walsh K. A. Application of sequenator analyses to the study of proteins. Biochemistry. 1972 Nov 21;11(24):4493–4502. doi: 10.1021/bi00774a011. [DOI] [PubMed] [Google Scholar]
- Hugli T. E., Vallota E. H., Müller-Eberhard H. J. Purification and partial characterization of human and porcine C3a anaphylatoxin. J Biol Chem. 1975 Feb 25;250(4):1472–1478. [PubMed] [Google Scholar]
- Jackson C. M. Characterization of two glycoprotein variants of bovine factor X and demonstration that the factor X zymogen contains two polypeptide chains. Biochemistry. 1972 Dec 19;11(26):4873–4882. doi: 10.1021/bi00776a001. [DOI] [PubMed] [Google Scholar]
- Jackson C. M., Hanahan D. J. Studies on bovine factor X. II. Characterization of purified factor X. Observations on some alterations in zone electrophoretic and chromatographic behavior occurring during purification. Biochemistry. 1968 Dec;7(12):4506–4517. doi: 10.1021/bi00852a047. [DOI] [PubMed] [Google Scholar]
- Jesty J., Nemerson Y. Purification of Factor VII from bovine plasma. Reaction with tissue factor and activation of Factor X. J Biol Chem. 1974 Jan 25;249(2):509–515. [PubMed] [Google Scholar]
- Jollès J., Schoentgen F., Alais C., Fiat A. M., Jollès P. Studies on the primary structure of cow kappa-casein. Structural features of para-kappa-casein; N-terminal sequence of kappa-caseinoglycopeptide studied with a sequencer. Helv Chim Acta. 1972;55(8):2872–2883. doi: 10.1002/hlca.19720550820. [DOI] [PubMed] [Google Scholar]
- Laursen R. A. Solid-phase Edman degradation. An automatic peptide sequencer. Eur J Biochem. 1971 May 11;20(1):89–102. doi: 10.1111/j.1432-1033.1971.tb01366.x. [DOI] [PubMed] [Google Scholar]
- Mattock P., Esnouf M. P. A form of bovine factor X with a single polypeptide chain. Nat New Biol. 1973 Mar 21;242(116):90–92. doi: 10.1038/newbio242090a0. [DOI] [PubMed] [Google Scholar]
- Radcliffe R. D., Barton P. G. Comparisons of the molecular forms of activated bovine factor X. Products of activation with Russell's viper venom, insoluble trypsin, sodium citrate, tissue factor, and the intrinsic system. J Biol Chem. 1973 Oct 10;248(19):6788–6795. [PubMed] [Google Scholar]
- Schroeder W. A., Shelton J. B., Shelton J. R. An examination of conditions for the cleavage of polypeptide chains with cyanogen bromide: application to catalase. Arch Biochem Biophys. 1969 Mar;130(1):551–556. doi: 10.1016/0003-9861(69)90069-1. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Powers J. C., Cohen G. H., Davies D. R., Wilcox P. E. Substrate binding site in bovine chymotrypsin A-gamma. A crystallographic study using peptide chloromethyl ketones as site-specific inhibitors. Biochemistry. 1971 Sep 28;10(20):3728–3738. doi: 10.1021/bi00796a014. [DOI] [PubMed] [Google Scholar]
- Shotton D. M., Hartley B. S. Amino-acid sequence of porcine pancreatic elastase and its homologies with other serine proteinases. Nature. 1970 Feb 28;225(5235):802–806. doi: 10.1038/225802a0. [DOI] [PubMed] [Google Scholar]
- Stenflo J., Fernlund P., Egan W., Roepstorff P. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2730–2733. doi: 10.1073/pnas.71.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenflo J. Vitamin K and the biosynthesis of prothrombin. IV. Isolation of peptides containing prosthetic groups from normal prothrombin and the corresponding peptides from dicoumarol-induced prothrombin. J Biol Chem. 1974 Sep 10;249(17):5527–5535. [PubMed] [Google Scholar]
- Stroud R. M., Kay L. M., Dickerson R. E. The crystal and molecular structure of DIP-inhibited bovine trypsin at2.7Angstrom resolution. Cold Spring Harb Symp Quant Biol. 1972;36:125–140. doi: 10.1101/sqb.1972.036.01.018. [DOI] [PubMed] [Google Scholar]
- Titani K., Hermodson M. A., Fujikawa K., Ericsson L. H., Walsh K. A., Neurath H., Davie E. W. Bovine factor X 1a (activated Stuart factor). Evidence of homology with mammalian serine proteases. Biochemistry. 1972 Dec 19;11(26):4899–4903. doi: 10.1021/bi00776a004. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- YAOI Y., TITANI K., NARITA K. N- AND C-TERMINAL RESIDUES IN BAKER'S YEAST CYTOCHROME C. J Biochem. 1964 Sep;56:222–229. doi: 10.1093/oxfordjournals.jbchem.a127984. [DOI] [PubMed] [Google Scholar]



