Abstract
Previously, using the single-prolonged stress (SPS) rat model of post-traumatic stress disorder, we reported that moderate treadmill exercise, via modulation of oxidative stress related mechanisms, rescued anxiety and depression-like behaviors and reversed SPS-induced memory impairment. In this study using the SPS model (2 h restrain, 20 min forced swimming, 15 min rest, and 1–2 min diethyl ether exposure), we hypothesized that antioxidant rich grape powder (GP) prevents SPS-induced behavioral and memory impairment in rats. Male Sprague Dawley rats were randomly assigned into: Control (CON; provided tap water), SPS (provided tap water), GP-SPS (provided 15 g/L GP in tap water for 3 wk followed by SPS), or GP-CON (3 wk of GP followed by control exposure). Anxiety and depression-like behaviors were significantly greater in SPS rats when compared to CON or GP treated rats and GP reversed these behavioral deficits. SPS rats made significantly more errors in both short- and long-term memory tests compared to CON or GP treated rats, which were prevented in GP-SPS rats. GP prevented SPS-induced increase in plasma corticosterone level. Furthermore, brain derived neurotrophic factor (BDNF) levels were significantly decreased in amygdala of SPS rats but not in GP-SPS rats compared to CON or GP-CON rats. Additionally, GP significantly increased acetylated Histone3, Histone deacetylase 5 (HDAC 5) in hippocampus and amygdala of SPS rats as compared to CON or GP-CON rats. In conclusion, we suggest protective role of GP in SPS-induced behavioral, cognitive and biochemical impairments in rats. Perhaps, epigenetic regulation of BDNF enables GP-mediated prevention of SPS-induced deficits in rats.
Keywords: single-prolonged stress, PTSD, depression, anxiety, cognition, grape powder
1. Introduction
Previously, our lab has reported that moderate treadmill exercise prevents SPS-induced anxiety- and depression-like behaviors and also prevents learning and memory deficits in rats [1]. We postulated that preventive effects of exercise are enabled via suppression of oxidative stress pathways [1]. Relevant to this, our work has previously suggested that antioxidants most likely serve as exercise mimetic [1–4]. Therefore, in the present study, we focused on directly testing whether treatment with antioxidants can prevent SPS-induced behavioral and cognitive impairments. This is important for several reasons. First, SPS is an excellent rodent model of Post-Traumatic Stress Disorder (PTSD) as it mimics clinical symptoms of PTSD, including anxiety, depression and cognitive impairment [5]. Second, traditional PTSD treatment including antidepressants, selective serotonin reuptake inhibitor (SSRIs), antipsychotics and anticonvulsants, have proved to be ineffective due to their negative side effects [6], therefore, studies to investigate alternative safe approaches must be conducted. Finally, poor compliance to exercise regimen due to PTSD-related physical disabilities or a general lack of discipline from combat or trauma fatigue, has also been reported [7, 8]. Therefore, research into alternative interventions seems all the more pertinent.
Grapes have been known for a long time for their potential health benefits [9] related to cardiovascular ailments [10, 11], diabetes [12, 13], aging [14–16], Alzheimer’s disease and other neurodegenerative disorders [17, 18]. Phytochemical analysis of grapes has revealed various constituents capable of mediating biological response, including the polyphenol resveratrol [19–21]. Recently, rodent studies including our pro-oxidant model and an estrogen depletion model, we reported that a freeze-dried grape powder (GP) provided by California Table Grape Commission (CTGC), prevents pro-oxidant and ovariactemoy-induced anxiety- and depression-like behaviors and also improves learning and memory deficits in rats [2, 22]. Hence, testing beneficial effects of grapes in an animal model of PTSD seem reasonable. While beneficial effects of grapes on anxiety and cognition [23, 24] have been reported, none have investigated its protective effect in an animal model of PTSD.
SPS, an acute stress model of PTSD, is known to offset Hypothalamus-Pituitary-Adrenal (HPA) axis and sympathoadrenal system. And, HPA axis activation is known to elevate plasma corticosterone levels [5, 25]. Therefore, plasma corticosterone was utilized as a systemic marker of stress. Furthermore, various clinical and animal studies report incidence of poor cognition and memory impairment in PTSD [1, 26–28] which is often associated with depleted levels of brain derived neurotrophic factor (BDNF) expression [29, 30]. And, it is believed that changes in BDNF transcription in the brain are partly regulated by epigenetic mechanism such as histone acetylation [31]. Here, we investigated potential involvement of oxidative stress and related epigenetic mechanisms in grape powder mediated protective effects in the rat SPS model. To investigate the involvement of oxidative stress, plasma 8-isoprostane levels were measured. 8-isoprostane is a known marker of oxidative stress. Isoprostanes are a family of eicosanoids of non-enzymatic origin produced by the random oxidation of tissue phospholipids by oxygen radicals [32]. Furthermore, protein expression levels of specific antioxidant enzymes, including glyoxalase (GLO)-1, glutathione reductase (GSR)-1, manganese superoxide dismutase (Mn SOD) and copper zinc (Cu/Zn) SOD were examined. BDNF levels were also evaluated. Stress in general and SPS in particular has been shown to decrease brain levels of BDNF and reportedly known to influence brain plasticity and cognition, involving epigenetic components [33] including histone acetylation and deacetylation. And, oxidative stress is known to regulate histone acetylation/deacetylation processes. Oxidative stress susceptible areas of the brain i.e. areas considered more prone to stressful stimuli namely amygdala, hippocampus, and pre-frontal cortex were selected for this study. By examining histone acetylation/deacetylation dependent BDNF expression in SPS rats, we also investigated the possible neuroprotective effects of grape powder.
We hypothesize that GP prevents SPS-induced behavioral and cognitive impairments in rats. The objectives are the following; i) To investigate the protective role of GP in SPS-induced PTSD-like behaviors in rats. ii) To reveal potential molecular mechanisms responsible for protective effects of GP. In order to test this hypothesis, rats were subjected to 3 weeks of GP treatment, followed by the SPS protocol. Following SPS procedure, anxiety- and depression-like behavior tests as well as short and long-term memory tests were conducted. Blood was withdrawn and selected brain areas isolated for analysis of specific biochemical parameters including corticosteroids, markers of oxidative stress and epigenesis.
2. Materials and Methods
2.1 Freeze Dried Grape Powder
Freeze dried grape powder was provided by the California Table Grape Commission (CTGC). The powder was received in small sealed plastic bags and stored at −80°C. Grape powder solution was prepared fresh daily as published previously by us [2] by dissolving the powder in tap water at a concentration of 15 g/L. This grape powder dose produced most pronounced effects on rat behavior as reported previously by us [2]. Detailed composition of this powder has been listed in Table 1 and also previously published by our research group [2].
Table 1.
Phytochemical analysis of freeze-dried grape powder published by CTGC
| Compounds | Total | Individual |
|---|---|---|
| Catechins | 57.2 mg/kg | |
| Catechin | 36.4 mg/kg | |
| Epicatechin | 20.8 mg/kg | |
| Anthocyanin | 566 mg/kg | |
| Peonidin | 38.3 mg/kg | |
| Cyanidin | 266 mg/kg | |
| Malvidin | 261.7 mg/kg | |
| Flavonols | ||
| Quercetin | 16 mg/kg | |
| Kaempferol | 3.4 mg/kg | |
| Isorhamnetin | 3.5 mg/kg | |
| Stilbenes | ||
| Resveratrol | 1.8 mg/kg | |
| Total Polyphenols | 448 mg/100g |
Abbreviations: CTGC, California Table Grape Commission
2.2 Animals
All experiments were conducted in accordance with the NIH guidelines using approved protocols from the University of Houston Animal Care and Use Committee. Male Sprague Dawley rats (175–200 g; Charles River, Wilmington, MA) were housed with a 12-h light, 12-h dark cycle (lights on at 0600 h) in a climate-controlled room with food and water provided ad libitum. PicoLab Rodent Diet 20 (catalog no. 5056) was purchased from LabDiet, Inc. and contains a mixture of 20% crude protein, 4.5% crude fat, 6% fiber, and 7% minerals. After arrival at the animal research facility, all rats were allowed 1 week to acclimate (Fig. 1).
Fig. 1.
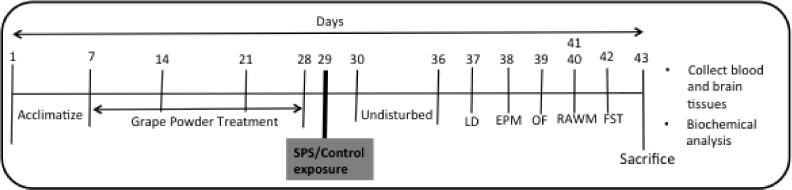
A schematic representation of the experimental regimen. Male Sprague Dawley rats were acclimatized for one week and assigned into 4 groups; group 1: Naïve Control (CON), group 2: grape powder- control exposure (GP-CON; no SPS exposure and provided with 15g/L grape powder dissolved in tap water for 3 wk), group 3: SPS, group 4: grape powder-SPS (GP-SPS; SPS exposure after grape powder treatment for 3 wk). The two SPS groups: SPS and GP-SPS (10 rats/group) were subjected to a one time combined stress paradigm [34, 35] for one day. One SPS group was supplemented with 3 weeks of grape powder while the other group was fed with tap water. The GP-control group was supplemented with grape powder for 3 weeks without the SPS procedure. Behavior tests were conducted one week after SPS protocol.
2.3 Single Prolonged Stress (SPS)
SPS procedure consisted of one time combined stress paradigm applied consecutively in a day [1, 34, 35]: immobilization (compression with double layered plastic Ziploc bag with edges covered in duct tape to prevent the rats from escaping) for two hours followed immediately by forced swimming for 20 minutes (in a tall cylindrical tank of water) then allowed to rest for 15 minutes, and finally exposure to ether anesthesia (with diethyl ether until loss of consciousness) (Fig. 1). The animals were then returned to their home cages and left undisturbed for 7 days. Control animals were not subjected to any stress, and were housed in the undisturbed environment during the SPS experiments. Male Sprague Dawley rats were assigned into 4 groups (10 rats/group); 1) Control (CON), 2) grape powder-control exposure (GP-CON; no SPS exposure and provided with 15g/L grape powder dissolved in tap water for 3 wk), 3) SPS (SPS exposure), 4) grape powder-SPS (GP-SPS; SPS exposure after grape powder treatment for 3 wk). The GP-CON and GP-SPS were pretreated with grape powder for 3 wk before control/SPS exposure and continued to receive grape powder-treated water until euthanized.
One week after the SPS/control exposure, all rats were subjected to anxiety-like behavior tests followed by memory test and depression-like behavior test. All rats were euthanized by decapitation 24-hours after the last behavior test. Blood and brain tissues were collected, indices of oxidative stress and corticosterone assays were conducted as previously by us [4, 36, 37].
2.4 Anxiety-like behavior tests
First, light-dark (LD) test was conducted followed by elevated-plus maze (EPM) and open-field (OF) tests as previously published by us [4, 37].
2.4.1 Light-Dark (LD) exploration
Less time rat spent in the lit area is considered as a measure of anxiety-like behavior. The light-dark box consisted of a light and a dark compartment separated with an opening for passage from one compartment to the other. Total time spent in the lit area was recorded during 5 min test, as previously published by us [4, 37].
2.4.2 Elevated plus-maze (EPM)
Less time rat spent in open arms is considered as a measure of anxiety-like behavior. A standard rat elevated plus-maze with two walled arms and two open arms extending 43 cm from a 10 cm central area (Med Associates Inc., St. Albans, VT) was used. The arms of the maze were approximately 90 cm above the floor. The rat’s movements were tracked visually. Each session was started by placing the rat in the central area facing the open arms of the maze and lasted 5 min. In between each test animal, the maze was wiped down with alcohol. The amount of time the rat spent in the open arms was noted [38].
2.4.3 Open-Field (OF) test
Rodents in general, have the tendency to explore novel areas. Normally, they spend equal amount of time in the center as well as in the periphery of open spaces. However, anxious rats spend more time towards the peripheral areas and do not explore new exposed areas. Rats were placed in the center of the OF (60×40 cm) and left free to explore the arena for 15 min and movement was quantified using Opto-Varimex Micro Activity Meter v2.00 system (Optomax, Columbus Instruments; OH) as previously published by us [4, 37]. Total activity, ambulatory activity and distance covered were recorded.
2.5 Memory Function Test
The radial arm water maze (RAWM) procedures were done as previously published by us [2]. The RAWM consisted of a black circular pool filled with water at 25°C containing six swim paths in a dimly lit room. Each rat was randomly assigned a goal arm which contains a hidden black platform near the end of the arm. The rats were randomly released at an arm different from the goal arm, allowed to swim and locate the platform which is submerged 1 cm under water. The rats were allowed 1 minute for each learning trial or memory test. An error was counted when the rat entered more than halfway into an arm other than the goal arm or if the rat entered more than half of the goal arm but failed to approach the platform. Number of errors ranged from 1 to 7, as the rat can only swim into a total of 7 arms within 1 minute. If the rat failed to locate the platform within 1 minute, it was manually guided to the platform and scored with 7 errors. Upon reaching the platform, the rat was allowed 15 seconds rest before the next trial began. Each rat was subjected to a set of six learning trials (trials 1–6) followed by a five min rest period and then another set of six learning trials (trials 7–12). The short-term memory test was conducted 30 min after the end of 12th trial. This was followed by the long-term memory test that was given 24 h later.
2.6 Depression-like behavior test
The forced swim test (FST) is performed to measure depression-like behavior in rodents [39]. Rats were individually placed in a water tank (24 cm in diameter and 50 cm high filled with 25°C water) for 5 min. At some point after being placed in the water, the rat assumes an immobile posture, marked by motionless floating and cessation of struggling. The total time spent immobile was recorded [40, 41]. The FST was performed two weeks after the completion of the SPS exposure.
2.7 Brain dissections and preparation of homogenates
Rats were anesthetized using isoflurane anesthesia (57319-479-06, Phoenix pharmaceuticals, USA) 24 hours after the conclusion of all behavioral tests. The brains were quickly removed and rapidly frozen at −80°C until analysis. The hippocampus, amygdala and pre-frontal cortex were identified according to Paxinos and Watson [42] and grossly dissected out and homogenized; protein concentration was determined as previously published by us [37].
2.8 Western blot analysis
Homogenates were subjected to SDS-PAGE and Western blotting. The following dilutions were used for detection of specific proteins: GLO-1; 1:200, GSR-1; 1:200, Mn-SOD 1:1000, Cu/Zn SOD; 1:1000, Acetylated Histone3 (H3 acetyl; 1:500, Histone deacetylase 2 (HDAC2); 1:500, Histone deacetylase 5 (HDAC5); 1:500, anti-acetyl-Histone H4 (Lys8) (H4K8) 1:500, BDNF; 1:500 and loading control β-actin; 1:1000 and Total Histone H3 (Total H3); 1:2000. Anti-rabbit HRP-conjugated (1:1000) or anti-mouse HRP-linked secondary antibody (1:1000) was used as needed. The intensity of each Immunoreactive band on immunoblots (normalized to the β-actin loading control) was determined using Alpha Ease FC 4.0 (Alpha Innotech Corp.).
2.9 Statistical analyses
Data are expressed as means ± SEM. For all behavioral tests significance was determined by one-way ANOVA and Tukey’s post hoc test (GraphPad Software, Inc.). For corticosterone, 8-isoprostane and western blot analysis; significance was determined using two-tailed t-test (GraphPad Software, Inc.). For all statistical analyses a value of P < .05 was considered significant.
3. Results
3.1 Anxiety-like behavior tests
Light–dark (LD), elevated plus maze (EPM) and open-field tests (OFT) were conducted to test anxiety-like behavior of rats. In light–dark test, a rat is exposed to a novel environment with protected (dark compartment) and unprotected (light compartment) areas. Unwillingness to explore the lit, unprotected area and more willingness to spend time in the dark compartment during a 5-min test session is indicative of high anxiety-like behaviors. The light–dark test results suggest that the amount of time SPS rats spent in the light compartment was significantly lower when compared with CON and GP-CON (P < .05) rats. Grape powder treatment caused a significant increase in the time spent in the light compartment by GP-SPS rats (P < .05) when compared to SPS rats (Fig. 2A).
Fig. 2.
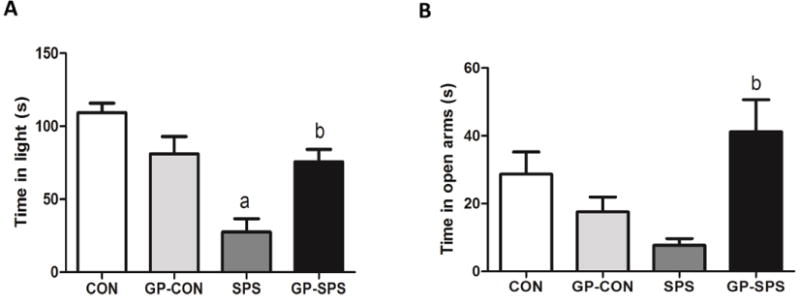
Examination of anxiety-like behavior tests including light-dark (A) and elevated plus maze (B) in rats treated with/without grape powder. ‘a’ represents significant difference from CON group and ‘b’ represents significant difference from SPS group, P < .05. Values are means ± SEM, n = 8–10 rats/group. CON: Naïve Control rats; GP-CON: rats pretreated with 3 weeks of grape powder prior to control exposure, SPS: rats pretreated with tap water prior to SPS exposure, GP-SPS: rats pretreated with grape powder prior to SPS exposure.
Elevated-plus maze test is based on rat’s aversion for open-elevated spaces. This aversion leads to the behavior termed as thigmotaxis, which means avoidance of open areas by restricting movements to enclosed spaces or to the edges of a confined space [43]. Increased amount of time spent in the closed arms during a 5-min session is indicative of high anxiety behavior. The EPM results suggest that SPS rats spent significantly decreased time in the open arms as compared to CON and GP-CON rats (P < .05). However, GP-SPS rats spent significantly increased time in the open arms as compared to SPS rats (P < .05) (Fig. 2B).
Rodents typically spend equal time exploring the periphery of the arena as well as unprotected center area. Rats that spend significantly more time exploring the unprotected center area demonstrate anxiolytic baseline behavior [44]. SPS rats demonstrated significantly lower total (P < .05) (Fig. 3A) and ambulatory (P < .05) (Fig. 3B) activity and covered less distance (P < .05) (Fig. 3C) than the CON, GP-CON or GP-SPS rats.
Fig. 3.
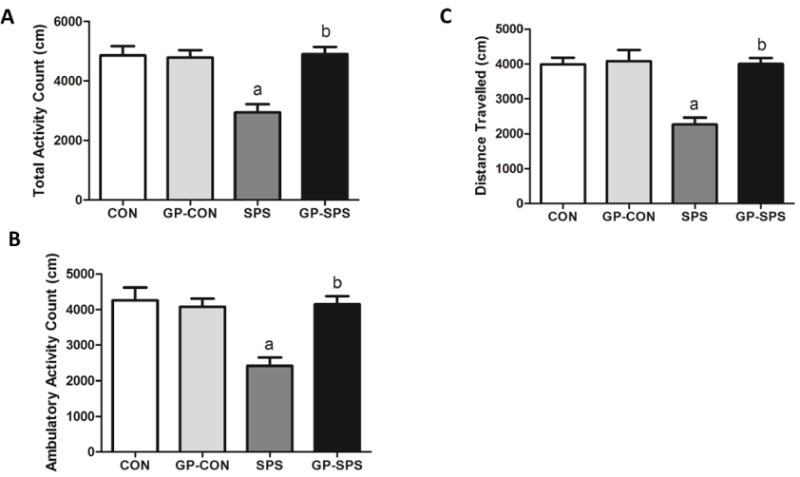
Examination of anxiety-like behavior in the open field test in the rats treated with/without grape powder. The open-field test determined total activity count (A), ambulatory activity count (B), and distance travelled (C). ‘a’ represents significant difference from CON group and ‘b’ represents significant difference from SPS group, P < .05. Values are means ± SEM, n = 8–10 rats/group. CON: Naïve Control rats; GP-CON: rats pretreated with 3 weeks of grape powder prior to control exposure, SPS: rats pretreated with tap water prior to SPS exposure, GP-SPS: rats pretreated with grape powder prior to SPS exposure.
3.2 Depression-like behavior test
The forced swim test was used to assess depression-like behavior in rats. The amount of time rat spends being immobile in the water tank during 5 minutes of forced swim session is indicative of depression-like behavior [45]. The SPS rats exhibited significant increase in time spent immobile when compared to CON, GP-CON and GP-SPS groups (P < .05) (Fig. 4). GP supplementation prevented depressive effect of SPS in GP-SPS rats.
Fig. 4.
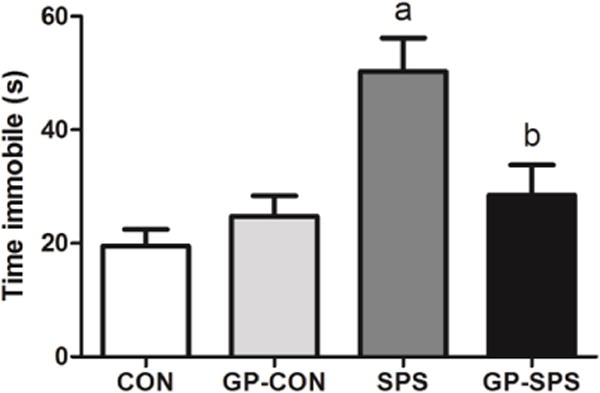
Examination of depression-like behavior using forced-swim test in rats treated with/without grape powder. ‘a’ represents significant difference from CON group and ‘b’ represents significant difference from SPS group, P < 0.05. Values are means ± SEM, n = 8–10 rats/group. CON: Naïve Control rats; GP-CON: rats pretreated with 3 weeks of grape powder prior to control exposure, SPS: rats pretreated with tap water prior to SPS exposure, GP-SPS: rats pretreated with grape powder prior to SPS exposure.
3.3 Memory function test
SPS rats made significantly higher number of errors both in the short-term (Fig. 5A) and long-term memory tests in the RAWM than all the other groups (P < .05) (Fig. 5B). GP treatment prevented both short-term and long-term memory impairment in GP-SPS rats.
Fig. 5.
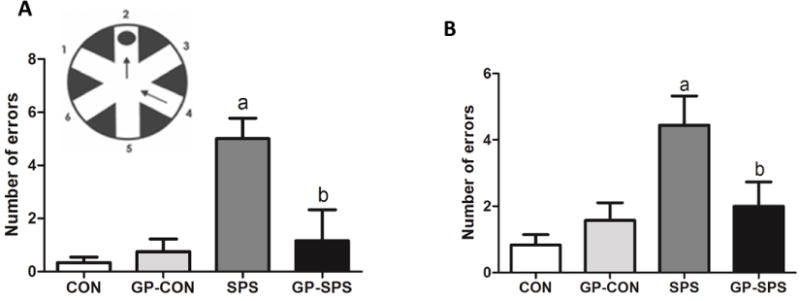
Examination of memory using radial-arm water maze (RAWM) memory test in rats treated with/without grape powder. Short-term (A) and long-term (B) memory was assessed using a series of 12 RAWM trials. The RAWM apparatus is shown as an insert containing a circular water pool with 6 swim paths. ‘a’ represents significant difference from CON group and ‘b’ represents significant difference from SPS group, P < .05. Values are means ± SEM, n = 8–10 rats/group. CON: Naïve Control rats; GP-CON: rats pretreated with 3 weeks of grape powder prior to control exposure, SPS: rats pretreated with tap water prior to SPS exposure, GP-SPS: rats pretreated with grape powder prior to SPS exposure.
3.4 Analysis of plasma corticosterone levels
Corticosterone level in plasma was measured using an EIA based kit [46]. SPS significantly increased plasma corticosterone levels when compared to CON and GP-CON rats (P < .05) (Fig. 6A). The corticosterone levels were significantly decreased in GP-SPS group (P < .05) when compared to SPS rats.
Fig. 6.
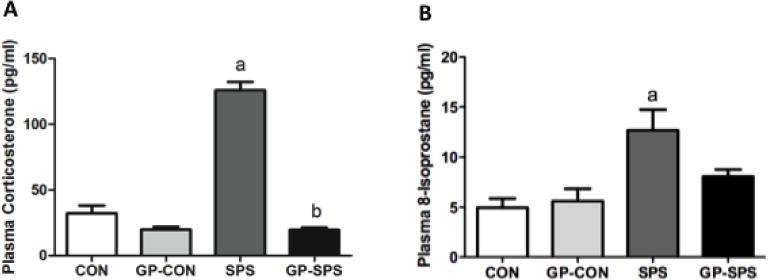
Analysis of plasma corticosterone and 8-isoprostane in rats treated with/without grape powder. The plasma corticosterone (A) and 8-isoprostane (B) levels were measured using an Enzyme Immuno Assay kit (Cayman). ‘a’ represents significant difference from CON group and ‘b’ represents significant difference from SPS group, P < .05. Values are means ± SEM, n = 8–10 rats/group. CON: Naïve Control rats; GP-CON: rats pretreated with 3 weeks of grape powder prior to control exposure, SPS: rats pretreated with tap water prior to SPS exposure, GP-SPS: rats pretreated with grape powder prior to SPS exposure.
3.5 Analysis of plasma 8-isoprostane levels
8-isoprostane levels in plasma were measured using an EIA based kit [4]. SPS significantly increased serum 8-isoprostane levels when compared to CON and GP-CON rats (P < .05) (Fig. 6B). GP treatment decreased SPS-induced elevated 8-isoprostane levels in plasma; however, the decrease was not statistically significant.
3.6 Assessment of anti-oxidative enzymes
Protein expression levels of GLO-1 (Fig. 7A), GSR-1 (Fig. 7B), Cu-Zn SOD (Fig. 7C) and Mn-SOD (Fig. 7D) were examined in the hippocampus, amygdala and pre-frontal cortex regions of the brain. While Mn-SOD, Cu-Zn SOD and GLO-1 protein expression levels remained unchanged in all the groups, GSR-1 protein levels were less in the amygdala of SPS rats when compared to CON and GP-CON rats but it did not reach significance. Grape powder treatment significantly increased SPS-induced decrease in GSR-1 protein levels in the amygdala only (P < .05).
Fig. 7.
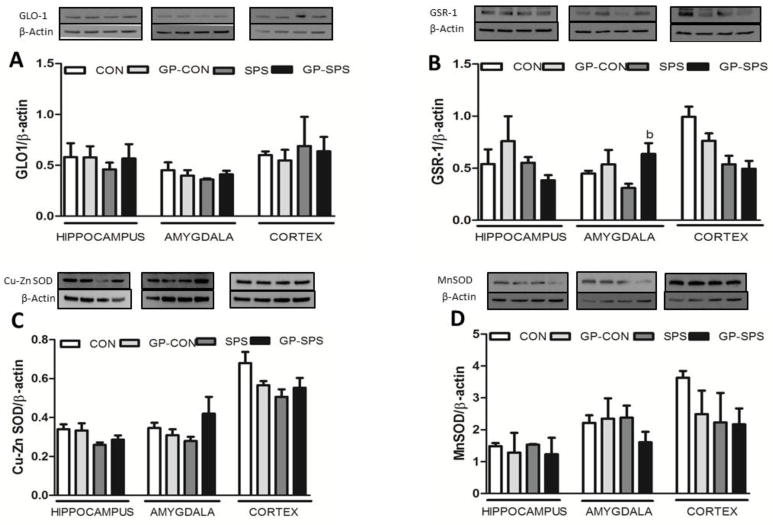
Examination of GLO-1, GSR-1, Cu-Zn SOD and MnSOD protein levels in the hippocampus, amygdala, and cortex of rats treated with/without grape powder. Protein levels of GLO-1 (A), GSR-1 (B), Cu-Zn SOD (C) and MnSOD (D) were determined by western blotting. The upper panels shown in (A – D) are representative blots for GLO-1 (A), GSR-1 (B), Cu-Zn SOD (C), MnSOD (D), and the protein loading control β-actin (A – D). The representative blots are presented in the same order (left to right) as the groups in the figure. Bar graphs in (A – D) are ratios of GLO-1 to β-actin, GSR-1 to β-actin, Cu-Zn SOD to β-actin and MnSOD to β-actin, respectively. ‘b’ represents significant difference from SPS group in the protein levels within the hippocampus, amygdala and cortex, P < .05. Values are means ± SEM, n = 4 rats/group. CON: Naïve Control rats; GP-CON: rats pretreated with 3 weeks of grape powder prior to control exposure, SPS: rats pretreated with tap water prior to SPS exposure, GP-SPS: rats pretreated with grape powder prior to SPS exposure.
3.7 Assessment of BDNF and epigenetic markers
BDNF protein expression levels (Fig. 8A) in the amygdala were significantly decreased as compared to CON rats (P < .05). GP treatment significantly increased SPS-induced decline in BDNF levels in GP-SPS rats (P < .05). Furthermore, Acetylated H3 protein expression levels (Fig. 8B) showed a decreased trend across all three brain regions in SPS rats. And, GP treatment in rats exposed to SPS rats significantly increased the Acetylated H3 protein levels in the hippocampus (P < .05) and amygdala (P < .05) but not in the pre-frontal cortex. H4K8 (Fig. 8C) protein expression levels showed decreased trend in the amygdala but not in the hippocampus or the pre-frontal cortex of SPS rats. GP treatment reversed the changes in H4K8 protein expression only in the amygdala of the GP-SPS rats (P < .05). HDAC5 (Fig. 8D) protein expression levels in amygdala were significantly decreased than that of CON rats (P < .05) and grape powder treatment blocked SPS-induced decrease in HDAC5 in the amygdala of GP-SPS rats (P < .05). HDAC2 (Fig. 8E) protein expression levels did not change in any of the groups across all three brain regions.
Fig. 8.
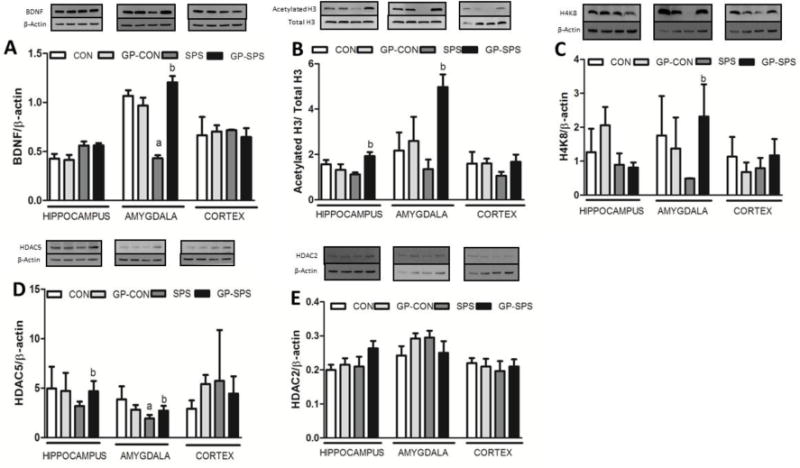
Examination of BDNF and epigenetic markers in the hippocampus, amygdala, and cortex of rats treated with/without grape powder. Protein levels of BDNF (A), Acetylated H3 (B), H4K8 (C), HDAC5 (D), and HDAC2 (E) were determined by western blotting. The upper panels shown in (A – E) are representative blots for BDNF (A), Acetylated H3 (B), H4K8 (C), HDAC5 (D), HDAC2 (E), and the protein loading control Total H3 (B) and β-actin (A, C – E). The representative blots are presented in the same order (left to right) as the groups in the figure. Bar graphs in (A – E) are ratios of BDNF to β-actin, Acetylated H3 to Total H3, H4K8 to β-actin HDAC5 to β-actin, and HDAC2 to β-actin, respectively. ‘a’ represents significant difference from CON group and ‘b’ represents significant difference from SPS group in the protein levels within the hippocampus, amygdala and cortex, P < .05. Values are means ± SEM, n = 4 rats/group. CON: Naïve Control rats; GP-CON: rats pretreated with 3 weeks of grape powder prior to control exposure, SPS: rats pretreated with tap water prior to SPS exposure, GP-SPS: rats pretreated with grape powder prior to SPS exposure.
4. Discussion
Grapes are commonly considered to promote good health [10]. Its beneficial effects are particularly associated with mental well-being [16, 17, 24]. Evidence from animal studies supporting this association is gradually increasing. Our laboratory in two separate studies reported that freeze-dried grape powder provided by CTGC prevented pharmacologically and ovariectomy-induced anxiety- and depression-like behaviors and also improved learning and memory function in rats [2, 22]. To more aggressively assess beneficial effects of this grape powder, it must be vigorously tested in other pre-clinical models. SPS-model of PTSD seems quite relevant.
In this study, SPS-induced anxiety- and depression-like behaviors as well as memory impairment were prevented with CTGC supplied GP treatment. This suggests beneficial effects of this freeze-dried GP on SPS-induced behavioral and cognitive deficits. This is in agreement with our previous studies in which we had reported protective effects of this GP in two separate models of stress [2, 22]. Protective effects are most likely attributable to the antioxidant rich nature of grape powder [10]. The rationale for this consideration is the following. Several laboratories including our own have established causal role of oxidative stress in behavioral as well as cognitive impairments in animals [1, 2, 22, 47–50]. And, studies suggest involvement of antioxidant mechanisms potentially enabling neuroprotective effect of grapes [51, 52]. Relevant to this, we observed that GP treatment prevented SPS-induced rise in oxidative stress examined via assessing plasma 8-isoprostane levels. Interestingly, a systemic marker of stress, corticosterone also showed similar results. Basically, GP treatment prevented SPS-induced increase in corticosterone levels. This is in tandem with our previous observations in a related rat model of psychological stress in which we had observed increased 8-isoprostane and corticosterone levels [53]. Elevated oxidative stress levels have also been reported in another animal model of PTSD, i.e. predator exposure model [54]. Therefore, it is clear from our data that SPS leads to erroneous physiological and behavioral outcomes and GP prevents occurrence of these negative outcomes.
Further results provide interesting information regarding the mechanistic underpinnings of these observations. We focused our attention on key antioxidant enzymes implicated earlier by us [3, 53] and others [55, 56] in specific behavioral and cognitive deficits. However, we did not observe significant differences in the protein expression of Mn SOD, Cu-Zn SOD, GLO-1 or GSR-1, when examined in key brain areas, i.e. hippocampus, amygdala and the pre-frontal cortex. This is not surprising as SPS is an acute stress lasting less than three hours, while changes observed in antioxidant expression in our previous studies were noted in chronic or sub-chronic stress models [1, 2, 22, 53]. Perhaps, antioxidant pathways are destabilized only when chronically challenged. Next, we examined levels of BDNF, an important factor that regulates memory and cognition [57]. Earlier, we had postulated that BDNF potentially confers neuroprotective properties of GP, as GP protected BSO-induced decline in BDNF levels while alleviating behavioral deficits in rats [2]. Role of BDNF in neuroprotection is also well established [58, 59]. Furthermore, BDNF is reported to be downregulated during stress in rodent hippocampus, and antidepressant treatment prevents stress-induced decreased BDNF levels [60]. Association of impaired memory and cognition with reduced levels of BDNF has also been reported in PTSD patients [26, 27, 29]. Overall, these findings suggest that impaired memory and cognition in PTSD occurs potentially via BDNF downregulation. Similarly, in this study we observed significant decrease in BDNF levels in amygdala of SPS rats that was prevented with GP treatment. Furthermore, epigenetic regulation of BDNF has also been reported [61]. Therefore, we examined various epigenetic markers to investigate their potential involvement in BDNF-mediated SPS-induced cognitive impairment. It is known that epigenetic changes and chromatin conformational changes regulate DNA accessibility for transcription factors, co-activators, and polymerases, thereby, resulting in either transcriptional gene activation or repression of various genes, including BDNF [31]. This was demonstrated in a study by M Fuchikami et al in which single immobilization stress significantly reduced BDNF mRNA expression in the rat hippocampus accompanied with significantly decreased level of acetylated H3 present within the promoter region of BDNF gene [33]. In agreement with this, herein we report that SPS shows a decreased trend for acetylated histone H3 and H4K8 protein expression in hippocampus and amygdala in rats. It is likely that decreased acetylated histone in SPS rats repressed transcription of BDNF and therefore, SPS rats showed impaired cognition and memory deficits. However, GP treatment reversed SPS-induced decreased histone acetylation, thereby activating BDNF gene transcription that could probably improve memory and cognition in GP-SPS rats. Furthermore, various studies reported that HDACs (Histone Deacetylases) repress transcription of genes by facilitating winding of DNA potentially because of decreased histone acetylation [61]. Interestingly, our data showed decreased level of HDAC5 in the hippocampus and the amygdala of SPS rats and GP prevented this decline. These data suggests that perhaps decreased expression of HDAC5 leads to increase in acetylation and therefore, higher expression of BDNF is observed in SPS rats. In contrast, we observed decreased expression of BDNF in SPS rats. The possible reason for such result could be the fact that SPS is an acute stress and therefore, would not affect HDAC5 associated BDNF regulation. Furthermore, we did not observe any change in HDAC2 levels in any of the three brain regions. A possible reason could be that acute stress is not sufficient to induce changes in the expression of HDAC2. Overall, our data suggests that grape powder-induced improved memory and cognition in SPS rats might be due to increased expression of acetylated H3 and H4K8 which in turn regulate BDNF expression in brain. Although GP exerted beneficial effects in SPS rats, this study has its own limitations. For example, bioavailability and bioactivity of GP is not known [62, 63]. Therefore, we cannot predict which specific compound(s) present in the GP used in this study, are responsible for its beneficial effects.
Finally, data obtained in this study supports our hypothesis that GP prevents SPS-induced behavioral and memory impairments in rats. Also, we suggest that psychological stress leads to excessive increase in oxidative stress due to suppression in antioxidant enzyme activities. This imbalance triggers epigenetic changes leading to transcriptional repression of BDNF. All of these effects combined, lead to behavioral deficits in rats. Grape powder with its antioxidant properties has exercise mimicking effects and helps maintain optimum oxidative stress levels preventing repression of BDNF. This proposition if proved accurate would have immense therapeutic benefit for those affected with PTSD. And, identifying the epigenetic mechanisms in specific neurocircuitries underlying PTSD-like behaviors is crucial for revealing molecular pathways amenable to nutritional intervention.
Fig. 9.
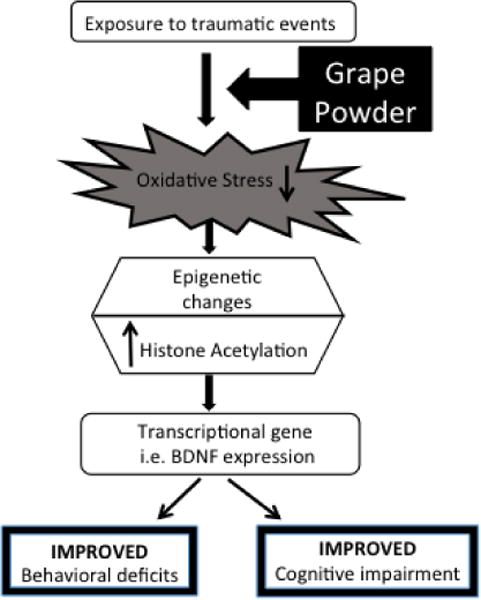
Schematic representation of the events potentially responsible for the protective effect of GP in SPS-induced behavioral and cognitive impairment.
Acknowledgments
Funding for this research was provided by NIH R15 G103327 grant awarded to Samina Salim. The authors thank the California Table Grape Commission for providing us with the grape powder and especially Ms. Courtney Romano for her help with prompt delivery arrangements.
Abbreviations
- SPS
single-prolonged stress
- PTSD
post-traumatic stress disorder
- GP
grape powder
- HPA
Hypothalamus-Pituitary-Adrenal
- SSRI
selective serotonin reuptake inhibitor
- CTGC
California Table Grape Commission
- GLO-1
glyoxalase-1
- GSR-1
glutathione reductase-1
- Mn-SOD
Manganese-Superoxide Dismutase
- Cu/Zn-SOD
Copper/Zinc-Superoxide Dismutase
- BDNF
brain derived neurotrophic factor
- LD
light-dark exploration
- EPM
elevated plus maze
- OF
open field test
- RAWM
radial arm water maze test
- FST
forced swim test
- BSO
L-buthionine-(S,R)-sulfoximine
- H4K8
anti-acetyl-Histone H4 (Lys8)
- HDAC
histone deacetylase
- HAT
histone acetyl transferase
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patki G, Li L, Allam F, Solanki N, Dao AT, Alkadhi K, et al. Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiology & behavior. 2014;130:47–53. doi: 10.1016/j.physbeh.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allam F, Dao AT, Chugh G, Bohat R, Jafri F, Patki G, et al. Grape powder supplementation prevents oxidative stress-induced anxiety-like behavior, memory impairment, and high blood pressure in rats. The Journal of nutrition. 2013;143:835–42. doi: 10.3945/jn.113.174649. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Patki G, Solanki N, Atrooz F, Allam F, Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain research. 2013;1539:73–86. doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh G. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behavioural brain research. 2010;208:545–52. doi: 10.1016/j.bbr.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, et al. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depression and anxiety. 2009;26:1110–7. doi: 10.1002/da.20629. [DOI] [PubMed] [Google Scholar]
- 6.Berger W, Mendlowicz MV, Marques-Portella C, Kinrys G, Fontenelle LF, Marmar CR, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33:169–80. doi: 10.1016/j.pnpbp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Assis MA, de Mello MF, Scorza FA, Cadrobbi MP, Schooedl AF, Gomes da Silva S, et al. Evaluation of physical activity habits in patients with posttraumatic stress disorder. Clinics. 2008;63:473–8. doi: 10.1590/S1807-59322008000400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zen AL, Whooley MA, Zhao S, Cohen BE. Post-traumatic stress disorder is associated with poor health behaviors: findings from the heart and soul study. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2012;31:194–201. doi: 10.1037/a0025989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pezzuto JM, Venkatasubramanian V, Hamad M, Morris KR. Unraveling the relationship between grapes and health. The Journal of nutrition. 2009;139:1783S–7S. doi: 10.3945/jn.109.107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lisa M, Vislocky MLF. Grapes and Grape Products: Their Role in Health. Nurtition Today. 2013;48:47–51. [Google Scholar]
- 11.Pezzuto JM. Grapes and human health: a perspective. Journal of agricultural and food chemistry. 2008;56:6777–84. doi: 10.1021/jf800898p. [DOI] [PubMed] [Google Scholar]
- 12.El-Alfy AT, Ahmed AA, Fatani AJ. Protective effect of red grape seeds proanthocyanidins against induction of diabetes by alloxan in rats. Pharmacological research : the official journal of the Italian Pharmacological Society. 2005;52:264–70. doi: 10.1016/j.phrs.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Pinent M, Blay M, Blade MC, Salvado MJ, Arola L, Ardevol A. Grape seed-derived procyanidins have an antihyperglycemic effect in streptozotocin-induced diabetic rats and insulinomimetic activity in insulin-sensitive cell lines. Endocrinology. 2004;145:4985–90. doi: 10.1210/en.2004-0764. [DOI] [PubMed] [Google Scholar]
- 14.Balu M, Sangeetha P, Murali G, Panneerselvam C. Modulatory role of grape seed extract on age-related oxidative DNA damage in central nervous system of rats. Brain research bulletin. 2006;68:469–73. doi: 10.1016/j.brainresbull.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Devi A, Jolitha AB, Ishii N. Grape seed proanthocyanidin extract (GSPE) and antioxidant defense in the brain of adult rats. Medical science monitor : international medical journal of experimental and clinical research. 2006;12:BR124–9. [PubMed] [Google Scholar]
- 16.Shukitt-Hale B, Carey A, Simon L, Mark DA, Joseph JA. Effects of Concord grape juice on cognitive and motor deficits in aging. Nutrition. 2006;22:295–302. doi: 10.1016/j.nut.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Thomas P, Wang YJ, Zhong JH, Kosaraju S, O’Callaghan NJ, Zhou XF, et al. Grape seed polyphenols and curcumin reduce genomic instability events in a transgenic mouse model for Alzheimer’s disease. Mutation research. 2009;661:25–34. doi: 10.1016/j.mrfmmm.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Wang YJ, Thomas P, Zhong JH, Bi FF, Kosaraju S, Pollard A, et al. Consumption of grape seed extract prevents amyloid-beta deposition and attenuates inflammation in brain of an Alzheimer’s disease mouse. Neurotoxicity research. 2009;15:3–14. doi: 10.1007/s12640-009-9000-x. [DOI] [PubMed] [Google Scholar]
- 19.Jang JH, Surh YJ. Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free radical biology & medicine. 2003;34:1100–10. doi: 10.1016/s0891-5849(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 20.Virgili M, Contestabile A. Partial neuroprotection of in vivo excitotoxic brain damage by chronic administration of the red wine antioxidant agent, trans-resveratrol in rats. Neuroscience letters. 2000;281:123–6. doi: 10.1016/s0304-3940(00)00820-x. [DOI] [PubMed] [Google Scholar]
- 21.Zhuang H, Kim YS, Koehler RC, Dore S. Potential mechanism by which resveratrol, a red wine constituent, protects neurons. Annals of the New York Academy of Sciences. 2003;993:276–86. doi: 10.1111/j.1749-6632.2003.tb07534.x. discussion 87–8. [DOI] [PubMed] [Google Scholar]
- 22.Patki G, Allam FH, Atrooz F, Dao AT, Solanki N, Chugh G, et al. Grape powder intake prevents ovariectomy-induced anxiety-like behavior, memory impairment and high blood pressure in female Wistar rats. PloS one. 2013;8:e74522. doi: 10.1371/journal.pone.0074522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuhrman B, Volkova N, Coleman R, Aviram M. Grape powder polyphenols attenuate atherosclerosis development in apolipoprotein E deficient (E0) mice and reduce macrophage atherogenicity. The Journal of nutrition. 2005;135:722–8. doi: 10.1093/jn/135.4.722. [DOI] [PubMed] [Google Scholar]
- 24.Sonmez U, Sonmez A, Erbil G, Tekmen I, Baykara B. Neuroprotective effects of resveratrol against traumatic brain injury in immature rats. Neuroscience letters. 2007;420:133–7. doi: 10.1016/j.neulet.2007.04.070. [DOI] [PubMed] [Google Scholar]
- 25.Yehuda R, Antelman SM. Criteria for rationally evaluating animal models of posttraumatic stress disorder. Biological psychiatry. 1993;33:479–86. doi: 10.1016/0006-3223(93)90001-t. [DOI] [PubMed] [Google Scholar]
- 26.Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–33. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- 27.Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN, Jr, Sutker PB. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- 28.Kohda K, Harada K, Kato K, Hoshino A, Motohashi J, Yamaji T, et al. Glucocorticoid receptor activation is involved in producing abnormal phenotypes of single-prolonged stress rats: a putative post-traumatic stress disorder model. Neuroscience. 2007;148:22–33. doi: 10.1016/j.neuroscience.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 29.Dell’Osso L, Carmassi C, Del Debbio A, Catena Dell’Osso M, Bianchi C, da Pozzo E, et al. Brain-derived neurotrophic factor plasma levels in patients suffering from post-traumatic stress disorder. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33:899–902. doi: 10.1016/j.pnpbp.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan GB, Vasterling JJ, Vedak PC. Brain-derived neurotrophic factor in traumatic brain injury, post-traumatic stress disorder, and their comorbid conditions: role in pathogenesis and treatment. Behavioural pharmacology. 2010;21:427–37. doi: 10.1097/FBP.0b013e32833d8bc9. [DOI] [PubMed] [Google Scholar]
- 31.Fuchikami M, Yamamoto S, Morinobu S, Takei S, Yamawaki S. Epigenetic regulation of BDNF gene in response to stress. Psychiatry investigation. 2010;7:251–6. doi: 10.4306/pi.2010.7.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 2003;124:1386–92. doi: 10.1378/chest.124.4.1386. [DOI] [PubMed] [Google Scholar]
- 33.Fuchikami M, Morinobu S, Kurata A, Yamamoto S, Yamawaki S. Single immobilization stress differentially alters the expression profile of transcripts of the brain-derived neurotrophic factor (BDNF) gene and histone acetylation at its promoters in the rat hippocampus. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2009;12:73–82. doi: 10.1017/S1461145708008997. [DOI] [PubMed] [Google Scholar]
- 34.Liberzon I, Krstov M, Young EA. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology. 1997;22:443–53. doi: 10.1016/s0306-4530(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 35.Yoshii T, Sakamoto H, Kawasaki M, Ozawa H, Ueta Y, Onaka T, et al. The single-prolonged stress paradigm alters both the morphology and stress response of magnocellular vasopressin neurons. Neuroscience. 2008;156:466–74. doi: 10.1016/j.neuroscience.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 36.Salim S, Asghar M, Chugh G, Taneja M, Xia Z, Saha K. Oxidative stress: a potential recipe for anxiety, hypertension and insulin resistance. Brain research. 2010;1359:178–85. doi: 10.1016/j.brainres.2010.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, et al. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behavioural brain research. 2011;224:233–40. doi: 10.1016/j.bbr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Bert B, Fink H, Huston JP, Voits M. Fischer 344 and wistar rats differ in anxiety and habituation but not in water maze performance. Neurobiology of learning and memory. 2002;78:11–22. doi: 10.1006/nlme.2001.4040. [DOI] [PubMed] [Google Scholar]
- 39.Calvo N, Cecchi M, Kabbaj M, Watson SJ, Akil H. Differential effects of social defeat in rats with high and low locomotor response to novelty. Neuroscience. 2011;183:81–9. doi: 10.1016/j.neuroscience.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beck CT. Secondary traumatic stress in nurses: a systematic review. Archives of psychiatric nursing. 2011;25:1–10. doi: 10.1016/j.apnu.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Cougle JR, Resnick H, Kilpatrick DG. Does prior exposure to interpersonal violence increase risk of PTSD following subsequent exposure? Behaviour research and therapy. 2009;47:1012–7. doi: 10.1016/j.brat.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paxinos GWC. The rat brain sterotaxic coordinates. 6 Academic Press; 1986. [Google Scholar]
- 43.Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacology, biochemistry, and behavior. 1994;49:171–6. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 44.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxietylike behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 45.Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology. 1988;94:147–60. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- 46.Patki G, Solanki N, Atrooz F, Allam F, Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain research. 2013 doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouayed J, Rammal H, Younos C, Soulimani R. Positive correlation between peripheral blood granulocyte oxidative status and level of anxiety in mice. European journal of pharmacology. 2007;564:146–9. doi: 10.1016/j.ejphar.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 48.de Oliveira MR, Silvestrin RB, Mello EST, Moreira JC. Oxidative stress in the hippocampus, anxiety-like behavior and decreased locomotory and exploratory activity of adult rats: effects of sub acute vitamin A supplementation at therapeutic doses. Neurotoxicology. 2007;28:1191–9. doi: 10.1016/j.neuro.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Masood A, Nadeem A, Mustafa SJ, O’Donnell JM. Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. The Journal of pharmacology and experimental therapeutics. 2008;326:369–79. doi: 10.1124/jpet.108.137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Souza CG, Moreira JD, Siqueira IR, Pereira AG, Rieger DK, Souza DO, et al. Highly palatable diet consumption increases protein oxidation in rat frontal cortex and anxiety-like behavior. Life sciences. 2007;81:198–203. doi: 10.1016/j.lfs.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Hwang IK, Yoo KY, Kim DS, Jeong YK, Kim JD, Shin HK, et al. Neuroprotective effects of grape seed extract on neuronal injury by inhibiting DNA damage in the gerbil hippocampus after transient forebrain ischemia. Life sciences. 2004;75:1989–2001. doi: 10.1016/j.lfs.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 52.Narita K, Hisamoto M, Okuda T, Takeda S. Differential neuroprotective activity of two different grape seed extracts. PloS one. 2011;6:e14575. doi: 10.1371/journal.pone.0014575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patki G, Solanki N, Atrooz F, Ansari A, Allam F, Jannise B, et al. Novel mechanistic insights into treadmill exercise based rescue of social defeat-induced anxiety-like behavior and memory impairment in rats. Physiol Behav. 2014;130:135–44. doi: 10.1016/j.physbeh.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson CB, McLaughlin LD, Nair A, Ebenezer PJ, Dange R, Francis J. Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. PloS one. 2013;8:e76146. doi: 10.1371/journal.pone.0076146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gingrich JA. Oxidative stress is the new stress. Nature medicine. 2005;11:1281–2. doi: 10.1038/nm1205-1281. [DOI] [PubMed] [Google Scholar]
- 56.Hovatta I, Juhila J, Donner J. Oxidative stress in anxiety and comorbid disorders. Neuroscience research. 2010;68:261–75. doi: 10.1016/j.neures.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 57.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. The European journal of neuroscience. 2004;20:2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 58.Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, et al. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell death and differentiation. 2005;12:1329–43. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- 59.Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nature medicine. 2009;15:331–7. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee BH, Kim YK. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry investigation. 2010;7:231–5. doi: 10.4306/pi.2010.7.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boulle F, van den Hove DL, Jakob SB, Rutten BP, Hamon M, van Os J, et al. Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Molecular psychiatry. 2012;17:584–96. doi: 10.1038/mp.2011.107. [DOI] [PubMed] [Google Scholar]
- 62.Halliwell B, Rafter J, Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not? The American journal of clinical nutrition. 2005;81:268S–76S. doi: 10.1093/ajcn/81.1.268S. [DOI] [PubMed] [Google Scholar]
- 63.Janle EM, Lila MA, Grannan M, Wood L, Higgins A, Yousef GG, et al. Pharmacokinetics and tissue distribution of 14C-labeled grape polyphenols in the periphery and the central nervous system following oral administration. Journal of medicinal food. 2010;13:926–33. doi: 10.1089/jmf.2009.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]


